SARS-CoV-2 as a Trigger in the Development of Tourette’s Like Symptoms
Received: 04-Feb-2022 / Manuscript No. JIDT-22-53540 / Editor assigned: 07-Feb-2022 / PreQC No. JIDT-22-53540 (PQ) / Reviewed: 18-Feb-2022 / QC No. JIDT-22-53540 / Revised: 21-Feb-2022 / Manuscript No. JIDT-22-53540 (R) / Published Date: 28-Feb-2022 DOI: 10.4172/2332-0877.1000491
Abstract
Background: CNS symptoms have been reported in individuals affected by Coronavirus disease 19 (COVID-19). Gut microbiota can affect central physiology via the microbiota-gut-brain axis. We describe Tourette’s-like symptoms resulting from Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection disrupting gut microbiota.
Materials and Methods: A 16-year-old female developed acute-onset Tourette’s-like and neuropsychiatric symptoms after exposure to and infection from SARS-CoV-2. The patient had negative Nasopharyngeal (NP) real-time Reverse Transcription-PCR (RT-PCR) tests for SARS-CoV-2 on five occasions from August of 2020 through June of 2021. The patient’s symptoms worsened over the next six months until Next-Generation Sequencing (NGS) revealed SARS-CoV-2 in her stool. Repair of the gastrointestinal microbiota, treatment with nutraceuticals and pharmaceuticals and changing her surroundings drastically improved her microbiome and sizably reduced symptoms.
Conclusion: The use of nasopharyngeal RT-PCR testing for SARS-CoV-2 may be inadequate and inaccurate for individuals exposed to the virus. The impact of SARS-CoV-2 infection to the GI tract may cause significant havoc in the gut microbiota which may lead to disruption of the blood brain barrier, disruption to the gut-microbiome-brain axis and neurological symptoms. Additional testing, eradication of infectious agents as well as restoration of the gut microbiome are needed to effectively treat this condition.
Keywords: SARS-CoV-2; Coronavirus disease; Infection; Microbiome; Tourette’s
Introduction
Coronavirus Disease 2019 (COVID-19) is a global pandemic resulting from the novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Coronaviruses (CoVs) are a family of enveloped viruses with a single-strand, positive-sense RNA genome approximately 26–32 kilobases in size, which is the largest known genome for an RNA virus [1]. In humans, coronavirus infections primarily involve the upper respiratory tract and/or the gastrointestinal tract, and symptoms vary from mild, self-limiting disease (e.g., the common cold diarrhea, nausea, and vomiting) to more severe manifestations (e.g., bronchitis and pneumonia with renal involvement) [2]. Since Angiotensin Converting Enzyme 2 (ACE2) is found in the absorptive enterocytes of the ileum and colon and ACE2 is also the cellular entry receptor of SARS-CoV-2, the digestive system could be invaded by SARS-CoV-2 and might serve as a route of infection [3].
Materials and Methods
Real-Time Polymerase Chain Reaction (RT-PCR) from nasopharyngeal swabs has been adopted as the “gold standard” test and remains the most common method used to identify SARS-CoV-2, the virus that causes COVID-19 [4]. Although the test was designed to diagnose actively infected individuals, testing results may remain positive after an individual is no longer infected, and individuals may test negative when the virus is present in areas other than the respiratory tract (e.g., digestive system). Acquired mutations of the virus may contribute to the evasion of detection from specifically targeted PCR primers and samples collected soon after infection, or after symptoms have resolved, have resulted in high false-negative rates [5]. Of interest, patients have tested positive for SARS-CoV-2 via Next- Generation Sequencing (NGS) from the stool as long as 40 days after positive nasopharyngeal RT-PCR test and some case studies indicate that the virus can proliferate in the digestive tract even though RT-PCR test results are negative for SARS-CoV-2 [6,7].
The Blood-Brain Barrier (BBB) controls the passage of molecules and nutrients into and out of the brain and ensures homeostasis of the Central Nervous System (CNS) [8]. Even with an intact BBB, inflammatory factors may exert indirect effects upon brain function. Evidence suggests that BBB permeability may be either enhanced or weakened by gut microbiota composition [9].
Bidirectional gut-brain communication ensures proper maintenance and coordination of GI functions to support behavior and physiological processes. Animal studies of amphetamineinduced tics demonstrate that oral administration of Lactobacillus plantarum, a beneficial bacterium with anti-inflammatory psychobiotic properties, can enhance brain levels of dopamine and norepinephrine and largely ameliorate the [murine] tics [10]. Similar to fluoxetine, pretreatment with Lactobacillus rhamnosus blocks the induction of RU 24969-induced OCD-like behaviour [11]. A recent animal study showed that fecal microbiota transplant can rescue age related memory deficits, potentially acting through a microbiome produced metabolite [12]. Finally, two strains of Bifidobacteria appear to reduce anxiety and depression more than the antidepressant escitalopram in innately anxious BALB/c mice [13].
Mounting evidence indicates that the gut microbiota populations may also underpin some neurobehavioral disorders in humans [14]. Dysbiosis may play a role in altered cognitive functions observed in patients with gastrointestinal diseases and some researchers have proposed that a “leaky gut” in humans leads to stress-related psychiatric disorders including anxiety, depression, impaired social function and cognitive dysfunction [10,15,16].
It has been shown that coronaviruses have neuroinvasive properties, and infected peripheral myeloid can subsequently be recruited or transmigrate to the CNS under conditions (e.g., inflammation, stress) which increase BBB permeability [17]. In addition, coronaviruses cause dysbiosis and PANDAS (Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections)/PANS (Pediatric Acute-onset Neuropsychiatric Syndrome) patients have been shown to have dysbiosis that could interfere with dopamine and norepinephrine production [18].
Since the onset of the SARS-CoV-2 pandemic, multiple geographically disparate groups caring for patients with Tourette’s and other tic disorders have noted an increase in these disorders as well as increased illness severity in patients with extant cases. One Canadian specialty group described more than a 15-fold increase in adolescents presenting to their clinic with tic disorders [19].
Results
Here we report a case involving a healthy 16-year-old female with no previous medical concerns who presented with the abrupt onset of Tourette’s-like symptoms and neuropsychiatric episodes after being infected with SARS-CoV-2.
Relevant family history
In late March 2020, the patient’s mother developed symptoms including shortness of breath, chest pressure, elevated heart rate, increased respiratory rate, diarrhea and night sweats. Although the patient’s mother had symptoms for 2.5 weeks, the first PCR test was negative for SARS-CoV-2 but later tested positive for SARS-CoV-2 on 9 April 2020 and was diagnosed with COVID-19 on 12 April 2020.
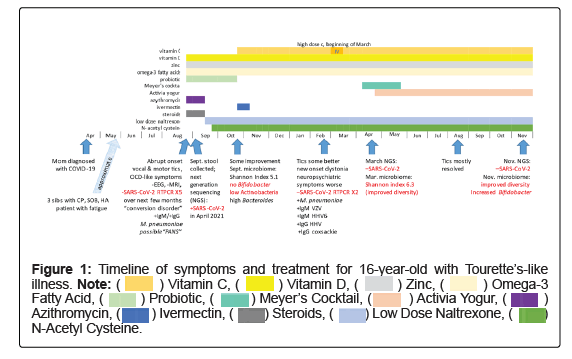
Several months later, the patient’s siblings reported intermittent mild chest pain, shortness of breath, and headaches. However, neither the patient nor the siblings were tested for SARS-CoV-2 at the time (Figure 1).
Patient timeline
A video alongside this patient’s case is included (Video 1). On 19 August 2020, the patient began complaining of a twitch on the side of her neck. Within 30 minutes, the motor tics increased in frequency and intensity and progressed to full body movements including repetitively and aggressively punching her head and abdomen. The patient also developed acute tachycardia (heart rate>140) and was rushed to the local Emergency Room (ER) where she was admitted to the hospital. While at the hospital, she began to have vocal tics and was monitored for seizures. The physicians ruled out physical/mental abuse and after an unremarkable one-hour Electroencephalogram (EEG), the physicians also ruled out seizures. In view of the dramatic nature of the movements and vocalizations, aside from a tic disorder, the possibility of a conversion disorder was also entertained.
Video 1: Video of patients case.
On 21 August 2020, the patient’s symptoms continued to worsen. She was seen at a local integrative medicine facility in Atlanta, GA. Bloodwork was ordered and she was prescribed azithromycin 500 mg for three days as well as a variety of nutraceuticals including vitamin C (1000 mg/day), vitamin D (5000 IU/day), curcumin (500 mg BID), zinc (15-20 mg/day), omega-3-fatty acids (2000 g/day), probiotic (2 capsules/day) and OptiMag Neuro™ (5 g/day).
Between 22 August 2020 to 24 August 2020, the patient displayed mild improvement in hyperkinetic movements and azithromycin was extended for another seven days. On 25 August 2020, lab results showed positive IgG and IgM antibodies for Mycoplasma pneumonia. A preliminary diagnosis of PANDAS/PANS was made. The patient was instructed to remain on azithromycin for the full 10-day course and to add prednisone (70 mg/day) for five days. The nutraceutical protocol remained the same.
Our first contact with the patient was on 27 August 2020 via telehealth at Ventura Clinical Trials/Malibu Specialty Center. After reviewing the patient’s history, tests were ordered to examine the patient’s gut microbiome including stool whole-genome sequencing. On 28 August 2020, the patient had a negative RT-PCR nasopharyngeal test. Throughout September 2020, the patient remained stable. Azithromycin × 3 weeks and two rounds of prednisone (70 mg first round, 100 mg second round) produced little to no change and were discontinued. Preliminary results of an MRI performed on 17 September 2020 demonstrated no significant structural abnormalities that would explain/contribute to the hyperkinetic movements and phonic tics. Treatment additions included CBD oil (20-25 mg before bed), low-dose naltrexone (4.5 mg before bed), N-acetylcysteine, manuka honey and casein-free/dairy-free yogurt.
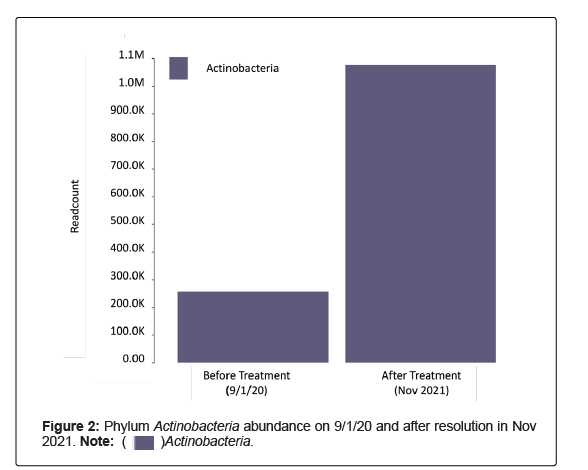
The first stool sample was collected 1 September 2020 into a Zymo Research Shield Fecal Collection Tube and shipped to the lab. DNA was then extracted and purified, then library prepped and sequenced on the NextSeq 550 to generate FASTQ files. These sequencing data were then analyzed using a bioinformatics pipeline for the microbiome composition and relative abundance, as well as alpha and beta diversity. The results were generated 18 October 2020 displaying no Bifidobacteria, low levels of Actinobacteria and limited microbiome diversity (Figures 2 and 3). Although reagents were not available to run testing for SARSCoV- 2 at the time, the presence of SARS-CoV-2 was highly suspicious. Therefore, the patient was placed on a three-day course of ivermectin (12 mg) to be taken on day 1, day 3 and day 7. It was later discovered in April 2021 that SARS-CoV-2 was present in the stool sample collected in September 2020.
On 30 October 2020, the patient reported feeling much better; her tics were significantly reduced, and she had no adverse reactions to the ivermectin. She was instructed to follow through with day 7 of ivermectin. Transitional follow-up visits from November 2020 to January 2021 were mostly unchanged. The tics continued and the patient had a difficult time focusing. A second RT-PCR nasopharyngeal test was negative for SARS-CoV-2 on 8 January 2021.
In February 2021, the patient began having more extreme episodes of anxiety and OCD-like symptoms (e.g., pacing 6 steps in each direction, fear of dolls in her room, inability to leave the corner of her room because she was afraid that the fan in her room would fall on her head), which she termed “neuro-psychotic episodes.” Hyperkinetic movements also continued. Updated bloodwork demonstrated elevated levels of Mycoplasma pneumonia, positive IgG antibodies for Coxsackievirus, positive IgM antibodies for Varicella-zoster, positive IgM antibodies for Human Herpes Virus (HHV) Type 6, positive IgG antibodies for HHV, low vitamin D level, as well as elevated levels for several allergens. Mycotoxin results were also positive for Ochratoxin A, Gliotoxin and Citrinin and stool samples showed low levels of Bifidobacteria.
RT-PCR tests for SARS-CoV-2 from 1 February 2021 and 17 February 2021 were again negative for SARS-CoV-2. Because of the positive viral antibodies and the elevated levels of allergens and mycotoxins, at her follow up visit on 23 February 2021, it was recommended that the patient change her environment, and high-dose intravenous vitamin C infusions were incorporated into the treatment plan.
On 9 March 2021, following the vitamin C infusions, the patient reported improvement, specifically abatement of her “psychotic episodes.” In addition, she stated that she and her mother were moving out of their home. Since hyperkinetic movements continued, the patient underwent a repeat fecal sample by enrichment nextgeneration sequencing. The sample was collected on 14 March 2021, received on 31 March 2021 and results generated on 19 April 2021. To generate these results, RNA was extracted and purified, then reverse transcribed, library prepped, enriched, and sequenced utilizing the Illumina NextSeq 550 System. The resulting FASTQ files were then processed using bioinformatics software to identify the presence of SARS-CoV-2. Results were reviewed on 20 April 2021. Sequencing showed improvement in microbiome diversity and further stool testing was negative for SARS-CoV-2 (Figures 1 and 3).
Follow up conversations between June 2021 to August 2021 revealed complete resolution of “neuro-psychotic episodes” and a significant reduction in Tourette’s-like symptoms following the incorporation of intravenous vitamin C, NAC, as well as the change in environment. Repeat stool samples performed in November 2021 continued to show negative results for SARS-CoV-2, increased Actinobacteria (specifically genus Bifidobacteria) and overall increased microbiome diversity.
Discussion
The sudden onset of Tourette’s, obsessive compulsive disorder, and other neuropsychiatric disorders have been associated with infectious agents such as bacteria, viruses, mold and parasites. The condition is diagnosed as PANDAS (Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections)/PANS (Pediatric Acute-onset Neuropsychiatric Syndrome) [20]. Many children with PANS are very ill, with extreme compulsions (licking shoes, barking), motor and phonic tics (whooping, wringing hands) and terrifying episodes of extreme anxiety or aggression [21]. Alleviation of symptoms associated with PANDAS/PANS may occur with proper treatment of the infectious agent as well as restoration of the gut microbiota.
Acne, eczema and psoriasis have been shown to involve an interaction between the mind and the skin and dysbiosis has also been shown to contribute to skin disorders [22,23]. In this case, treatment of dysbiosis also resulted in the resolution of acne.
It is known that enteric pathogens and the gut microbiome impact the brain in several ways, which may produce systemic and/ or central nervous system inflammation. These include increasing intestinal permeability; cross-reactions with human antigens to stimulate dysfunctional responses of the adaptive immune system; altered neurotransmitter balance; production of metabolites from microbial enzymes that may produce neurotoxicity; and stimulation of host immune responses leading to diverse patterns of systemic cytokine activation [24]. Antiviral immune responses induced by acute viral respiratory infections are associated with dysbiosis, which may subsequently alter immune function against secondary microbial infection or modify the dynamics of inter-microbial interactions, thereby enhancing the proliferation of potentially pathogenic species [25].
The case that SARS-CoV-2 infection precipitates changes in gut microbial composition, which could be involved in the pathogenesis of neuropsychiatric symptoms via the gut-brain axis has been shown to be mechanistically feasible [26]. It has been reported that dysbiosis occurs during SARS-COV-2 infection altering normal intestinal functions such as barrier function and nutrient absorption and affecting the optimal functioning of the immune system. Therapies such as probiotics might help decrease the inflammatory response of viral pathogenesis and respiratory symptoms by strengthening the host immune system, amelioration of the gut microbiome and improvement of gut barrier function [27]. A healthy gut microbiota is largely responsible for the overall health of the host.
Studies have demonstrated that the oral administration of Bifidobacterium longum BB536 significantly alleviated symptoms, reduced the loss of body weight, inhibited viral proliferation in the lungs and improved cytokine production against viruses and oral administration with Bifidobacterium breve YIT4064 increased anti-influenza virus IgG antibodies in serum and protected against infection [28,29]. Data from our lab has revealed that decreased microbial diversity, decreased Bifidobateria, as well as depletion of Faecalibacterium increase susceptibility to viral infections and may lead to dysfunction of the gut-brain axis. It has been demonstrated that SARS-CoV-2 crosses the BBB and any dysfunction of the BBB increases the risk of significant neurological symptoms [30].
Since the beginning of the pandemic, there has been a significant rise in tick disorders and Tourette’s-like symptoms with sudden onset of motor and verbal tics, especially in teenage girls [31]. Considering this case, it would be prudent to perform stool assessments on all individuals with a recent onset of Tourette’s-like symptoms for SARSCoV- 2 as well as potentially repairable dysbiosis.
Conclusion
This case demonstrates that SARS-CoV-2 has the potential to cause significant havoc in the enteric flora, which damages the intestinal barrier, disrupts communication along the GMBA and impairs immune function, leading to neuropsychiatric symptoms. Low Bifidobateria as well as decreased GI microbial diversity sets up the ability for viruses to increase infectiousness and potentially lead to more significant illness. Further testing, including NGS, may be necessary to ensure the presence as well as the alleviation of SARS-CoV-2 from the body. In addition, it is essential to eliminate toxic organisms, reseed the GI tract and optimize gut health for complete healing.
Author Contributions
Conceptualization, S.H.; methodology, S.H. investigation, S.H.; resources, S.H.; writing S.H and S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board, E&I Review Services (IRB study#20110, date of approval: 8/21/2020).
Informed Consent Statement
Informed consent was obtained from the subjects involved in the study and guardian. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Data is not publically published. Data available upon reasonable request from corresponding author.
Acknowledgments
The authors would like to thank Dr. Kate Hendricks for insightful discussion and editorial assistance. The authors would like to thank Dr. Sonya Dave for editing and medical writing assistance.
Conflicts of Interest
SH declares that she has pecuniary interest in Topelia Pty Ltd in Australia, and Topelia Pty Ltd in USA where development of COVID-19 preventative/treatment options are being pursued. She has also filed patents relevant to Coronavirus treatments. She is the Founder and owner of Microbiome research foundation, Progenabiome and Ventura Clinical Trials.
References
- Weiss SR, Navas-Martin S (2005) Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev 69:635-664.
[Crossref] [Google Scholar] [PubMed]
- Wevers BA, van der Hoek L (2009) Recently discovered human coronaviruses. Clin Lab Med 29:715-724.
[CrossRef] [Google Scholar] [PubMed]
- Zhang H, Kang Z, Gong H, Xu D, Wang J, et al. (2020) Digestive system is a potential route of COVID-19: An analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 69:1010-1018.
- LeBlanc JJ, Gubbay JB, Li Y, Needle R, Arneson SR, et al. (2020) Real-time PCR-based SARS-CoV-2 detection in Canadian laboratories. J Clin Virol 128:104433.
[CrossRef] [Google Scholar] [PubMed]
- Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med 382:2302-2315.
[CrossRef] [Google Scholar] [PubMed]
- Papoutsis A, Borody T, Dolai S, Daniels J, Steinberg S, et al. (2021) Detection of SARS-CoV-2 from patient fecal samples by whole genome sequencing. Gut Pathog 13:7.
[CrossRef] [Google Scholar] [PubMed]
- Chen L, Lou J, Bai Y, Wang M (2020) COVID-19 disease with positive fecal and negative pharyngeal and sputum viral tests. Am J Gastroenterol 115:790.
[CrossRef] [Google Scholar] [PubMed]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, et al. (2014) The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6:263ra158.
[CrossRef] [Google Scholar] [PubMed]
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, et al. (2021) Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 9:392.
[CrossRef] [Google Scholar] [PubMed]
- Liao JF, Cheng YF, Li SW, Lee WT, Hsu CC, et al. (2019) Lactobacillus plantarum PS128 ameliorates 2,5-Dimethoxy-4-iodoamphetamine-induced tic-like behaviors via its influences on the microbiota-gut-brain-axis. Brain Res Bull 153:59-73.
[CrossRef] [Google Scholar] [PubMed]
- Kantak PA, Bobrow DN, Nyby JG (2014) Obsessive-compulsive-like behaviors in house mice are attenuated by a probiotic (Lactobacillus rhamnosus GG). Behav Pharmacol 25:71-79.
[CrossRef] [Google Scholar] [PubMed]
- Mossad O, Nent E, Woltemate S, Folschweiller S, Buescher JM, et al. (2021) Microbiota-dependent increase in δ-valerobetaine alters neuronal function and is responsible for age-related cognitive decline. Nature Aging 1:1127-1136.
- Savignac HM, Kiely B, Dinan TG, Cryan JF (2014) Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol Motil 26:1615-1627.
[CrossRef] [Google Scholar] [PubMed]
- Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13:701-712.
[CrossRef] [Google Scholar] [PubMed]
- Nikolov RN, Bearss KE, Lettinga J, Erickson C, Rodowski M, et al. (2009) Gastrointestinal symptoms in a sample of children with pervasive developmental disorders. J Autism Dev Disord 39:405-413.
[CrossRef] [Google Scholar] [PubMed]
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI, et al. (2005) Host-bacterial mutualism in the human intestine. Science 307:1915-1920.
[CrossRef] [Google Scholar] [PubMed]
- Desforges M, Coupanec AL, Dubeau P, Bourgouin A, Lajoie L, et al. (2019) Human coronaviruses and other respiratory viruses: Underestimated opportunistic pathogens of the central nervous system?. Viruses 12:14.
[CrossRef] [Google Scholar] [PubMed]
- Quagliariello A, Chierico FD, Russo A, Reddel S, Conte G, et al. (2018) Gut microbiota profiling and gut-brain crosstalk in children affected by pediatric acute-onset neuropsychiatric syndrome and pediatric autoimmune neuropsychiatric disorders associated with Streptococcal infections. Front Microbiol 9:675.
[CrossRef] [Google Scholar] [PubMed]
- Walla, Watching TikTok videos might cause you to develop tics-Here’s why, The Jerusalem post, 2021.
- Wilbur C, Bitnun A, Kronenberg S, Laxer RM, Levy DM, et al. (2019) PANDAS/PANS in childhood: Controversies and evidence. Paediatr Child Health 24:85-91.
[CrossRef] [Google Scholar] [PubMed]
- Chang K, Frankovich J, Cooperstock M, Cunningham MW, Latimer ME, et al. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): Recommendations from the 2013 PANS consensus conference. J Child Adolesc Psychopharmacol 25:3-13.
[CrossRef] [Google Scholar] [PubMed]
- Koo J, Lebwohl A (2001) Psycho dermatology: the mind and skin connection. Am Fam Physician 64:1873-1878.
- Balato A, Cacciapuoti S, Caprio RD, Marasca C, Masarà A, et al. (2019) Human microbiome: Composition and role in inflammatory skin diseases. Arch Immunol Ther Exp (Warsz) 67:1-18.
[CrossRef] [Google Scholar] [PubMed]
- Galland L (2014) The gut microbiome and the brain. J Med Food 17:1261-1272.
[CrossRef] [Google Scholar] [PubMed]
- Hanada S, Pirzadeh M, Carver KY, Deng JC (2018) Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front Immunol 9:2640.
[CrossRef] [Google Scholar] [PubMed]
- Li N, Ma WT, Pang M, Fan QL, Hua JL (2019) The commensal microbiota and viral infection: A comprehensive review. Front Immunol 10:1551.
[CrossRef] [Google Scholar] [PubMed]
- Din AU, Mazhar M, Waseem M, Ahmad W, Bibi A, et al. (2021) SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed Pharmacother 133:110947.
[CrossRef] [Google Scholar] [PubMed]
- Iwabuchi N, Xiao JZ, Yaeshima T, Iwatsuki K (2011) Oral administration of Bifidobacterium longum ameliorates influenza virus infection in mice. Biol Pharm Bull. 34:1352-1355.
[CrossRef] [Google Scholar] [PubMed]
- Yasui H, Kiyoshima J, Hori T, Shida K (1999) Protection against influenza virus infection of mice fed Bifidobacterium breve YIT4064. Clin Diagn Lab Immunol 6:186-192.
[CrossRef] [Google Scholar] [PubMed]
- Zhang L, Zhou L, Bao L, Liu J, Zhu H, et al. (2021) SARS-CoV-2 crosses the blood-brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduct Target Ther 6:337.
[CrossRef] [Google Scholar] [PubMed]
- Heyman I, Liang H, Hedderly T (2021) COVID-19 related increase in childhood tics and tic-like attacks. Arch Dis Child 106:420-421.
[CrossRef] [Google Scholar] [PubMed]
Citation: Hazan S, Jordan S (2022) SARS-CoV-2 as a Trigger in the Development of Tourette’s-Like Symptoms. J Infect Dis Ther 10: 491. DOI: 10.4172/2332-0877.1000491
Copyright: © 2022 Hazan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6857
- [From(publication date): 0-2022 - Dec 07, 2025]
- Breakdown by view type
- HTML page views: 6136
- PDF downloads: 721

 N-Acetyl Cysteine.
N-Acetyl Cysteine.