Short Communication Open Access
Risk Communication Regarding Drug Safety in Japan
Michiko Yamamoto*Education Center for Clinical Pharmacy Practice, Showa Pharmaceutical University, 3-3165, Higashi-Tamagawagakuen, Machidashi, Tokyo, Japan
- *Corresponding Author:
- Michiko Yamamoto
Education Center for Clinical Pharmacy Practice
Showa Pharmaceutical University
3-3165, Higashi-Tamagawagakuen
Machidashi, Tokyo 194-8543, Japan
Tel: +81-42-721-1511
Fax: +81-42-721-1588
E-mail: m-yamamoto@ac.shoyaku.ac.jp
Received date: February 25, 2014; Accepted date: March 20, 2014; Published date: March 27, 2014
Citation: Michiko Yamamoto (2014) Risk Communication Regarding Drug Safety in Japan. Occup Med Health Aff 2:152. doi: 10.4172/2329-6879.1000152
Copyright: © 2014 Michiko Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Occupational Medicine & Health Affairs
Background
For individual and for public health, drug safety has significant implications on a large scale. Given the inherently uncertain safety and effectiveness of drugs, discussion of the significance of benefit/risk communication with stakeholders including healthcare professionals and patients has been repeated [1-4]. In pharmacovigilance, risk communication has an enormous role with the object of measures for drug safety and its importance would be recognized. According to WHO Glossary of terms used in pharmacovigilance, risk is defined as the probability of harm being caused; the probability (chance, odds) of an occurrence [5]. Risk communication is one of the composing element in risk analysis along with risk assessment and risk management in the field of food and environmental issues [6]. Risk communication is an interactive process of exchange of information and opinion on risk among risk assessors, risk managers, and other interested parties in protecting the public health [7]. All stakeholder groups should be ideally involved from the start. The communication is aimed to offer essential information so that consumers can make an independent decision about risks. People need to know the risk which affects their health and safety and they need to decide a proper way to cope with the risk. Regulatory bodies and companies responsible for public health and safety should identify potential risk, assess, and decide the appropriate measures to execute them. Therefore, it becomes important for the government to grasp the level of the understanding of the risk or what kind of behavior has been performed [8]. Along with risk evaluation and risk management, risk communication should also be performed for the public from the regulatory bodies and companies even if the risk would be gray information. Furthermore, in case of the drug safety, the verification is required for the way of the risk communication of the drugs in consideration of the risk benefit of drugs in the medical care.
Situation in Japan
In Japan, a number of serious public health crisis involving environmental pollution, food-borne diseases, and health hazards due to pharmaceuticals have occurred in the past 50 years. Pharmaceutical serious incidents are specially called "Yakugai” [9]. There is a word called “Ishokudougen” in Japan meaning that medicine (I) and one's daily food (shoku) are equally important in human life since these components are essential to treat diseased body, develop life and maintain health conditions. The source of drug and food is thought to be same in origin (dougen). Actually there is lot of medicinal plants which people use to eat as food for health. Therefore, we use to think that the treated medicine should be safe like food. Whereas in Europe and America, medicines were made from poison or chemicals, and there is the thought that a risk and benefit are inevitable.
Historically in a flow of these cultural background and paternalism in the medical care, Japanese people tend to believe that the approved pharmaceutical products must be safe. They did not receive enough information related to risk and benefits of the products by medical doctors. The companies and regulatory bodies also disseminated the lack of safety information to the healthcare professionals, so-called risk communication was insufficient. In such cases, regulatory bodies should have a primary responsibility for the company’s information. I am referring here to the recent example that became a merkmal (characteristic) of safe measures for the government in Japan. The patients who received the unheated fibrinogen preparation or coagulation factor IX products were approximately 290,000 people after 1980 and it is said that more than 10,000 people of those were infected with hepatitis C virus (HCV) [10]. The following issues are considered to be important for this particular Yakugai happened in Japan from drug safety point of view.
Medical Representatives (MR) in the pharmaceutical companies promoted an off-label use (hemostasis) of the fibrinogen preparations to the healthcare professionals and abundant preparations were consumed.
The risk information of HCV infection was not sufficiently distributed as the package leaflet to the healthcare professionals.
US FDA decided to withdraw the preparation in 1977, but Japanese companies neither reported this fact in time to the Ministry of Health and Welfare (MHW) nor quickly responded to do so.
In spite of the fact that cases of hepatitis C were recognized by the companies one after another after administration of the fibrinogen preparations, they did not deal with them properly and the MHW did not have an efficient framework to collect and analyze such cases systemically.
This incident was positioned as one of the big drug disasters (Yakugai). In response to it, the inspection committee was established finally in May 2008 by the Ministry of Health, Labour and Welfare. A final report had been published in 2010 for the restructuring of drug regulatory administration to prevent a recurrence of drug-related incidents, and one of the key recommendations was the improvement of drug-related safety risk communication [11]. The report presents the fundamental view related to the cause. Information based in the fact has not reached doctors and patients. For this reason, risk communication is focused in the report. The proposal related to risk communication and patient involvement has two principles: To provide the information in a proactive and consistent manner
Involvement of patients and consumers in risk communication
The main contents are as follows:
The regulatory bodies, healthcare professionals and pharmaceutical companies should develop a system for risk communication with patients in order to disseminate the information on adverse reactions and promote the proper use of the drugs for patients.
The scheme for Patient reporting of Adverse Drug Reactions should be established.
The regulatory bodies should review and improve several safety information which also includes “Dear Healthcare Professional Letter (DHPL)" which is currently provided to healthcare professionals.
The regulatory bodies should also consider various measures to provide information for patients, including dissemination of the information focused on adverse drug reactions.
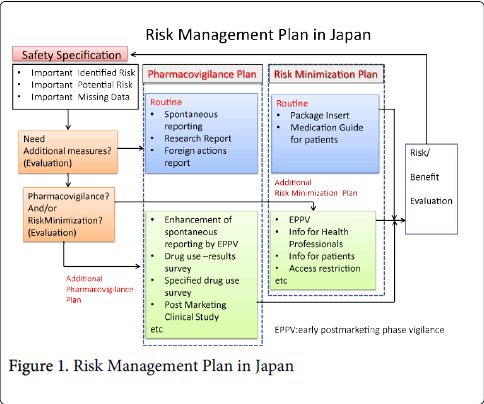
The regulatory bodies should confirm whether pharmaceutical companies appropriately implement safety measures, including provision of information to healthcare professionals. According to these concrete proposals, the operation of the drug adverse reaction reporting system by patients was started and the other issues would be in process and an effort would be made. Since April 2012, Risk Management Plan (RMP) has been imposed to the pharmaceutical companies (Figure 1).
From the drug development, drug approval to post-marketing in all periods, the risk evaluation and review of the drugs are carried out. Safety measures for post marketing with an explicit prospect should be implemented by pharmaceutical companies.
Approach Risk Communication in Japan
It has been considered that to ensure the safety of drugs in Japan, there were several items which were not efficiently executed in terms of involvement of patients, though the framework or law had been set up. The following issues are considered that should be improved; Risk information should be provided to patients and consumers as well as healthcare professionals concurrently by regulatory agencies and pharmaceutical companies in order to share it with them.
1) Risk information should be provided to patients and consumers as well as healthcare professionals concurrently by regulatory agencies and pharmaceutical companies in order to share it with them.
2) Risk information should be made easily available and accessible by public through web sites including regulatory agencies in any communication tools.
3) Considering the patient’s health literacy level, appropriate risk information should be provided by regulatory agencies.
4) The leaflets containing the patient information should be evaluated for understandability and accessibility by conducting user testing periodically.
For the risk communication, transparency, interactivity and sharing the evidence based information are significant which composes elements and consumers or patients to be involved in the risk communication. With introduction and advancement of IT technologies, wide range of drug information is easily available for the people. But as the information contents are multifarious, it is necessary to show what appropriate and evaluated information for them is. The drug information has high specialty, hence it would be difficult for consumers or patients to understand correctly. Therefore, it is necessary for healthcare professionals to make it easy for them to understand. Under this situation for the drug safety, the importance of risk communication has been recognized and it should be reinforced in future.
References
- Urushihara H, Kobashi G, Masuda H, Taneichi S, Yamamoto, et al.(2014) Pharmaceutical company perspectives on current safety risk communications in Japan. Springerplus, 24:3-51
- Bahri P, Harrison-Woolrych M (2012) How to Improve Communication for the Safe Use of Medicines?: Discussions on Social Marketing and Patient-Tailored Approaches at the Annual Meetings of the WHO Programme for International Drug Monitoring. Drug Saf, 35:1073-1079.
- Bahri P, Harrison-Woolrych M(2012) Focusing on Risk Communication About Medicines: Why Now? Drug Saf, 35:971-975.
- Avorn J(2008) Drug warnings that can cause fits--communicating risks in a data-poor environment. N Engl J Med,359 :991-994.
- WHO Glossary of terms used in Pharmacovigilance
- Codex schematic framework for risk analysis(2005). A primer on risk assessment modeling:focus on seafood products.
- Morgan MG,Fischhoff B, Bostrom A, Atman CJ(2001) Risk Communication : A Mental Models Approach. Cambridge University Press, pp:1-14
- Yamamoto M(2012), Risk communication efforts regarding drug safety in the US and EU, and Japan. YakugakuZasshi,132: 533-548
- Imamura T, Ide H, Yasunaga H (2007) History of public health crises in Japan.J Public Health Policy, 28:221-37.
- Yakugai Hepatitis national plaintiff's group webpage.
- Committee on Regulatory Restructuring for Inspection and Recurrence Prevention of Drug-Induced Hepatitis Disaster Case (2010). The Final Recommendation on Restructuring Drug Regulatory Administration for Drug Disaster Prevention [in Japanese]. General Affairs Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare, Tokyo.
Relevant Topics
- Child Health Education
- Construction Safety
- Dental Health Education
- Holistic Health Education
- Industrial Hygiene
- Nursing Health Education
- Occupational and Environmental Medicine
- Occupational Dermatitis
- Occupational Disorders
- Occupational Exposures
- Occupational Medicine
- Occupational Physical Therapy
- Occupational Rehabilitation
- Occupational Standards
- Occupational Therapist Practice
- Occupational Therapy
- Occupational Therapy Devices & Market Analysis
- Occupational Toxicology
- Oral Health Education
- Paediatric Occupational Therapy
- Perinatal Mental Health
- Pleural Mesothelioma
- Recreation Therapy
- Sensory Integration Therapy
- Workplace Safety & Stress
- Workplace Safety Culture
Recommended Journals
Article Tools
Article Usage
- Total views: 16491
- [From(publication date):
May-2014 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 11790
- PDF downloads : 4701