Residual Hepatic HCV-RNA in the Native Liver of Living Donor Liver Transplant Recipients with Chronic Hepatitis C: Mini-Review
Received: 08-Sep-2021 / Published Date: 12-Sep-2022
Abstract
Chronic Hepatitis C Virus (HCV) infection is the leading risk factor for Hepatocellular Carcinoma (HCC), with an annual HCC risk of 2%-4% in cirrhotic patients. With the highly effective and safe direct-acting antiviral (DAA) therapy, HCV infection would be considered “cured and eliminated” successfully in >95% treated patients. However, even with the widespread use of direct-acting antivirals, Living Donor Liver Transplantation (LDLT) plays an important role in advanced liver disease. According to our recent observation, the pre-transplant serum HCV RNA levels may give an underestimate of the number of positive HCV RNA cases and that hepatic HCV RNA data may be more accurate.
Herein, we would like to have a mini review to detailed address issues including the significance of hepatic HCV RNA and the discrepancy between hepatic HCV RNA and HCV core antigen in native liver of chronic hepatitis C recipients undergoing LDLT, a new insight into cure and complete elimination of HCV infection and the utilization of HCV-aviremic organs into aviremic recipients in liver transplantation.
Keywords: Hepatitis C virus; Hepatocellular carcinoma; HCV RNA; Direct-acting antiviral agents; Liver transplantation
Abbreviations
AFP: Alpha Fetoprotein; Anti-HCV: Antibody for Hepatitis C Virus; BCLC: Barcelona Clinic Liver Cancer; DAA: Direct-Acting Antiviral Agents; DNA: Deoxyribonucleic Acid; HCC: Hepatocellular Carcinoma; HCV: Hepatitis C Virus; LDLT: Living Donor Liver Transplantation; LT: Liver Transplantation; RNA: Ribonucleic Acid; RTPCR: Reverse Transcription-Polymerase Chain Reaction
Introduction
In clinical practice, despite Hepatitis C Virus (HCV)-infected patients achieved a Sustained Viral Response (SVR) with Direct-Acting Antiviral Agents (DAAs), they remained at risk of Hepatocellular Carcinoma (HCC) development [1-3]. A recent study showed unusually high rates of HCC after treatment with DAA therapy for hepatitis C-related liver cirrhosis [4]. Just as the similar phenomenon occurred in patients diagnosed to have COVID-19, the residual virus reservoirs in tissue specimens is warranted to be explored due to the potential replication and transmissibility in recovered individuals [5,6]. Hence, it is important to determine whether a SVR in patients who have underwent DAA treatment is equal to disease cured or persistent HCV infection which will progress in target organ, leading to future liver cirrhosis and HCC development.
A recent study conducted by Wang presented that HCV-RNA would persist in hepatocytes and/or PBMC in a certain of patients who achieved spontaneous or treatment-induced HCV RNA clearance from serum [7]. Furthermore, detection of occult hepatitis C Virus infection in patients with SVR to DAAs for recurrent infection after liver transplantation also has been reported in 2017 [8,9].
The clearances of HCV and treatment response to anti-viral therapy were associated with genetic variants [10]. In the setting of our liver transplant program, Chiu has investigated the association of IL28B SNPs rs12979860 and rs8099917 on HCV RNA status in donors and recipients of LDLT [11]. In the IL28B SNP rs12979860, high expression of unfavorable genotype CT in both recipient and donor led to high HCV-RNA recurrence after LDLT.
Notably, with the availability of DAA therapy, the number of liver transplants for HCV had decreased by more than one-third over the past decade worldwide [12]; however, there still remain some controversies to be clarified. The purpose of this article is to make an evident-based mini review of current issues and findings related to HCV patients undergoing LDLT in our liver transplantation program.
Identification of residual hepatic HCV-RNA in the native liver
In recent years, the introduction of DAA has made HCV become an easily treatable disease, with high rates of SVR, which is associated with improvement of liver dysfunction, promotion of fibrosis regression and reduction the risk of HCC development [1-3].
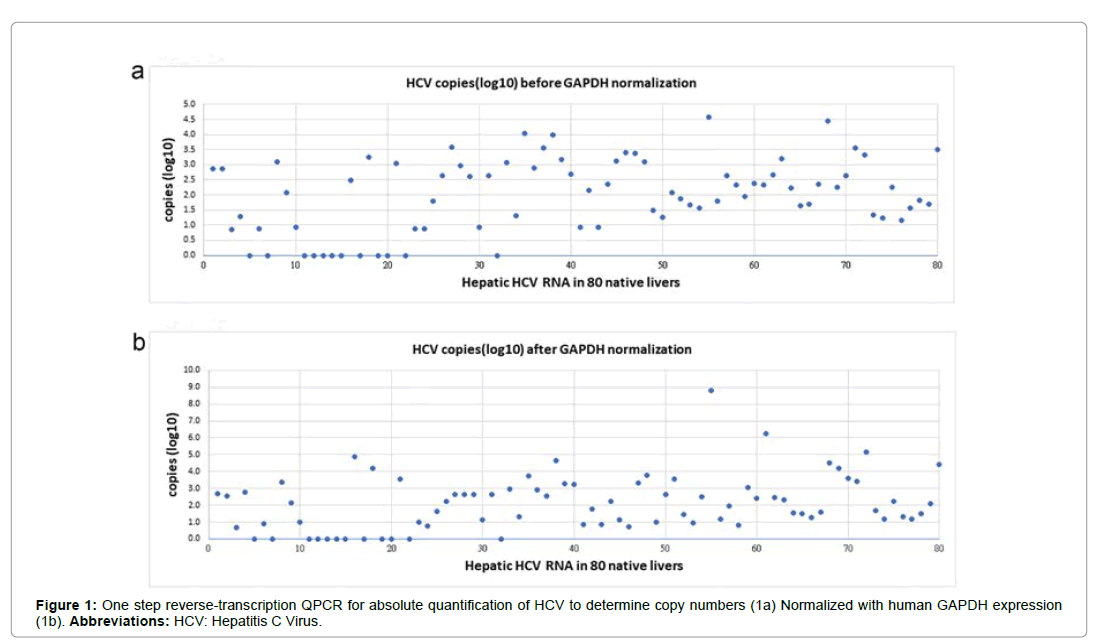
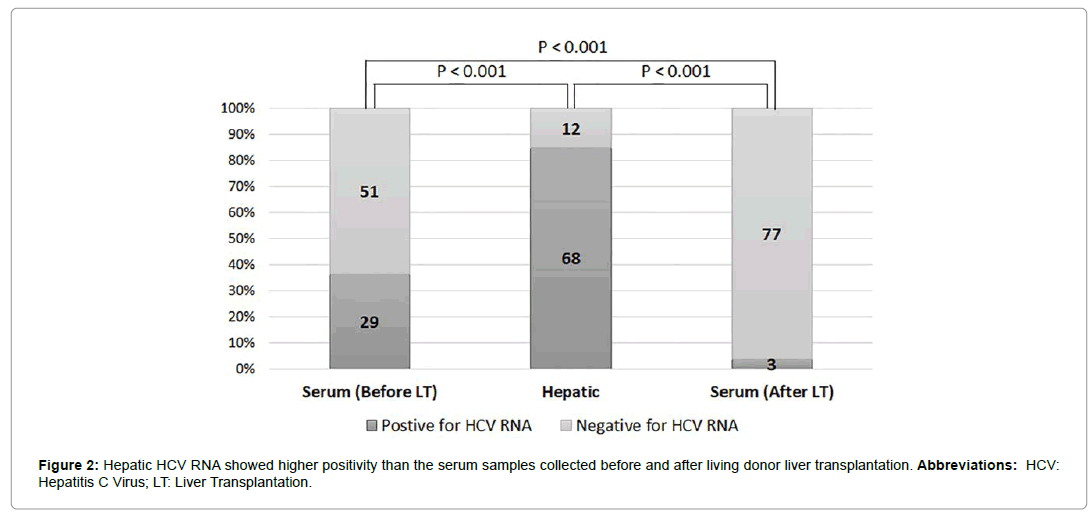
In recent study, we further evaluated 80 recipients with chronic hepatitis C [13]. Before LDLT, 36.25% (29/80) were serum positive and 63.75% (51/80) serum negative for HCV RNA (Table 1). After LDLT, we performed one-step reverse-transcription QPCR to generate a standard curve for the absolute quantification of hepatic HCV RNA. Quantification of relative expression (reported as arbitrary units (copy number)) was performed using the 2-ΔΔCt relative quantification method. Quantitative PCR data showed that the variability coefficient of Ct was always lower than 2% of the mean values (Figure 1). After LDLT, 85% (68/80) of native livers were positive for hepatic HCV RNA, whereas 15.0% (12/80) were negative for hepatic HCV RNA (Table 1). Among 68 recipients with positive hepatic HCV RNA, 3.75% (3/80) remained serum positive for HCV RNA (Figure 2). There was a statistically significant difference in HCV RNA identification between the serum HCV RNA and hepatic HCV RNA (p<0.001) before LT and between the serum HCV RNA and hepatic HCV RNA (p<0.001) after LT (Table 1).
| Category | Native liver | p value | |
|---|---|---|---|
| Hepatic HCV RNA (+) | Hepatic HCV RNA (-) | ||
| N=68 (85%) | N=12 (15.0%) | ||
| Serum HCV RNA before LT | 29 (36.3) | 51 (63.7) | <0.001 |
| Serum HCV RNA after LT | 3 (3.7) | 77 (96.3) | <0.001 |
HCV: Hepatitis C virus; LT: Liver Transplantation; Fisher’s exact test
Table 1: Comparison of the HCV RNA status of the native liver and serum before/after liver transplantation in 80 recipients who underwent living donor liver transplantation.
Among 68 recipients with positive hepatic HCV RNA, 39.7% (27/68) had been treated with DAA before LDLT, of which 51% (15/27) were positive for serum HCV RNA and 30.8% (12/27) were negative for serum HCV RNA. In total, 44% (3/68) cases remained positive for serum HCV RNA after LDLT. All three positive serum HCV RNA recipients sustained viral response because of DAA treatment after LDLT. There was no significant difference in hepatic HCV RNA levels between samples from patients pretreated with DAA and serum HCV RNA levels before and after LDLT (Table 2).
| Native liver | Hepatic HCV RNA (+) | Hepatic HCV RNA (-) | p value | |
|---|---|---|---|---|
| N=68 (%) | N=12 (%) | |||
| Pre-Tx DAA | 27 (39.7) | 7 (58.3) | 0.187 | |
| 15 (51.7)a | 12 (30.8)a’ | |||
| Serum HCV RNA before LT | Positive | Negative | Negative | 0.067* |
| 29 (42.7)b | 39 (57.3)b’ | 12 (100) | ||
| Serum HCV RNA after LT | Positive | Negative | ||
| 3 (4.4) | 65 (95.6) | |||
| Post-Tx DAA | 1 (3.5)c | 2 (5.1)c’ | 0.414** | |
* =aa’:bb’; ** =aa’:cc’; Fisher’s exact test; Pre-Tx: pre-treatment; Post-Tx: Post-Treatment; DAA: Direct-Acting Antiviral Agent.
Table 2: The relationship between the hepatic HCV RNA levels in patients pre-treated with/without direct-acting antiviral agents and the outcome of serum HCV RNA after living donor liver transplantation.
Our recent study concluded that the significant underestimation of HCV RNA in the serum was identified by measuring the hepatic HCV RNA levels (p<0.001) followed by their comparison with the pre-LDLT serum HCV RNA and the removed native liver hepatic HCV RNA levels.
HCV core antigen (HCV Ag) in native liver of chronic hepatitis C recipients
According to guidelines from European Association for the Study of the Liver (EASL) recommended HCV core Antigen (HCV Ag) testing to be a less expensive alternative to HCV RNA for determination of HCV viraemia when HCV RNA testing is not available [14]. In 2018, Tilborg conducted a research to support the use of HCV Ag testing to document HCV viraemia in a cost-saving diagnostic algorithm (sensitivity 94%, 95% CI 86-98; specificity 100%, 95% CI 94-100); it also could be used for documentation of treatment adherence, but it might not be adequate to determine SVR [15]. Recently, a study in Taiwanese cohort analyzing serum samples from 110 patients treated with DAAs suggested that HCV Ag assay may be a feasible alternative to HCV RNA for the determination of SVR12 in patients treated with DAAs [16].
In our liver transplant setting, we investigated the discrepancy between hepatic HCV RNA and HCV Ag in the liver tissue by using one step reverse transcribed QPCR for identification of hepatic HCV RNA and HCV Ag assay based on the protocol provided by Human Hepatitis C Virus Core Antigen ELISA Kit (Cat. No: MBS 167758) in the explanted native liver of CHC patients undergoing LDLT. Among 80 HCV related recipients, 85% (68/80) positive HCV-RNA was significantly higher in the native liver tissues than in the serum before (29/80, 36.3%; p=0.000) and after LDLT (3/80, 4.4%; p=0.000). In contrast, hepatic HCV Ag was 100 % negative identified in all 80 removed native liver.
Based on our research, we suggested HCV Ag assay may have lack of sensitivity and negative predictive value in liver tissues. In contrast to the serum HCV RNA and HCV Ag, a great discrepancy might be described between hepatic HCV RNA and HCV Ag in the liver tissue.
New insights into cure of HCV infection
Similar to the mechanism of antiviral treatment for hepatitis B infection, patients with negative serum HCV RNA may not have HCV clearance in the liver. In contrast, the high percentage of positive hepatic HCV RNA represents that HCV returns to the hepatocyte as well as a target organ for its replication. It may not be beneficial for the long-term outcomes of chronic hepatitis C infection. Recent reports have shown unusually high rates of HCC after treatment with DAA for hepatitis C-related liver cirrhosis [17,18]. Therefore, high-viral carriers target organs, advanced cirrhosis and HCC are completely cured simultaneously through liver transplantation. Obtaining safe results is a real benefit for patients. In our study, although there were no statistically significant differences in clinical liver function before and after LDLT between patients positive and negative for hepatic HCV RNA, 4.4% of recipients with positive hepatic HCV RNA remained positive for serum HCV RNA after LDLT. All three recipients had already been treated with DAA after LDLT and had a sustained viral response in the serum. The outcome of DAA treatment after LDLT may be different from that of the other 77 recipients, as HCV is driven back to a new liver graft. The high load of HCV RNA in a liver graft may be an entirely new concern. Owing to hepatocyte infection with HCV, cell surface levels of sulfate proteins increase and subsequently, the virus enters the liver cells by regulating SMAD6 and SMAD7 [19]. In contrast, DAA does not inhibit HCV in liver cells. The new liver graft may become a target organ for HCV infection after DAA treatment. In our recent studies, we showed that HCV enhanced expression of micro-RNA 122, micro-RNA 92b and PNPLA3 variants, as well as increased the risk of developing HCC; these form the clinical evidence of the recurrence of HCC [20-22]. In addition to environmental factors such as alcohol and tobacco [23,24], hepatic HCV RNA has been reported to play a role in the severity of HCV infection and the response to antiviral treatment [25]. We suggest that the chronic liver injury concomitant residual hepatic HCV-RNA and long-term outcomes with DAA therapy could still remain an issue after SVR [7].
HCV-positive donors to HCV-negative recipients
Liver transplantation has been hampered by the shortage of available donors in relation to the number of people on the waiting list. To decrease the number of deaths on the waiting list and overcome the problems of lack of donors, many transplant centers aggressively use marginal donors or even HCV seropositive, non-aviremic donors to HCV-seronegative recipients [26]. Recent research from Kapila showed a real-world experience of the transplantation of HCV-aviremic organs into aviremic recipients and they stated that in carefully selected patients, the use of HCV-aviremic grafts in the DAA era appears to be efficacious and well tolerated [27].
However, based on our study, hepatic HCV RNA might still be found after LDLT even in patients with pre-transplant negative serum HCV RNA. Serum HCV-RNA load might represent an underrated estimation to entire HCV viral loads in patients receiving anti-viral therapy. Nowadays, there is no strong evidence published yet to correlate high de novo HCC occurrence or recurrence after LT in hepatic HCV RNA patients, but given the constant exposure to immunosuppressant in this organ-transplant cohort in the real-world setting, the oncologic safety of aviremic/viraemia HCV-infected organ donor may need longer duration of observation, even in the DAA era.
Conclusion
A complete eradication of HCV in patients treated with antiviral agents after clinical diagnosis of SVR seems unlikely despite of substantially improved anti-HCV efficacy. Based on the results of our study, the importance of pre-transplant antiviral therapy to attain negative HCV RNA needs to be emphasized here; it has proven to be far more beneficial than post-transplant antiviral therapy.
Acknowledgments
This work was supported by the grant number CMRPG8F1541 and CMRPG8H1131 from the Chang Gung Memorial Hospital of Taiwan. We gratefully acknowledge all the participants who participated in the study and the study team for their support.
Authors Contributions
Study concept and design: King-Wah Chiu and Chih-Chi Wang; data collection: Shu-Hsien Lin and Chih-Che Lin; data analysis and interpretation: King-Wah Chiu and Chih-Chi Wang; performed experiments: Kuang-Den Chen, Kuang-Tzu Huang and Li-Wen Hsu; and manuscript drafting and critical revisions: Shu-Hsien Lin, King- Wah Chiu.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Singal AG, Lim JK, Kanwal F (2019) AGA Clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis c infection and hepatocellular carcinoma: Expert review. Gastroenterology 156: 2149-2157.
- European Association for the Study of the Liver (2018) EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 69: 182-236.
- Ghany MG, Morgan TR (2020) Hepatitis C guidance 2019 update: American association for the study of liver diseases-infectious diseases society of america recommendations for testing, managing, and treating hepatitis c virus infection. Hepatology 71: 686-721.
- Ravi S, Axley P, Jones D, Kodali S, Simpson H, et al. (2017) Unusually high rates of hepatocellular carcinoma after treatment with direct-acting antiviral therapy for hepatitis c related cirrhosis. Gastroenterology 152: 911-912.
- Zuo T, Liu Q, Zhang F, GCY Lui, Tso EYK, et al. (2021) Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut 70: 276-284.
- Cheung CCL, Goh D, Lim X, Tien TZ, Lim JCT, et al. (2021) Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut.
- Wang Y, Rao H, Chi X, Li B, Liu H, et al. (2019) Detection of residual HCV-RNA in patients who have achieved sustained virological response is associated with persistent histological abnormality. EBioMedicine 46: 227-235.
- Enomoto M, Murakami Y, Kawada N (2017) Detection of HCV RNA in sustained virologic response to direct-acting antiviral agents: Occult or science fiction? Gastroenterology 153(1): 327-328.
- Elmasry S, Wadhwa S, Bang BR, Jerome KR, Kahn JA, et al., (2017) Detection of occult hepatitis c virus infection in patients who achieved a sustained virologic response to direct-acting antiviral agents for recurrent infection after liver transplantation. Gastroenterology 152: 550-553.
- O'Brien TR, Yang HI, Groover S, Jeng WJ (2019) Genetic factors that affect spontaneous clearance of hepatitis c or b virus, response to treatment, and disease progression. Gastroenterology 156: 400-417.
- Chiu KW, Nakano T, Chen KD, Lin CC, Hu TH, et al. ( 2016) Association of il28b snps rs12979860 and rs8099917 on hepatitis c virus-rna status in donors/recipients of living donor liver transplantation. Plos one 11: e0156846.
- Cotter TG, Paul S, Sandikc B, Couri T, Bodzin AS, et al., (2019) Improved graft survival after liver transplantation for recipients with hepatitis c virus in the direct-acting antiviral era. Liver Transpl 25: 598-609.
- Lin SH, Wang CC, Huang KT, Chen KD, Lin CC, et al. (2021) HCV RNA in serum and liver samples of patients undergoing living donor liver transplantation. J Int Med Res 49: 3000605211034945.
- European Association for the Study of the Liver, EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol 73: 1170-1218.
- Tilborg MV, AL Marzooqi SH, Wong WWL, Maan R, Vermehren J, et al. (2018) HCV core antigen as an alternative to HCV RNA testing in the era of direct-acting antivirals: Retrospective screening and diagnostic cohort studies. The Lancet Gastroenterology & Hepatology 3: 856-864.
- Lin SF, Fletcher GJ, Anantharam R, Varughese S , David VG, et al., (2020) Clinical utility of hepatitis c virus core antigen assay in the monitoring of direct-acting antivirals for chronic hepatitis c. PLoS One 15: e0229994.
- Ioannou GN, Green PK, Beste LA, Mun EJ, Kerr KF, et al. (2018) Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis c. J Hepatol 69: 1088-1098.
- Guarino M, Viganò L, Ponziani FR, Giannini ED, Lai Q, et al. (2018) Recurrence of hepatocellular carcinoma after direct acting antiviral treatment for hepatitis c virus infection: Literature review and risk analysis. Dig Liver Dis 50: 1105-1114.
- Zhang F, Sodroski C, Cha H, Li Q, Liang TJ (2017) Infection of hepatocytes with HCV increases cell surface levels of heparan sulfate proteoglycans, uptake of cholesterol and lipoprotein, and virus entry by up-regulating smad6 and smad7. Gastroenterology 152: 257-270.
- Yen YH, Tsai MC, Wu CK, Chang KC, Hung CH, et al. (2018) Association between PNPLA3 (rs738409 C>G) variant and hepatocellular carcinoma in asian chronic hepatitis c patients: A longitudinal study. J Formos Med Assoc 117: 833-840.
- Nakano T, Chen IH, Wang CC, Chen P-J, Tseng HP, et al. (2019) Circulating exosomal miR-92b: Its role for cancer immunoediting and clinical value for prediction of posttransplant hepatocellular carcinoma recurrence. Am J Transplant 19: 3250-3262.
- Chen KD, Lin CC, Tsai MC, Huang KT, Chiu KW, et al. (2018) Tumor microenvironment mediated by suppression of autophagic flux drives liver malignancy. Biomed J 41: 163-168.
- Hou W, Bukong TN, Kodys K, Szabo G (2013) Alcohol facilitates HCV RNA replication via up-regulation of miR-122 expression and inhibition of cyclin G1 in human hepatoma cells. Alcohol Clin Exp Res 37: 599-608.
- Zhao L, Li F, Taylor EW (2013) Can tobacco use promote HCV-induced miR-122 hijacking and hepatocarcinogenesis? Med Hypotheses 80: 131-133.
- Maylin S, Laouénan C, Peignoux MM, Panhard X, Lapalus M, et al. (2012) Role of hepatic HCV-RNA level on the severity of chronic hepatitis c and response to antiviral therapy. J Clin Virol 53: 43-47.
- Hughes CB, Humar A(2021) Liver transplantation: Current and future. Abdom Radiol 46: 2-8.
- Kapila N, Menon KVN, Al-Khalloufi K, Vanatta JM, Murgas C, et al. (2020) Hepatitis C Virus NAT-positive solid organ allografts transplanted into hepatitis c virus-negative recipients: A Real-world experience. Hepatology 72: 32-41.
Citation: Lin SH, Wang CC, Huang KT, Chen KD, Lin CC (2021) Residual Hepatic HCV-RNA in the Native Liver of Living Donor Liver Transplant Recipients with Chronic Hepatitis C: Mini-Review. J Oncol Res Treat 6:167.
Copyright: © 2021 Lin SH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 3307
- [From(publication date): 0-2021 - Dec 23, 2025]
- Breakdown by view type
- HTML page views: 2473
- PDF downloads: 834