Role of Bacterial Translocation in the Progressive and Delayed Irinotecan Induced Diarrhea.
Received: 30-Aug-2018 / Accepted Date: 19-Sep-2018 / Published Date: 29-Sep-2018 DOI: 10.4172/2161-0681.1000350
Keywords: Irinotecan; Diarrhea; Bacteria translocation
Introduction
Irinotecan (CPT-11, Camptosar®) is a semi-synthesized watersoluble prodrug of 7-Ethyl-10-hydroxy-camptothecin (SN-38) derived from camptothecin, a natural compound isolated from the bark and stem of Camptotheca acuminate [1]. Irinotecan is approved by the FDA as the first-line drug to treat metastatic colon cancer (mCRC) and is currently under active investigations to treat different types of malignant such as lung, pancreatic, ovarian, cervical, prostate, and gastrointestinal cancers [2-6]. The mechanism of action is that the drug (i.e., its active form SN-38) can inhibit topoisomerase I to interrupt DNA synthesis in cancer cells [7,8]. Irinotecan can be used alone or in combination with other drugs such as in combination with 5-FU/leucovorin [9,10].
Despite the promising efficacy, irinotecan clinical usage is limited due to side effects including vomiting, nausea, diarrhea, constipation, neutropenia, weakness, fever, pain, abnormal liver function, hair loss, etc. Among these side effects, diarrhea is one of the major doselimiting side effects that may affect clinical outcomes. A few of papers have reviewed the incidences, possible mechanism, and preventive/ therapeutic approaches [11-13]. The aim of this paper is to review the role of bacterial translocation (BT) in the progressive and delayed irinotecan gut toxicity.
Irinotecan gut toxicity
Diarrhea is one of the major dose-limiting toxicities of irinotecan: Irinotecan can cause early-onset and late-onset diarrhea. The earl-onset, which is characterized by rapid-onset diarrhea and may also include abdominal cramping and diaphoresis, occurs within 24 hours of drug administration, while the late-onset occurs after 24 hours of drug administration. The early-onset diarrhea can be effectively controlled by atropine [14,15]. However, the late-onset diarrhea, which is inconsistent, unpredictable, non-cumulative, dose dependent, with wide interpatient and intra-patient variability, is a much more serious problem that may affect patients’ quality of life and may cause early death either directly from life-threatening sequelae or indirectly from adjustments in chemotherapy plan [16,17].
Incidence of diarrhea induced by irinotecan is significant: The overall incidence of diarrhea ranges from 60% to 87%, including up to 40% of severe late-onset diarrhea (grade 3 and 4), which appears to be dose-dependent [18,19]. The median time to onset ranged from 5 to 11 days after drug administration and the diarrhea duration last for 2 to 5 days depend on the dosing schedule [16,20]. The incidence of diarrhea using the common regimens is listed in Table 1.
| Dosage | and | Dose Regimens | Diarrhea Incidence | References |
|---|---|---|---|---|
| Administration | (total/grade 3-4) | |||
| Irinotecan | Weekly, 125 mg/m2 intravenous infusion over 90 | >82 % / >36% | [93] | |
| minutes on days 1, 8, 15, 22 then 2-week rest | ||||
| Irinotecan | Every 3 weeks 350 or 300 mg/m2 intravenous infusion | >76 % / >19% | [93] | |
| over 90 minutes on day 1 every 3 weeks | ||||
| Irinotecan/LV/5-FU | Irinotecan 180 mg/m2 intravenous infusion over 90 | <51%/<17% | [94-98] | |
| minutes on days 1, 15, 29 with LV 200 mg/m2 intravenous infusion over 2 hours on days 1, 2, 15, 16,29, 30 followed by 5-FU 400 mg/m2 intravenous bolusinfusion on days 1, 2, 15, 16, 29, 30 and 5-FU 600 mg/m2 intravenous infusion over 22 hours on days 1, 2, 15, 16, 29, 30. |
||||
Table 1: Diarrhea incidence using the FDA approved irinotecan dosage and administration for the treatment of colorectal cancer.
Most of the patients need diarrhea treatment using anti-diarrhea agents (e.g., loperamide, octreotide). Although standard diarrhea management is recommended, around 10% of patients who developed diarrhea require hospitalization or even change the chemotherapy plan (e.g., dose reduction), which significantly affect the clinical outcomes and even cause early death [16,21].
Neutropenia is another dose-limiting toxicity of irinotecan: Neutropenia, which can be ameliorated or prevented using growth factors such as G-CSF, is a short duration and reversible dose limiting side effect of irinotecan. Compared to diarrhea, neutropenia is less frequent and easily managed.
For example, a clinical study showed that grade 3-4 diarrhea affected 22% of the metastatic colorectal cancer patients receiving irinotecan plus 5-FU/leucovorin treatment, while only 5% of the patients developed grade 3-4 neutropenia [22]. Another clinical study showed that even at a dosage of 750 mg/m2 of irinotecan, the maximum tolerated dose for the risk of severe neutropenia was not reached [23].
Dose reduction is an approach in managing irinotecan-induced diarrhea: Dose reduction and/or changing in dosing regimens are frequently used to manage irinotecan-induced diarrhea. For example, it is reported that the grade 3-4 diarrhea incidence reduced from 57.1 % to 10.8 % in colonic cancer patients carrying TA7/TA7 allele (UGT1A1 promoter) when irinotecan’s dose was reduced from 350 mg/m2 3-weeky to 250 mg/m2 3-weeky [24]. However, it is highly suspected that dose reduction will affect the therapeutic effect as tumor suppression is correlated with in vivo exposure of SN-38. In fact, recent clinical reports clearly indicate that many patients could benefit from higher doses [23,25-30].
Mechanisms of gut toxicity
Diarrhea is caused by GI damage: The mechanisms underlying diarrhea induced by irinotecan is not entirely understood. The complex etiology of irinotecan-induce diarrhea seems to involve changes in the absorption of fluids and electrolytes, intestinal motor dysfunction, and inflammation of the mucosal membranes lining the gastrointestinal tract [12,31]. Molecular mechanism studies revealed that early-onset diarrhea induced by irinotecan is associated with parasympathetic discharge, stimulation of serotonin receptors, and release of thromboxane A2 (TX-A2),[12,20] while the molecular mechanism of late-onset diarrhea is not fully understood but is correlated with direct damage to the intestinal mucosa by SN-38. Understanding the disposition of irinotecan provides insight into the mechanism of late-onset diarrhea.
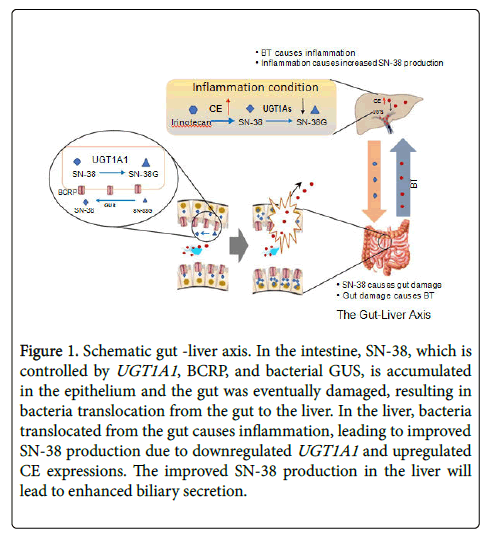
Irinotecan disposition is associated with colonic exposure of SN-38: Irinotecan’s disposition has been well studied [32]. The drug is administrated through i.v. route and is activated to SN-38 by carboxylesterases (CEs) in the plasma or liver. The active drug (i.e., SN-38) is then transferred and distributed to different organs including the tumor tissue. The free SN-38 can be conjugated into SN-38 glucuronide (SN-38G, a non-effect, non-toxic metabolite) mediated by uridine 5'-diphospho-glucuronosyl-transferase (UGT) 1A subfamily. SN-38 was shown to induce intestinal inflammation and oxidative stress, leading to gut damage [12,33,34]. The colonic SN-38 comes from different sources. SN-38 in the liver can be excreted via different hepatic efflux transporters (e.g., i.e., P-gp, MRP2, BCRP) through bile into small intestine and then enters the colon (Figure 1).
Figure 1: Schematic gut -liver axis. In the intestine, SN-38, which is controlled by UGT1A1, BCRP, and bacterial GUS, is accumulated in the epithelium and the gut was eventually damaged, resulting in bacteria translocation from the gut to the liver. In the liver, bacteria translocated from the gut causes inflammation, leading to improved SN-38 production due to downregulated UGT1A1 and upregulated CE expressions. The improved SN-38 production in the liver will lead to enhanced biliary secretion.
Moreover, irinotecan and SN-38G can also be secreted from the liver into the small intestine, where these two non-toxic compounds can be converted into free SN-38 by intestinal CEs or by microflora, respectively. Mass balance study using 14-carbon labelled irinotecan have demonstrated that the fecal route of excretion, mainly from biliary excretion, is the major route eliminating more than 60% of the administered drug,[35] although there are other competing metabolic/ excretion pathways of irinotecan facilitated by cytochrome P450 (CYP) enzymes [36].
Other than biliary secretion, the enterocytes also secrete these three compounds, [37,38] however, the amounts of these compounds secreted from intestine are significantly lower than those from bile [38]. In addition, free SN-38 can be re-absorbed in the intestine and reach the liver through the portal vein to form an enterohepatic recycling (EHR) (Figure 1), resulting in prolonged half-life and repeated exposure in the gut. For example, it is reported that SN-38 presented in human plasma for 3 weeks after a single irinotecan i.v. infusion at a dose of 350 mg/m2 [39]. More importantly, when the gut is damaged by SN-38, viable bacteria or endotoxins will translocate from the gut to the liver, where CE and UGT1A1 expression will be affected, resulting in enhanced SN-38 biliary secretion and more gut damage (see detail later). This endocrine cycle aggravates diarrhea condition.
SN-38-induced intestinal microbiota changes. Studies using animal models have indicated that changes in the microflora of the gut as possible cause, at least in part, for late-onset diarrhea [40]. It has been shown that chemotherapy treatment is associated with a deregulated intestinal microbial homeostasis and a decreased microbial diversity [41]. Likewise, unbalanced bacterial population (dysbiosis) was observed with irinotecan treatment. In rats a relative increase or decrease in the presence of certain bacteria in stomach, jejunum, colon and feces were observed in the irinotecan-treated group compared to the control group. In colon of irinotecan-treated rats, an increasing trendin Escherichia spp., Clostridium spp., Enterococcus spp., Serratia spp.and Staphylococcus spp. was noticed, whereas, in fecal samples, increase in Proteus spp., Clostridium spp., and Peptostreptococcus spp. was associated with a decrease in Bacillus spp., and Bifidobacterium spp. [42]. Irinotecan treatment reduced the colony of good bacteria, Clostridium cluster IV (cluster which contains Butyrate‐producing Clostridium spp.) and in contrast increased β-glucuronidases (GUS) producing bacteria (such as Enterobacteriaceae spp.), [43] that deconjugates irinotecan metabolite, SN-38 glucuronide (SN-38G) back to the toxic metabolite SN-38 in the intestine. Also, a decrease in total bacterial count has also been reported with irinotecan injection [43]. There are few studies which have shown direct influence of intestinal microbiota on the pathogenesis of irinotecan-induced mucositis [44,45]. Patient data has shown that there is a link between irinotecan metabolism, GUS and gut microbiome signatures in individual patients [46]. It has been shown that differences in gut microflora composition can lead to variability in GUS catalytic activity and inhibition potential.
Although, studies indicate strongly the direct role of GUS [47,48] relationship between GUS activity and mucositis is still controversial. In a study by Pedroso et al. E. coli producing GUS was found to have a direct relationship with the increase of intestinal permeability with irinotecan, but any recruitment of neutrophils and eosinophils, nor were histology changes observed. Moreover, antibiotic treatment improved irinotecan-induced mucositis in Gunn rats (animals have an inherent deficiency in the UGT1A1 enzyme) [49] and a specific GUS inhibitor (D-saccharic acid 1.4-lactone) failed to alleviate diarrhea with irinotecan [50]. Thus, indicating involvement of possible mechanisms other than GUS.
Irinotecan is known to cause injury to the tight junction leading to BT in rats, [51] BT is the passage of viable bacteria and endotoxins from gastrointestinal to mesenteric lymph nodes (MLN), bloodstream, and other organs [52]. The tight junction of the intestinal epithelial barrier is essential in preventing BT [53]. Evidence is increasing that the intestinal microbiota plays a critical role in modulating the efficacy and toxicity of chemotherapeutic agents. The microbiota provides their host with metabolic capabilities [54]. Variation of microbiota may cause mucositis and sepsis [51,55].
Diarrhea attenuation through modulating gut microbiome: The intestinal microbiota are thus potential targets to improve the therapeutic efficacy and mitigate the toxicity of irinotecan. Current tools designed to manipulate the gut microbiota include dietary intervention with glutamine, probiotics, or non-digestible carbohydrates to protect epithelia barrier [56-58]. Another approach is to co-administer with antibiotics to decrease the level of microbiota and decrease the activity of GUS to alleviate chemotoxicity [59,60]. Amoxapine, a known inhibitor of GUS, has been shown to suppress irinotecan induced diarrhea in a rat model [61]. The deeper understanding of the interactions between irinotecan, intestinal microbiota, and tumor is warranted to come up with new therapies and preventions to reduce adverse effects and improve therapeutic efficacy.
Limitations of current strategies being investigated to manage irinotecan gut toxicity: Various molecular and pharmacological approaches have been tested to alleviate irinotecan-induced diarrhea through reducing the intestinal accumulation of SN-38 including: 1) UGT1A1 induction (e.g., phenobarbital) [62], 2) CEs inhibition (e.g., benzene sulfonamide) [63], 3) CYP3A4 induction (e.g., phenytoin, carbamazepine) [64], 4) GUS inhibition (e.g., baicalin, probiotics, antibiotics) [65-67], 5) Hepatic transporter inhibition (e.g., cyclosporine) [62,68-70], and binding SN-38 using absorbents (e.g., aluminum silicate clay, activated charcoal) [71,72]. Other approaches have also been evaluated to protect gut damage such as using anti-inflammatory agents (e.g., pentoxifylline, thalidomide, celecoxib, RDP58, etc.), antibiotics, probiotics, oxidative stress inhibitors, intestinal alkalization, and herbal medicines [13,65,73]. Despite encouraging results in animal models or small scale of clinical trials, diarrhea is still a challenge for irinotecan therapy. Therefore, there is a critical need for fully mechanism investigation and for effective approaches to solve this urgent problem.
Gut-liver axis and EHR of irinotecan
The concept of the gut-liver axis was initially demonstrated in alcohol induced liver disease (ALD). Studies in rat demonstrated that acute alcohol transiently increased systemic levels of gut-derived endotoxin and associated detrimental effect could be protected by antibiotics [74]. Tight junction prevents both bacteria and toxin in the intestinal lumen and from the paracellular space getting into deep tissues. Recent studies have shown that SN-38 damaged the tight junction proteins, claudin-1 and occludin to disrupt the intestinal barrier,[75] and increase permeability (Figure 1). This results in the translocation of bacteria and bacterial products (such as lipopolysaccharide, LPS and unmethylated CpG containing DNA) from the gut lumen to the liver via the portal vein [76-78]. Recent studies have shown that SN-38 gut accumulation leads to disruption of the epithelial barrier promoting BT [51,79-81]. The transport of bacteria and bacterial products to the liver is known to induce hepatic inflammation including cytokines and other inflammatory markers [82-84]. We and others have shown that hepatic drug metabolizing enzymes and drug transporters are primarily reduced during inflammation [85-87]. Therefore, it is possible that SN-38 exposure to the gut will be increased due to the effect of the gut-liver axis on the EHR of irinotecan caused by increased BT-mediated hepatic inflammation. This novel mechanism of irinotecan gut toxicity needs to be investigated further. The liver is exposed to both gut microbes and SN-38 from the gut through the portal vein. Gut microbial components can cause inflammation in the liver, however SN-38 by itself can also induced inflammatory pathways. Future studies need to be conducted to elucidate the mechanism of hepatic inflammation in the gut-liver axis during irinotecan treatment.
Clinical implications of bacterial translocation
The concept of BT being the key to the gut-liver-gut dysfunction is interesting and it provides a better understanding of irinotecan-induced diarrhea than the currently hypothesized mechanisms. There is evidence in humans of the potential for BT from injured intestinal mucosa [88,89]. Indirect evidence with irinotecan is provided by the GERCOR study. This was a trial in which patients were treated with FOLFIRI compared to FOLFOX6 for metastatic colorectal cancer. Interestingly in patients who were treated with FOLFIRI (which is an irinotecan-based regimen) as a first-line therapy, the rate of grade 3-4 neutropenia rate was 25%, whereas with the FOLFOX6 the rate was 44% [90]. So irinotecan was less myelosuppressive, however when looking at the grade 3-4 neutropenia rate was 7% for patients receiving FOLFIRI vs only 1% for patients receiving FOLFOX [91]. This evidence would seem to indicate that while it produces less severe neutropenia, irinotecan likely has increased the rate of BT due to the increase in neutropenic febrile episodes.
The above statement is dependent on the assumption that BT plays a role in febrile neutropenia and there is evidence that this is the case. A study in patents with febrile neutropenia episodes looked at endotoxin and CD14 as a marker. The data showed that CD14 was higher in patient in gram-negative bacteremia than in gram-positive bacteremia. And this pattern was observed in cases where there was neutropenic fever without cultures indicating more likely gram-negative bacteremia. This would again seem to indicate BT as a more likely source [92-98].
The evidence presented above does seem to indicate that BT is an issue with irinotecan. Therefore, studies looking at BT-mediated effects on irinotecan EHR and consequently enteric toxicity of irinotecan is interesting and could provide further targets for intervention and aid patients in tolerating the therapy better.
Future Research Needed
Despite ongoing studies, there are no effective medications to efficiently manage irinotecan-induced late-onset diarrhea in patients. The major impediment is the lack of comprehensive understanding of the mechanism of this gut toxicity. To date all research have focused on gut-specific factors such as enzymes, inflammation, microbiome, etc. in order to understand the mechanism of toxicity. Future research is needed to identify the role of BT on hepatic SN-38 detoxifying enzymes, and their role in exacerbating irinotecan gut toxicity. Future research will focus on understanding the novel cross-talk between the gut and liver in order to develop new approaches to reduce/prevent irinotecan-induced diarrhea in patients.
References
- Vincent RM, Lopez-Meyer M, McKnight TD, Nessler CL (1997) Sustained harvest of camptothecin from the leaves of Camptotheca acuminate. J Nat Prod 60: 618-619.
- Farhat FS (2007) A general review of the role of irinotecan (CPT11) in the treatment of gastric cancer. Med Oncol 24: 137-146.
- Pizzolato JF, Saltz LB (2003) Irinotecan (Campto) in the treatment of pancreatic cancer. Expert Rev Anticancer Ther 3: 587-593.
- Gershenson DM (2002) Irinotecan in epithelial ovarian cancer. Oncology (Williston Park) 16:29-31.
- Verschraegen CF (2002) Irinotecan for the treatment of cervical cancer. Oncology (Williston Park) 16: 32-34.
- Reese DM, Tchekmedyian S, Chapman Y, Prager D, Rosen PJ (1998) A phase II trial of irinotecan in hormone-refractory prostate cancer. Invest New Drugs 16: 353-359.
- Mathijssen RH, Loos WJ, Verweij J, Sparreboom A (2002) Pharmacology of topoisomerase I inhibitors irinotecan (CPT-11) and topotecan. Curr Cancer Drug Targets 2: 103-123.
- Fujita K, Kubota Y, Ishida H, Sasaki Y (2015) Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J Gastroenterol 21: 12234-12248
- Wulaningsih W, Wardhana A, Watkins J, Yoshuantari N, et al. (2016) Irinotecan chemotherapy combined with fluoropyrimidines versus irinotecan alone for overall survival and progression-free survival in patients with advanced and/or metastatic colorectal cancer. Cochrane Database Syst Rev 12: CD008593.
- Ducreux M, Kohne CH, Schwartz GK, Vanhoefer U (2003) Irinotecan in metastatic colorectal cancer: Dose intensification and combination with new agents, including biological response modifiers Ann Oncol. 14 Suppl 2:ii17-23.
- Alimonti A, Gelibter A, Pavese I, Satta F, Cognetti F, et al. (2004) New approaches to prevent intestinal toxicity of irinotecan-based regimens. Cancer Treat Rev 30: 555-562
- Ribeiro RA, Wanderley CW, Wong DV, Mota JM, Leite CA, et al. (2016) Irinotecan- and 5-fluorouracil-induced intestinal mucositis: Insights into pathogenesis and therapeutic perspectives. Cancer Chemother Pharmacol 78: 881-893.
- Swami U, Goel S, Mani S (2013) Therapeutic targeting of CPT-11 induced diarrhea: a case for prophylaxis. Curr Drug Targets 14: 777-797.
- Yumuk PF, Aydin SZ, Dane F, Gumus M, Ekenel M, et al. (2004) The absence of early diarrhea with atropine premedication during irinotecan therapy in metastatic colorectal patients. International journal of colorectal disease 19: 609-610.
- Cheng C, Lau JE, Earl MA (2015) Use of atropine-diphenoxylate compared with hyoscyamine to decrease rates of irinotecan-related cholinergic syndrome. J Community Support Oncol 13: 3-7.
- Richardson G, Dobish R (2007) Chemotherapy induced diarrhea. J Oncol Pharm Pract 13: 181-198.
- Smith NF, Figg WD, Sparreboom A (2006) Pharmacogenetics of irinotecan metabolism and transport: An update. Toxicol in vitro 20: 163-175.
- Lam W, Bussom S, Guan F, Jiang Z, Zhang W, et al. (2010) The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci Transl Med 2:45ra59.
- Conti JA, Kemeny NE, Saltz LB, Huang Y, Tong WP, et al. (1996) Irinotecan is an active agent in untreated patients with metastatic colorectal cancer. J Clin Oncol 14: 709-715.
- Hecht JR (1998) Gastrointestinal toxicity or irinotecan. Oncology (Williston Park) 12: 72-78.
- Stein A, Voigt W, Jordan K (2010) Chemotherapy-induced diarrhea: Pathophysiology, frequency and guideline-based management. Ther Adv Med Oncol 2:51-63.
- Douillard JY, Group VS (2000) Irinotecan and high-dose fluorouracil/leucovorin for metastatic colorectal cancer. Oncology (Williston Park) 14: 51-55.
- Abigerges D, Chabot GG, Armand JP, Herait P, Gouyette A, et al. (1995) Phase I and pharmacologic studies of the camptothecin analog irinotecan administered every 3 weeks in cancer patients. J Clin Oncol 13: 210-221.
- Kweekel D, Guchelaar HJ, Gelderblom H (2008) Clinical and pharmacogenetic factors associated with irinotecan toxicity. Cancer Treat Rev 34:656-669.
- Han B, Jung JY, Kim HS, Cho JW, Kim KC, et al. (2016) A dose-finding study for oxaliplatin, irinotecan, and S-1 (OIS) in patients with metastatic or recurrent gastrointestinal cancer. Cancer Chemother Pharmacol 78: 949-958.
- Marcuello E, Paez D, Pare L, Salazar J, Sebio A, et al. (2011) A genotype-directed phase I-IV dose-finding study of irinotecan in combination with fluorouracil/leucovorin as first-line treatment in advanced colorectal cancer. Br J Cancer 105: 53-57.
- Lu CY, Huang CW, Wu IC, Tsai HL, Ma CJ, et al. (2015) Clinical implication of UGT1A1 promoter polymorphism for irinotecan dose escalation in metastatic colorectal cancer patients treated with bevacizumab combined with FOLFIRI in the first-line setting. Transl Oncol 8: 474-479.
- Yeh YS, Tsai HL, Huang CW, Wang JH, Lin YW, et al. (2016) Prospective analysis of UGT1A1 promoter polymorphism for irinotecan dose escalation in metastatic colorectal cancer patient treated with bevacizumab plus FOLFIRI as the first-line setting: study protocol for a randomized controlled trial. Trials 17: 46
- Armand JP (1996) CPT-11: Clinical experience in phase I studies. Semin Oncol 23: 27-33.
- Abigerges D, Armand JP, Chabot GG, Da Costa L, Fadel E, et al. (1994) Irinotecan (CPT-11) high-dose escalation using intensive high-dose loperamide to control diarrhea. J Natl Cancer Inst 86: 446-449.
- Lima-Junior RC, Figueiredo AA, Freitas HC, Melo ML, Wong DV, et al. (2012)Â Â Â Â Â Involvement of nitric oxide on the pathogenesis of irinotecan-induced intestinal mucositis: Role of cytokines on inducible nitric oxide synthase activation. Cancer Chemother Pharmacol 69: 931-942
- Lee CS, Ryan EJ, Doherty GA (2014) Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: The role of inflammation. World J Gastroenterol 20: 3751-3761.
- Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, et al. (2017) Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol 14: 356-365.
- Yin T, Yang G, Ma Y, Xu B, Hu M, et al. (2015) Developing an activity and absorption-based quality control platform for Chinese traditional medicine: Application to Zeng-Sheng-Ping(Antitumor B). J Ethnopharmacol 172: 195-201.
- Ma MK, McLeod HL (2003) Lessons learned from the irinotecan metabolic pathway. Curr Med Chem 10: 41-49.
- Takemoto I, Itagaki S, Chiba M, Itoh T, Hirano T, et al. (2006) Characterization of secretory intestinal transport of the lactone form of CPT-11. Cancer Chemother Pharmacol 57: 129-133.
- Arimori K, Kuroki N, Kumamoto A, Tanoue N, Nakano M, et al. (2001) Excretion into gastrointestinal tract of irinotecan lactone and carboxylate forms and their pharmacodynamics in rodents. Pharma Res 18: 814-822
- Mathijssen RH, Verweij J, Loos WJ, de Bruijn P, Nooter K, et al. (2002) Irinotecan pharmacokinetics-pharmacodynamics: the clinical relevance of prolonged exposure to SN-38. Br J Cancer 87: 144-150
- Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, et al. (2014) Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther 40: 409-421.
- van Vliet MJ, Tissing WJ, Dun CA, Meessen NE, Kamps WA, et al. (2009) Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis 49: 262-70.
- Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, et al. (2007) Chemotherapy-induced diarrhea is associated with changes in the luminal environment in the DA rat. Exp Biol Med (Maywood) 232: 96-106
- Lin XB, Dieleman LA, Ketabi A, Bibova I, Sawyer MB, et al. (2012) Irinotecan (CPT-11) chemotherapy alters intestinal microbiota in tumour bearing rats. PloS one 7:e39764.
- Pedroso SH, Vieira AT, Bastos RW, Oliveira JS, Cartelle CT, et al. (2015) Evaluation of mucositis induced by irinotecan after microbial colonization in germ-free mice. Microbiology 161: 1950-1960.
- Brandi G, Dabard J, Raibaud P, Di Battista M, Bridonneau C, et al. (2006) Intestinal microflora and digestive toxicity of irinotecan in mice. Clin Cancer Res 12: 1299-1307.
- Guthrie L, Gupta S, Daily J, Kelly L (2017) Human microbiome signatures of differential colorectal cancer drug metabolism. NPJ Biofilms Microbiomes 3: 27.
- Stringer AM, Gibson RJ, Bowen JM, Logan RM, Ashton K, et al. (2009) Irinotecan-induced mucositis manifesting as diarrhoea corresponds with an amended intestinal flora and mucin profile. Int J Exp Pathol 90: 489-499.
- Takasuna K, Hagiwara T, Hirohashi M, Kato M, Nomura M, et al. (1996) Involvement of beta-glucuronidase in intestinal microflora in the intestinal toxicity of the antitumor camptothecin derivative irinotecan hydrochloride (CPT-11) in rats. Cancer Res 56: 3752-3757.
- Kurita A, Kado S, Matsumoto T, Asakawa N, Kaneda N, et al. (2011) Streptomycin alleviates irinotecan-induced delayed-onset diarrhea in rats by a mechanism other than inhibition of beta-glucuronidase activity in intestinal lumen. Cancer Chemother Pharmacol 67: 201-213.
- Fittkau M, Voigt W, Holzhausen HJ, Schmoll HJ (2004) Saccharic acid 1.4-lactone protects against CPT-11-induced mucosa damage in rats. J Cancer Res Clin Oncol 130: 388-394
- Nakao T, Kurita N, Komatsu M, Yoshikawa K, Iwata T, et al. (2012) Irinotecan injures tight junction and causes bacterial translocation in rat. J Surg Res 173: 341-347.
- Zulfikaroglu B, Zulfikaroglu E, Ozmen MM, Ozalp N, Berkem R, et al. (2003) The effect of immunonutrition on bacterial translocation, and intestinal villus atrophy in experimental obstructive jaundice Clin Nutr 22: 277-281
- Ellis M (2004) Preventing microbial translocation in haematological malignancy. Br J Haematol 125: 282-293.
- David L. A, Maurice C. F, Carmody RN, Gootenberg DB, Button JE, et al. (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559-563
- van Vliet MJ, Harmsen HJ, de Bont ES, Tissing W.J. (2010) The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog 6:e1000879.
- Lin X. B, Dieleman LA, Ketabi A, Bibova I, Sawyer MB, et al. (2012) Irinotecan (CPT-11) Chemotherapy alters intestinal microbiota in tumour bearing rats. Plos One 7.
- Bowen JM, Stringer AM, Gibson RJ, Yeoh ASJ, Hannam S, et al. (2007) VSL#3 probiotic treatment reduces chemotherapy-induced diarrhea and weight loss. Cancer Biol Ther 6: 1449-1454.
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, et al. (2012) Host-gut microbiota metabolic interactions. Science 336: 1262-1267.
- Takasuna K, Hagiwara T, Hirohashi M, Kato M, Nomura M, et al. (1998) Inhibition of intestinal microflora beta-glucuronidase modifies the distribution of the active metabolite of the antitumor agent, irinotecan hydrochloride (CPT-11) in rats. Cancer Chemother Pharmacol 42: 280-286
- Takasuna K, Hagiwara T, Watanabe K, Onose S, Yoshida S, et al. (2006) Optimal antidiarrhea treatment for antitumor agent irinotecan hydrochloride (CPT-11)-induced delayed diarrhea Cancer Chemother Pharmacol 58: 494-503.
- Kong R, Liu T, Zhu X, Ahmad S, Williams AL, et al. (2014) Old drug new use--amoxapine and its metabolites as potent bacterial beta-glucuronidase inhibitors for alleviating cancer drug toxicity Clin Cancer Res 20: 3521-3530.
- Innocenti F, Undevia SD, Ramirez J, Mani S, Schilsky RL, et al. (2004) A phase I trial of pharmacologic modulation of irinotecan with cyclosporine and phenobarbital. Clin Pharmacol Ther 76: 490-502.
- Hyatt JL, Tsurkan L, Wierdl M, Edwards CC, Danks MK, et al. (2006) Intracellular inhibition of carboxylesterases by benzil: Modulation of CPT-11 cytotoxicity. Mol Cancer Ther 5:2281-2288.
- Prados MD, Yung WK, Jaeckle KA, Robins HI, Mehta MP, et al. (2004) Phase 1 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: A North American Brain Tumor Consortium study. Neuro Oncol 6: 44-54.
- Takasuna K, Kasai Y, Kitano Y, Mori K, Kobayashi R, et al. (1995) Protective effects of kampo medicines and baicalin against intestinal toxicity of a new anticancer camptothecin derivative, irinotecan hydrochloride (CPT-11), in rats. Jpn J Cancer Res 86: 978-984.
- Mego M, Chovanec J, Vochyanova-Andrezalova I, Konkolovsky P, Mikulova M, et al. (2015) Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complement Ther Med 23: 356-362.
- Flieger D, Klassert C, Hainke S, Keller R, Kleinschmidt R, et al. (2007) Phase II clinical trial for prevention of delayed diarrhea with cholestyramine/levofloxacin in the second-line treatment with irinotecan biweekly in patients with metastatic colorectal carcinoma. Oncology 72: 10-06.
- Desai AA, Kindler HL, Taber D, Agamah E, Mani S, et al. (2005) Modulation of irinotecan with cyclosporine: A phase II trial in advanced colorectal cancer. Cancer Chemother Pharmacol 56: 421-426.
- Chester JD, Joel SP, Cheeseman SL, Hall GD, Braun MS, et al. (2003) Phase I and pharmacokinetic study of intravenous irinotecan plus oral ciclosporin in patients with fuorouracil-refractory metastatic colon cancer. J Clin Oncol 21: 1125-1132.
- Horikawa M, Kato Y, Sugiyama Y (2002) Reduced gastrointestinal toxicity following inhibition of the biliary excretion of irinotecan and its metabolites by probenecid in rats. Pharm Res 19: 1345-1353.
- Kee BK, Morris JS, Slack RS, Crocenzi T, Wong L, et al. (2015) A phase II, randomized, double blind trial of calcium aluminosilicate clay versus placebo for the prevention of diarrhea in patients with metastatic colorectal cancer treated with irinotecan. Support Care Cancer 23: 661-670.
- Sergio GC, Felix GM, Luis JV (2008) Activated charcoal to prevent irinotecan-induced diarrhea in children. Pediatr Blood Cancer 51: 49-52
- Valenti Moreno V, Brunet Vidal J, Manzano Alemany H, Salud Salvia A, Llobera Serentill M, et al. (2006) Prevention of irinotecan associated diarrhea by intestinal alkalization. A pilot study in gastrointestinal cancer patients. Clin Transl Oncol 8: 208-212
- Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG (1995) Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 108: 218-224.
- Nakao T, Kurita N, Komatsu M, Yoshikawa K, Iwata T, et al. (2012) Irinotecan injures tight junction and causes bacterial translocation in rat. J Surg Res. 173: 341-347.
- Crispe IN (2009) The liver as a lymphoid organ. Annu Rev Immunol 27: 147-163
- Seki E, Brenner DA (2008) Toll-like receptors and adaptor molecules in liver disease: Update. Hepatology 48: 322-335.
- Seki E, Schnabl B (2012) Role of innate immunity and the microbiota in liver fibrosis: Crosstalk between the liver and gut. J Physiol 590: 447-458.
- Takasu C, Yismaw WG, Kurita N, Yoshikawa K, Kashihara H, et al. (2017) TU-100 exerts a protective effect against bacterial translocation by maintaining the tight junction. Surg Today 47: 1287-1294.
- Wardill HR, Gibson RJ, Van Sebille YZ, Secombe KR, Coller JK, et al. (2016) Irinotecan-induced gastrointestinal dysfunction and pain are mediated by common TLR4-dependent mechanisms. Mol Cancer Ther 15: 1376-1386.
- Wong DV, Lima-Junior RC, Carvalho CB, BorgesVF, Wanderley CW, et al. (2015) The adaptor protein Myd88 Is a key signaling molecule in the pathogenesis of irinotecan-induced intestinal mucositis. PLoS One 10: e0139985.
- Bibbo S, Ianiro G, Dore MP, Simonelli C, Newton EE, et al. (2018) Gut microbiota as a driver of inflammation in nonalcoholic fatty liver disease Mediators Inflamm 2018: 9321643.
- Rotman Y, Sanyal AJ (2017) Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut 66:180-190.
- Miura K, Ohnishi H (2014) Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease World J Gastroenterol 20: 7381-7391.
- Mallick P, Taneja G, Moorthy B, Ghose R (2017) Regulation of drug-metabolizing enzymes in infectious and inflammatory disease: Implications for biologics-small molecule drug interactions. Expert Opin Drug Metab Toxicol 13: 605-616
- Gerbal-Chaloin S, Iankova I, Maurel P, Daujat-Chavanieu M (2013) Nuclear receptors in the cross-talk of drug metabolism and inflammation. Drug Metab Rev 45:122-144.
- Vugmeyster Y, Harrold J, Xu X (2012) Absorption, distribution, metabolism, and excretion (ADME) studies of biotherapeutics for autoimmune and inflammatory conditions. AAPS J 14: 714-727.
- Du Plessis J, Vanheel H, Janssen CE, Roos L, Slavik T, et al. (2013) Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J Hepatol 58:1125-1132.
- Schnabl B, Brenner DA (2014) Interactions between the intestinal microbiome and liver diseases. Gastroenterology 146: 1513-1524.
- Folprecht G, Pericay C, Saunders MP, Thomas A, Lopez Lopez R, et al. (2016) Oxaliplatin and 5-FU/folinic acid (modified FOLFOX6) with or without aflibercept in first-line treatment of patients with metastatic colorectal cancer: the AFFIRM study. Ann Oncol 27: 1273-1279.
- Tournigand C, Andre T, Achille E, Lledo G, Flesh M, et al. (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol 22: 229-237.
- Wong M, Barqasho B, Ohrmalm L, Tolfvenstam T, Nowak P (2013) Microbial translocation contribute to febrile episodes in adults with chemotherapy-induced neutropenia. PLoS One 8: e68056.
- Fuchs CS, Moore MR, Harker G, Villa L, Rinaldi D, et al. (2003) Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J Clin Oncol 21: 807-814.
- Souglakos J, Pallis A, Kakolyris S, Mavroudis D, Androulakis N, et al. (2005) Combination of irinotecan (CPT-11) plus 5-fluorouracil and leucovorin (FOLFIRI regimen) as first line treatment for elderly patients with metastatic colorectal cancer: A phase II trial. Oncology 69: 384-390.
- Souglakos J, Androulakis N, Syrigos K, Polyzos A, Ziras N, et al. (2006) FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial. from the Hellenic Oncology Research Group (HORG). Br J Cancer 94: 798-805.
- Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini, G, et al. (2007) Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 25: 1670-1676.
- Vamvakas L, Athanasiadis A, Karampeazis A, Kakolyris S, Polyzos A, et al. (2010) Clinical outcome of elderly patients with metastatic colorectal cancer treated with FOLFOXIRI versus FOLFIRI: Subgroup analysis of a randomized phase III trial from the Hellenic Oncology Research Group (HORG). Crit Rev Oncol Hematol 76: 61-70.
- Santoro A, Comandone A, Rimassa L, Granetti C, Lorusso V, et al. (2008) A phase II randomized multicenter trial of gefitinib plus FOLFIRI and FOLFIRI alone in patients with metastatic colorectal cancer. Ann Oncol 19: 1888-1893.
Citation: Kee BK, Overman MJ, Gao S, Chityala PK, Yu He, et al. (2018) Role of Bacterial Translocation in the Progressive and Delayed Irinotecan-Induced Diarrhea. J Clin Exp Pathol 8: 350. DOI: 10.4172/2161-0681.1000350
Copyright: © 2018 Kee B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6368
- [From(publication date): 0-2018 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 5390
- PDF downloads: 978