Rewarding the Autistic through Understanding the Neural Cognitive, Emotional, Motor binding Circuitry in the Developmental Brain
Received: 26-Oct-2018 / Accepted Date: 01-Nov-2018 / Published Date: 09-Nov-2018 DOI: 10.4172/2572-4983.1000171
Keywords: Autism spectrum disorder syndrome; Cerebellar corticoemotional motor neural circuits; Reward; Adaptive time delay; Theory of mind; Early detection
Introduction
Autism is a complex spectrum disorder; mostly observed at 2-3 years of age with characteristic anomalous, deviant cognitive, emotional, behavioural and neuro-motor developments till adulthood. Each autistic is different. Increasing prevalence up to 2% in children with a greater association and severity in male gender is noted. Asperger’s syndrome, Rett syndrome, childhood disintegrative disorder, pervasive developmental disorder and autism are all grouped under Autism Spectrum Disorders (ASD) [1]. ASD sufferers can be readily recognised by their repetitive, sometimes aloof and servant behaviour; echolalia, inappropriate language response and impaired attention to other. The overall consequences deprived the ASD individuals with adaptive learning, social, relationship and communication skills. ASD are frequently stigmatized as mentally insane in our community. The American Academy of Pediatrics (AAP) recommends children be screened for developmental disorders at wellchild preventive visits before age three [2]. The Centres for Disease Control and Prevention (CDC) of United States have identified possible clinical alerts or red flags for ASD [1,2] (Table 1).
| S.N. | Possible Clinical Alerts or Red Flags for ASD |
|---|---|
| 1 | Not responding to his/her name by 12 months of age |
| 2 | Not pointing at objects to show interest by 14 months |
| 3 | Not playing "pretend" games by 18 months |
| 4 | Avoiding eye contact or preferring to be alone |
| 5 | Getting upset by minor changes |
| 6 | Flapping their hands, rocking their body or spinning in circles |
| 7 | Having unusual and sometimes intense reactions to the way things smell, taste, feel and/or look |
Table 1: Clinical signs that parents should alert the possibilities of ASD.
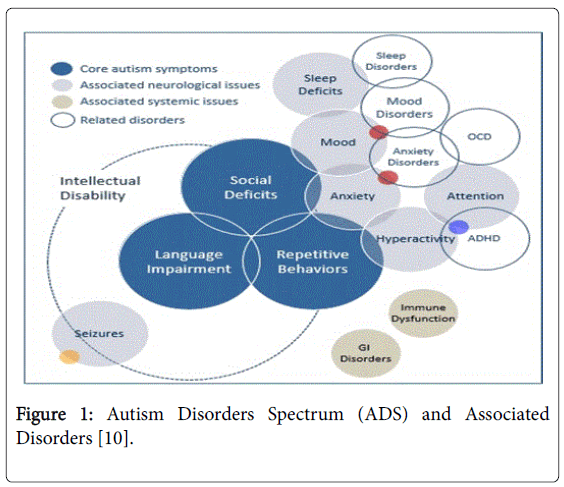
Despite its increasing prevalence, researchers are still unable to unveil the exact aetiologies of ASD. Genetics with chromosome 1q, 2q, 7q, 2q, 15q11-13, 16q, TRIO gene mutations and other hereditary conditions like fragile X syndrome, tuberous sclerosis and phenylketonuria (PKU), early in-utero environmental insults like viral infections, metabolic imbalances and exposure to chemicals like aluminium, mercury, ethyl alcohol, pesticides, thalidomide, misoprostol and valproic acid are all reported [3,4]. Interestingly, ASD individuals also have an increase odd with immune dysfunction resulting allergic sensitivities, skin allergies like eczema, infections, gastrointestinal symptoms like constipation and diarrhoea, low muscle tone, pain, pica, epileptic fits, sensory and hearing impairments, seizures, anxieties and sleep problems [5] (Figure 1). Triangulating these epidemiological data, one may put forward a falsifiable model on the pathogenesis of ASD: multiple related genetic mutations and developmental insults from the environment resulting in an incoherent, fragmented excitatory and inhibitory neuronal circuitries in the responsible anatomical brain structures; namely, the cerebellum, neocortex, thalamus and its adjacent limbic structures especially the amygdala which primary physiological function are to facilitate motor planning, cognition, attention, emotion, body basic autonomic nervous system and neuro-endocrine pathways (Figure 2).
Figure 1: Autism Disorders Spectrum (ADS) and Associated Disorders [10].
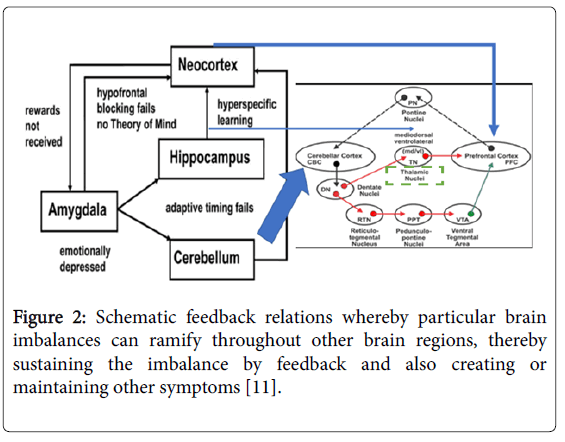
Figure 2: Schematic feedback relations whereby particular brain imbalances can ramify throughout other brain regions, thereby sustaining the imbalance by feedback and also creating or maintaining other symptoms [11].
The cerebellum, orbitofrontal cortex of the neocortex, thalamus, amygdala and hypothalamus and its neuro circuits are implicated in the pathogenesis of ASD. The integrated closeness and adaptation of these multidimensional circuitry sent matched coherent excitatory and inhibitory signals mainly in form of the glutamate GABA, acetylcholine, dopamine and serotonin neurotransmitters chemically and bind these relatively distantly located parts of the brain together through their multi-dimensional synaptic networks so as to maintain a state of stability, survival adaptiveness, learning, socialization and learning. Disruption of this binding or incoherence of neuronal networks due to genetic mutations, chemicals intoxications, miswiring of neuronal circuitry and other yet undiscovered causes may result ASD [6-9]. Embryogenesis of the human brain in a normal, healthy symmetrical and timely multiple synaptic development following the natural ontogeny will produce a coherent cognitively neural motor bind well-being with evolutionary survival advantage.
The cerebellum
In humans, the cerebellum is involved in motor control, cognitive functions such as attention and language as well as in regulating fear and pleasure responses. It is essential for several types of motor learning especially changes in sensorimotor relationships. The cerebellum is a complicated structure consisting of the anterior lobe, posterior lobe and flocculonodular lobe. The medial zone of the anterior and posterior lobes receives proprioceptive input from the spinal cord, cranial trigeminal nerve, visual and auditory systems. It sends sensory afferents to deep cerebellar nuclei which project to both the cerebral cortex and the brain stem, thus providing modulation of descending motor systems. The lateral zone, also known as neocerebellum contributes the cerebrocerebellum receives input exclusively from the cerebral parietal cortex via the pontine nuclei; forming the important cortico-ponto-cerebellar pathways; and sends output to the ventrolateral thalamus which in turn connected to motor areas of the cerebral cortex and to the red nucleus. Thus, the lateral cerebellum is involved in planning future movement, evaluating sensory information for future action and higher cognitive functions.
Post mortem anatomical studies showed that ASD individuals have diminished Purkinje cell in terms of size, number and branching patterns especially in cerebellum VI and VII lobules. Functional MRI (f-MRI) showed dysplasia; hypo or hyperplasia of the cerebellar vermis in ASD compared with norm. Purkinje cells have rich serotonin contents, reduction in serotonin neurotransmitters may disinhibited arousal and emotional behaviour in autism [12-14]. Low serotonin level predicts anxiety, unhappiness and depression. Emotional facial expressions using f-MRI in high functioning autistic adults showed under activation of the left cerebellum [15]. Magnetic resonance spectroscopy (MRS) showed decreased cerebellar N-acetyl-aspartame (NAA); a neuronal indicator of function and maturity [16]. An inability to shift attention in a timely manner is one of the primary problems in autism. Adaptive timing response which critically involved the cerebellum was absent as illustrated in the classical eye blink reflex was impaired in ASD. Autistic may take more time to shift their attention from one stimulus to another in the changing environment full of multiple stimuli. This delay disables the autistic important contents and context informational cues which is essential for their adaptive learning. The difficulty of ASD in controlling timely response to stimuli is a motor behavioural function of cerebellum in associative learning. Auditory, visual and oculomotor response also failed to be modulated and reproduced consistently independent of the stimulus duration again strengthened the assertion that adaptive timing function as controlled by cerebellum was missing in ASD. The defective lesions are believed to be localized to a specific part of the interposed nucleus in the deep cerebellar nuclei (DCN) [17-20]. Lastly, individuals resulted from cerebellar injuries clinically behave with similar cognitive developmental impairments as in autism [21,22]. At this juncture, emotional disinhibition, associative learning, motor planning, visual, auditory and verbal disability was evidently shown to be correlated with cerebellar developmental aberrancy, dysfunction and morphological abnormalities in ASD which may take place as early as in-utero neonatal period.
The pathogenetic role of the cerebellum in the development in ASD is also in consistent with its long embryological ontological development as endogenous sonic hedgehog signalling stimulates rapid proliferation of cerebellar granule neuron progenitors (CGNPs) in the external granule layer (EGL) during early in utero neonatal development into late neonatal period [23]. This prolonged period of embryogenesis theoretically provides a long window to mutagenesis and probable exposure to environmental toxic insults. The importance of the cerebellum in the motor development in ASD is also related to the evolution of humans into bipedalism which is believed to be a requisite for higher cognitive functions as explained by the cerebellum expansion theory. An upright posture mandated Homo sapiens to have a smaller rostral neocortex but bigger volume cerebellum caudally. An expansion of the lateral lobes of the cerebellum act as a back-fire station actively feedforward input to the neocortex in motor planning, higher cognitive activities, sensorimotor and emotional modulation.
The most recent period of human evolution showed a disproportionate increase in the relative size of the cerebellum to neocortex. The cerebellum compared to rest of the brain, has been increasing while the cerebral cortex decreasing in size. The cerebellum lateral hemispheres are three times bigger in size in humans than monkeys. Thus, the expansion of the cerebellum is an important milestone during Hominidae evolution especially in the evolvment of motor tasks, visual-spatial skills, higher cognitive emotional skills and learning which is tightly bind to the rest of the brain [24,25].
The neocortex
Anatomical mapping of the cerebellum using functional MRI suggest more than half of the cerebellar cortex is interconnected with association zones of the cerebral cortex [26]. Post mortem anatomical survey has shown smaller cortical cell column in the prefrontal and temporal cortex in ASD [27]. Brain volume growth is also different compared with the norm with a smaller head circumference at birth followed by a rapid increase in size and volume of brain especially the white matter after one month of age; 37% of autistic children were reported to have developmental macrocephaly by 4 years of age [28,29]. This is in contrast with the previously discussed evolutionary reduction in size of neocortex compared to cerebellum. The most striking cortical feature in ASD is neocortical asymmetries with the greatest amounts of cortical cells in higher-order association cortices and a disproportional rightward asymmetrical cortex in the autistic individuals [30]. Reversal of the normal language-related frontal cortex asymmetry was also noted [31]. fMRI showed a reduction of activation in the dorsolateral prefrontal cortex and reduction of both superior temporal cortex and temporal frontal cortical areas in making inferences from viewing another individual eye; hence absence of eye contact with other [32,33]. More delicate visually paced finger movements which needed refined coordination was reduced like writing in the activated cortical area but involved in a different area [34]. During sentence comprehension task, a reverse weighted activation in Wernicke’s area more than the normally used Broca’s area was seen [35].
Event-related potential (ERP) studies, which have been used to study brain dynamics, showed that higher brain cognitive functions in ASD were altered. ERP P300 covaries with stimulus probability and task relevance showed that language stimuli at left hemisphere were smaller and not at right hemisphere. However, no differences were found for musical chord stimuli consistent with the notion that adaptive timing for musical cord stimuli (non-language) develop normally in ASD and involved in different circuitry whereas phonetic stimuli (language-related) auditory stimuli is affected in autism [36]. This inappropriateness of phonetic language response greatly affected the ASD individual’s socialization, communication, restricted patterns behaviour and learning skills [37]. Cortical deficiencies also impaired the ASD from symbolic thinking, learning, imaginary play to even mental retardation.
The thalamus, amygdala and limbic system
Post mortem studies revealed abnormalities in the amygdala, hippocampus and ventral temporal cortex. fMRI has shown altered in the size and volume in amygdala in autism [38,39]. The amygdala is of particular significance in ASD as negative emotional reactivity secondary to noise, smells or light will cause instantaneous arousal in some ASD. It has been proposed that an under aroused emotional depression in the amygdala and related affected area typify the neural dynamics autistic behaviour [11,40,41].
The limbic system which better described as the paleomammalian cortex, consists of the thalamus, olfactory bulbs, hippocampus, hypothalamus, anterior thalamic nuclei, mammillary body and the septum pellucidum, the amygdala and its associated nuclei control emotions, body internal motivation, drive like desires, olfaction and long-term memory. The anterior nuclei of the thalamus are actively involved in the memory process. The thalamus with its different nuclei regulates many body vital autonomic functions like sleep, autonomic nervous, cardiac, respiratory, intestinal and auto-immune functions. As the thalamus is involved in ASD, many bodily innate unconscious autonomic defense and survival neuro-endocrine pathways may be affected in various degrees like autoimmunity, allergic hypersensitization, gut motility, pain and ultimately sleep disturbances.
Neuronal circuits aberrancy between the cerebellum, neocortex, thalamus, amygdala associated with cognitive, motor and emotional behaviour in ASD
The existence of a cerebello-thalamic circuitry with increasing activation was seen in ASD [42]. Activation of the ipsilateral anterior cerebellar hemisphere is increased in magnitude in spatial extent during motor task in ASD individuals in addition to atypical activation in contralateral and posterior cerebellar regions. Moreover, increased activation was correlated with the degree of cerebellar structural abnormality. This provides good evidence dysfunction of the autistic cerebellum may be reflection of cerebellar anatomical abnormality. Neurofunctional deficit might be a key contributor to the development of certain diagnostic features of autism like impaired communication and social interaction, restricted interests, and repetitive behaviours [43]. Social impairment was negatively correlated with the degree of anisotropy of the superior cerebellar peduncle, the primary output path of the cerebellum. Thus, cerebellar white matter decreases, ASD symptoms increases [44,45]. The thalamic nuclei like the relay nuclei receive specific definitive electrical signals and project this to the corresponding functional domains of distinct area in the cerebral cortex. The ventral posterolateral nuclei (VPL), ventral posteromedial nuclei (VPM), medial geniculate (MG) and lateral geniculate (LG) nuclei are thalamic nuclei which relay primary sensations to the cortex. The ventral lateral (VL) nuclei give feedback signals to the cerebellum while the ventral anterior nucleus (VAN) feedback basal ganglion output. Thus, a synaptic connective wiring networks are in place between the motor regions; cerebellum, basal ganglion and the conscious executor of the brain; the cerebral cortex. The association nuclei receive signals input from the cerebral cortex which can be inhibitory and project back to the latter in cortex association areas so as to regulate activity. The reticular nucleus encapsulated most of the thalamus and receives input from the cerebral cortex and dorsal thalamic nuclei. The thalamic reticular nucleus receives neuronal projections from the external segment of the Globus Pallidus. The Globus Pallidus is a component of basal ganglion of the brain which involved in the regulation of voluntary movement. Through disinhibition, neuronal signals were sent to the thalamus resulting in initiation of movement [46]. Thus, each nucleus of the thalamus is capable at rest in dispatching electrical signals and communications to specific anatomical domains to the corresponding cerebral cortex and areas of motor function. Through its association neurons, the signals are feedback to the thalamus and wired to the other structures like the amygdala which in turn flow back to the cortex. However, in the case of ASD with its multifactorial aetiologies, hypoactivity of orbitofrontal cortex or amygdala which provides motivationally directed goals, incentives or intentions drive improperly function, and this initially movement driven, thalamus modulated cortical emotional conscious motivational neuronal circuit will be disrupted and may result an arena of behaviour, emotional, cognitive, movement and autonomic nervous system pathological states.
Some authorities have reviewed this as an exhibition of failure of reward processing noted in individuals diagnosed with ASD [47-49]. As the ASD amygdala emotionally depressed, hypofrontal blocking fails; individuals become mindless; pleasantness, security and reward signals are not feedback and received by the amygdala; without reward subsequent social learning become non-existent. Individuals with ASD exhibited a specific behavioral insensitivity to social rewards like nonresponse to a smiling greeting face. ASD individual autonomic response was also impaired during social reward studies as children with autism exhibited decreased pupillary dilation when looking at happy faces [50,51]. This may be due to inactivity of the amygdala and incoherence and mismatching of thalamocortical circuitry. Similarly, children with autism failed to gaze orient to naturally occurring social stimuli also provide further evidence this reward circuits interconnecting, the neocortex, thalamus, cerebellum and amygdala are deficit in ASD. Even in the case of hunger as a drive to amygdala is emotionally depressed with compared to normal control children. All these suggested that the impaired social skills, learning and motor behavior may result from a reduced reward feeling in response to social stimuli. The involvements of cerebellum in deficits in social reward has previously exemplified by the paradigm of eyeblink conditioning which is absent in the afferents to the cerebellum can be readily explained by the diminution of the adaptive timely response. This absence of adaptive timely response which is crucial in a vast array of motor behavioural function of cerebellum in associative, auditory, visual and oculomotor learning may be secondary to inactivation of the cerebellar dentate nuclei operant to a food reward within its interposed nuclei in deep cerebellar complex.
Conclusion
In sum, the above revisit of the inter-connectiveness and relationship between human cerebellum, neocortex, thalamus, amygdala and its associated excitatory feed forward and inhibitory feedback circuitries incorporating the rewarding hypothesis, emotionally depressed amygdala, adaptive timing failed processing in cerebellum and no theory of mind is not to imply that this is what exactly happening in the pathogenesis of ASD. In fact, as ASD is such a diverse, complex and progressive cognitive, emotional, motor and autonomic syndrome, it is difficult to solve all the observed phenomenon in one coherent theory. On the contrary, it is hope that this mini-review may spark discussion, falsification and better models through scientific arguments and repeatable controlled experiments; serve as a move forward exploration and enquiry about the entangled puzzling causes of ADS, in addition to the already known genetic, neurotransmitters and toxins corroboration.
References
- Copeland JN (2018) What Is Autism Spectrum Disorder? American Psychiatric Association. US.
- Weitzman C, Wegner L (2015) Promoting Optimal Development: Screening for Behavioral and Emotional Problems. Pediatrics 135: 384-395.
- Chast P, Leboyer M (2012) Autism risk factors; genes environment and gene-environment interactions. Diagogues Clini Neurosci 14: 281-292.
- Modabbernia A, Velthorst E, Reichenberg A (2017) Environmental risk factors for autism; an evidence-based review of systematic reviews and meta-analyses. Mol Autism 8: 13.
- http://www.mensahmedical.com/autism-spectrum-disorders-biochemical-imbalances/
- Rubenstein JL, Merzenich MM (2003) Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2: 255-267.
- Choudhury PR, Lahiri S, Rajamma U (2012) Glutamate mediated signaling in the pathoâ€physiology of autism spectrum disorders. Pharmacol Biochem Behav 100: 841-849.
- Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, et al. (2012) GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neurosci Biobehav Rev 36: 2044–2055.
- Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J (2001) Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology 57: 1618-1628.
- http://www.mensahmedical.com/autism-spectrum-disorders-biochemical-imbalances/
- Grossberg S, Seidman D (2006) Neural dynamics of Autistic Bahaviors; Cognitive, Emotional, and timing substrates. Psychol Rev 113: 483-525.
- Fatemi SH, Halt AR, Realmuto G, Earle J, Kist DA, et al. (2002) Purkinje cell size is reduced in cerebellum of patients in autism. Cell Mol Neurobiol 22: 171-175.
- Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL (1988) Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med 318: 1349-1354.
- Boldu M, Du Plessis AJ, Sullivan N, Khwaja OS, Zhang X, et al. (2011) Spectrum of neurodevelopmental disabilities in children with cerebellar malformations. Dev Med Child Neurol 53: 409-416.
- Critchley HD, Daly EM, Bullman ET, Williams SCR, Van Amelsvoort T, et al. (2000) The functional neuro-anatomy of social behaviour: Changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain 123: 2203-2212.
- Chugani DC, Sundram BS, Beham M, Lee M, Moore GJ (1999) Evidence of altered energy metabolism in autistic children. Progress in Neuropsychopharmacology and Biological Psychiatry. 23: 635-641.
- Szelag E, Kowalska J, Galkowski T, Poppel E (2004) Temporal processing deficits in high-functioning children in autism. Br J Psychol 95: 269-282.
- Kloth AD, Badura A, Li A, Cherskov A, Connolly SG, et al. (2015) Cerebellar associative sensory learning defects in five mouse autism models. eLife 4: e06085.
- Bullock D, Fiala JC, Grossberg S. (1994) A neural model of timed response learning in the cerebellum. Neural Networks 7: 1101-1114.
- Fiala JC, Grossberg S, Bullock D (1996) Metabotropic glutamate receptor activation in cerebellar Purkinje cells as substrate for adaptive timing of the classically conditioned eye blink response. Journal of Neuroscience 16: 3760-3774.
- Baillieux H, De Smet HJ, Dobbeleir A, Paquier PF, De Deyn PP, et al. (2010) Cognitive and affective disturbances following focal cerebellar damage in adults; a neuropsychological and SPECT study. Cortex 46; 869-879.
- Bautista JR, Rubin SA, Moran TH, Schwartz GJ, Carbone KM (1995) Developmental injury to the cerebellum following perinatal Borna diseases virus infection. Brain Res Dev Brain Res 90: 45-53.
- Corrsles JD, Rocco GL, Blaess S, Guo Q, Joyner AL (2004) Spatial pattern of sonic hedgehog signaling through Gli genes during cerebellum development. Development 131: 5581-5590.
- Leisman G, Moustafa AA, Shafir T (2016) Thinking, Walking, Talking: Integratory Motor and Cognitive Brain Function. Front Public Health 4: 94.
- Mendoza G, Merchant H (2014) Motor system evolution and the emergence of high cognitive functions. Prog Neurobiol 122: 73-93.
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT (2011) The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106: 2322-2345.
- Casanova MF, Buxhoeveden DP, Switela AE, Roy E (2002) Minicolumnar pathology in autism. Neurology 58: 428-432.
- Courchesne E, Carper R, Akshoomoff N (2003) Evidence of Brain overgrowth in the first year of life in autism. JAMA 290: 337-344.
- Lainhart JE, Piven J, Wzorek M, Landa R, Santangelo SL, et al. (1997) Macrocephaly in children and adults with autism. J Am Acad Child Adolesc Psychiatry 36: 282-290.
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, kennedy D, et al. (2005) Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain 128: 213-226.
- De Fosse L, Hodge SM, Makris N, Kennedy DN, Cavines VS Jr, et al. (2004) Language-association cortex asymmetry in autism and specific language impairment. Ann Neurol 56: 755-756.
- Luna B, Minshew NJ, Garver KE, Lazar NA, Thulborn KR, et al. (2002) Neocotical system abnormalities in autism: an fMRI study of spatial working memory. Neurology 59: 834-840.
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, et al. (1999) Social intelligence in the normal and autistic brain. An fMRI study. Eur J Neurosci 11: 1891-1898.
- Muller RA, Pierce K, Ambrose JB, Allen G, Courchesne E (2001) Atypical patterns of cerebral motor activation in autism: a functional magnetic resonance study. Biol Psychiatry 49: 665-676.
- Just MA, Cherkassky VL, Keller TA, Minshew NL (2004) Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain 127: 1811-1821.
- Dawson G, Finley C, Phillips S, Galpert L, Lewy A (1998) Reduced P3 amplitude of the event-related brain potential: Its relationship to language ability in autism. J Autism Dev Disord 73: 345-358.
- Boeckx C (2017) The language-ready head; Evolutionary considerations. Psychon Bull Rev 24: 194-199.
- Sweeten TL, Posey DJ, Shekar A, McDougle CJ (2002) The amygdala and related structures in the pathophysiology of autism. Pharmacol Biochem Behav 71: 449-455.
- Howard MA, Cowell PE, Boucher J, Broks P, Mayes A, et al. (2000) Convergent neuroanatomical and behavioural evidence of an amygdala hypothesis of autism. NeuroReport 11: 2931-2935.
- Adolhs R, Tranel D, Damasio AR (1998) The human amygdala in social judgement. Nature 393: 470-474.
- Aggleton JP (1993) The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci 16: 328-333.
- Ide JS, Li CR (2011) A cerebellar thalamic cortical circuit for error-related cognitive control. Neuroimage 54: 455-464.
- Allen G, Muller R, Courchesne E (2004) Cerebellar function in autism: functional magnetic resonance image activation during a simple motor task 56: 269-278.
- Catani M, jones DK, Daly E, Embiricos N, Deeley Q, et al. (2008) Altered cerebellar feedback projections in Asperger syndrome. Neuroimage 41: 1148-1191.
- Zhou J, Liu X, Song W, Yang Y, Zhan Z, et al. (2011) Specific and Nonspecific Thalamocortical Functional Connectivity in Normal and Vegetative States. Conscious Cogn 20: 257-268.
- Whalley K (2010) Inhibition: too much of a good thing? Nature Reviews Neuroscience 11: 6-7.
- Kohls G, Schulte-Ruther M, Nehrkorn B, Muller K, Fink GR (2013) Reward system dysfunction in autism spectrum disorders. Soc Cogn Affect Neurosci 8: 565-572.
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY (2010) Reward Processing in Autism. Autism Res 3: 53-67.
- Delmonte S, Balsters JH, McGrath J, Fitzgerald J, Brennan S, et al. (2012) Social and monetary reward processing in autism spectrum disorders. Mol Autism 3: 7.
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, et al. (2002) Visual scanning of faces in autism. J Autism Dev Disord 32: 249-261.
- Corbett BA, Carmean V, Ravizza S, Wendelken C, Henry ML, et al. (2009) A functional and structural study of emotion and face processing in children with autism. Psychiatry Res 173: 196-205.
Citation: Chan KTM (2018) Rewarding the Autistic through Understanding the Neural Cognitive, Emotional, Motor binding Circuitry in the Developmental Brain. Neonat Pediatr Med 4: 171. DOI: 10.4172/2572-4983.1000171
Copyright: © 2018 Chan KTM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6072
- [From(publication date): 0-2018 - Dec 18, 2025]
- Breakdown by view type
- HTML page views: 5145
- PDF downloads: 927