Scope of Adsorption of Acid Dyes by Chitosan-Viscose Hybrid Material from an Aqueous Solution
Received: 03-Apr-2018 / Accepted Date: 24-Apr-2018 / Published Date: 30-Jun-2018
Abstract
In this paper, an establishment of easy and economical way to prepare chitosan-viscose hybrid (CH-viscose) materials is done followed by a pad-dry process in which the cure step was performed in a microwave oven to offer a viscose fabric with greatly enhanced affinity for acid dyes. FTIR spectroscopy showed the evidence of successful interaction between viscose and CH through formation of hydrogen bonding and/or ion dipole interaction. Influence of cure time onto the weight per surface unit of samples has been studied and three CH-viscose hybrid materials with different %CH content namely (I-III) were prepared. Cross-linking of cellulosic chain molecules of viscose fiber through CH segments was confirmed by tensile properties measurements. Applications of CH on viscose samples were then tested as adsorbent by dyeing with two acid dyes (Acid Red N-HFS, Acid Blue N-HFS) in a nonelectrolyte aqueous solution. Dyeing results were investigated using spectrophotometer analyses. Data obtained that treated viscose showed better dye exhaustion and fixation (if alkali be provided) than obtained with untreated one. In a nutshell, the limitation of using acid dyes for viscose may overcome by the use of low %CH content ranging from 0.8 to 1.0.
Keywords: Synthesis; π Conjugation; Spectra; Radiation
Introduction
Due to carrying positive charges generally, acid dyes are only applied to fibers such as polyamide and wool in an acidic condition. They are not used for cellulosic materials (cotton, viscose, jute, etc.) coloration owing to their low affinity for the fiber and small molecular size that makes it easy withdrawing from fiber intermolecular space. However, some literature has been suggested the fact that only cationic cotton can be effectively dyed with acid dyes. But, in case of regenerated cellulose like viscose still we have only dependency on reactive dye for traditional dyeing process (continuous and exhaust) [1].
A number of studies on cotton dyeing have been carried out to improve the dye uptake and fastness properties created affinity in cotton for acid dyes by introducing cationic sites in the fiber [2-4]. In one case, Discofix DBA was used in cationization of cotton fabrics and then dyed. It was found that even without mordanting the dyed cationized cotton exhibited a good colour yield and wet fastness properties. Demonstration of cationization of cotton is done bypolyethylenemine (PEI) has been used successfully [5]. Result shown that PEI increased the dye adsorption on cotton and also decreased the dye desorption from the fiber have investigated the adsorption and thermodynamics of acid dyeing of cotton pretreated with CH compared with untreated cotton. The effect of variation of dyeing parameters like electrolyte, pH were also studied [6].
In case of viscose, previously a process has been developed where viscose modification (cationization) is done by the help of polydiallyldimethyl ammonium chloride (poly-DMDAAC) to the dope [7]. Another area where N-modified fibres could be of value is in the dyeing of polyester/viscose blends [8]. Further, a number of patents claim the production of acid dyeable viscose rayon by the incorporation of additives in the dope. Recently dyeing studies have been performed with acid and reactive dyes on commercial viscose rayon fibre which had chitosan incorporated, such as Crabyon, Chitopoly and Danufil [7].
However, high initial cost and longer existing procedure is the main barrier of dope process. That’s why, a pad dry method may be considered as an easy and economical to prepare chitosan viscose hybrid material without spinneret composite where viscose fabric can show the affinity for acid dyes. In such case, chitosan (CH) may be used instead of electrolytes to overcome the negative charge on the viscose fiber. The increase of CH content in CH-Viscose hybrid material might indicate increased amount of amine groups. This paper aims to investigate the dye adsorption and other properties of dyed modified viscose fiber (modification done by chitosan in pad dye process) dyeing with acid dye.
Materials and Methods
Viscose fabric was collected from Divine Fabrics Ltd. Gazipur, Bangladesh. Two types of acid dyes were selected, namely Red N-HFS and Blue N-HFS provided by Texco Tech Bangladesh Ltd. Chitosan was collected from Institute of Radiation and Polymer Technology (IRPT), Savar, Dhaka. The physical and chemical characteristics of t he selected dyes are given in the Table 1 and the types of machines used for the experiment are given in the Table 2 as well.
| Dyes Name | Supplier | ƛmax (mm)1 | Purity(%)1 | Molecular weight (g/mol)2 |
|---|---|---|---|---|
| Red N-HFS | Texco Tech | 498 | 86 | 585.7 |
| Blue N-HFS | Texco Tech | 610 | 89 | 415.2 |
Table 1: Properties of the selected dyes (1Given by the Supplier, 2Measured experimentally).
Viscose fabric preparation
The viscose fabric used was supplied by Divine Textiles limited, Gazipur, Bangladesh. To remove the lubricants and other impurities then the fabric was put in 95°C water (liquor ratio of 1:10) to which 1.0 mg/L of mild alkali and other pretreatment chemicals were added. The mixture was boiled for 45 min. The fabric was then removed, washed with hot water and cold water in order to avoid break down of the emulsion and precipitation of the impurities on the viscose, squeezed to remove excess liquor and then air dried. Finally, it was washed with distilled water. The purified viscose was then dried at room temperature. Auxiliaries were all laboratory grade chemicals and purchased from Mark.
Dye stuffs
Two different commercially available textile dye stuffs were used in this study. All dye stuffs were purchased from Texco Tech Bangladesh Ltd. (A sole agent of Chinese dye in Bangladesh) and used without further purification. The characteristics of the selected dyestuffs and the machineries related to dyeing are listed in Tables 1 and 2, respectively.
| Name of machine | Model | Brand | Origin |
|---|---|---|---|
| Padding mangle | P-AO | Co power | Taiwan |
| Infra-Red Lab Dyeing m/c | Supermat | Co-power | Taiwan |
| Microwave-oven | 415/8 | James H. Heal | UK |
| Spectrophotometer | 650 | Data Color | USA |
Table 2: List of used machineries.
Preparation of CH-viscose hybrid material by pad dry cure process
Chitosan (CH) was collected from the Institute of radiation and polymer technology (IRPT), Atomic Energy Commission, Bangladesh. CH solution was prepared by impregnating and stirring 1 g of CH powder in 1% (v/v) aqueous acetic acid solution at 90°C. Immersion of approximately 13.0 cm2 woven bleached viscose fabric in 100 mL aqueous solutions of CH was done in padder and squeezed to a wet pick up of 100%. Then, with tension dried at 90°C for 5 min and finally placed in a microwave oven at 800 W for different cure times. After being restored to room temperature, samples were thoroughly rinsed to remove uncured CH from surface for 5-7 min and finally oven-dried at 40°C for 24 h. Three samples of CH-viscose hybrid where CH applied 0.8%, 1.0% and 1.2 % (depending on fabric weight) on viscose titled as CH-viscose (I-III) (depending on amount of CH applied).
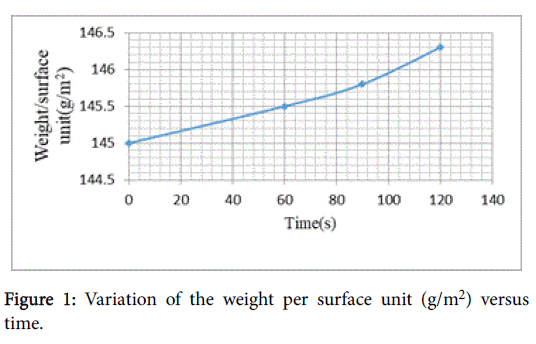
which shown different weight per surface unit [M (g/m2)] listed in Table 3.
| Samples | Time (s) | M (g/m2) | %CH | Color Coordinates | ||
|---|---|---|---|---|---|---|
| DL* | Da* | Db* | ||||
| Viscose | 0 | 145 | 0 | - | - | - |
| I-CH-Viscose | 60 | 145.5 | 2.34 | -7.4 | 3.5 | 1.9 |
| II-CH-Viscose | 90 | 145.8 | 4.48 | -8.9 | 6.3 | 3.9 |
| III-CH-Viscose | 120 | 146.3 | 4.98 | -8.9 | 7.3 | 4 |
Table 3: Color coordinate comparison of untreated viscose and CH-viscose hybrid materials.
According to a standard method (NF G 07-104), the weight per surface unit was calculated using the below mentioned equation for untreated sample and prepared hybrid sample.
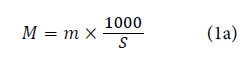
Where m is the mass of the fabric sample (g) and s is the surface of the same sample (cm2). %CH add-on was defined according to equation:
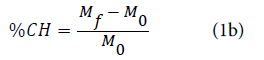
Where Mf and M0 signifies the weight per surface unit of the treated and untreated material, respectively. Higher weight per surface unit obtained by CH application to viscose fabric with the increased cure time shown in Figure 1.
Characterization of CH-viscose composite material by FTIR spectroscopy
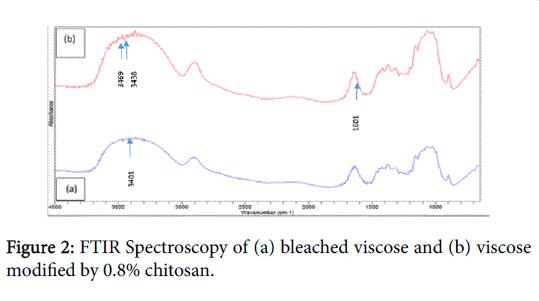
Our present study is going to show the interaction between the two polymers like CH and viscose. It’s obvious that FTIR has often been used in determining specific functional groups or chemical bonds that exist in a material. The presence of a peak at a specific wavenumber would indicate the presence of a specific chemical band [9]. For CH and CH-viscose hybrid material, if specific interactions took place between the two polymers, the most obvious and significant difference would be the appearance of new peaks or shift of existing peaks. Figure 2 showing the FTIR spectra of bleached viscose (a) and CH-viscose (cationization by chitosan) (b) were obtained by FTIR. The FTIR spectrum of CH-viscose in Figure 2b shows a little sharp peak assigned at around 1601 cm-1 indicates the existence of amine groups come from CH. Additionally, examination of the FTIR spectra of bleached viscose in Figure 2a shows peak for the OH functional groups at 3413 cm-1, whereas in case of treated viscose (b) observed to be the shift and created more new broad peak for OH and NH groups from 3413 to 3434 and 3469 cm-1 with the increase of CH content in CH-viscose hybrid material which indicates the presence of amine groups were incorporated into the viscose matrix and interacted with the OH groups of viscose [10].
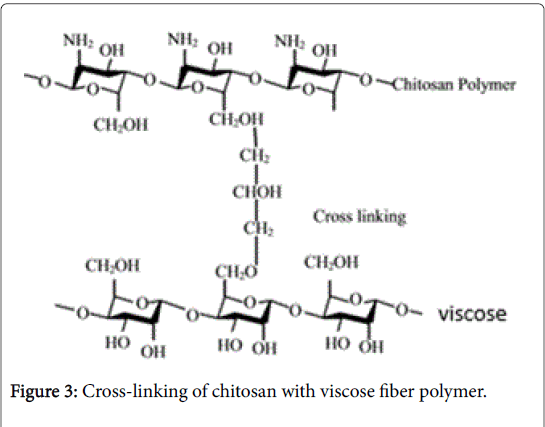
Figure 3 deals a sort of interaction of CH with viscose under acidic condition. In fact, the protonated cation of CH (-NH3 +) cross-linked with viscose polymers via hydrogen bonding and/or ion-dipole interactions.
Results and Discussion
Tensile properties CH-viscose hybrid material
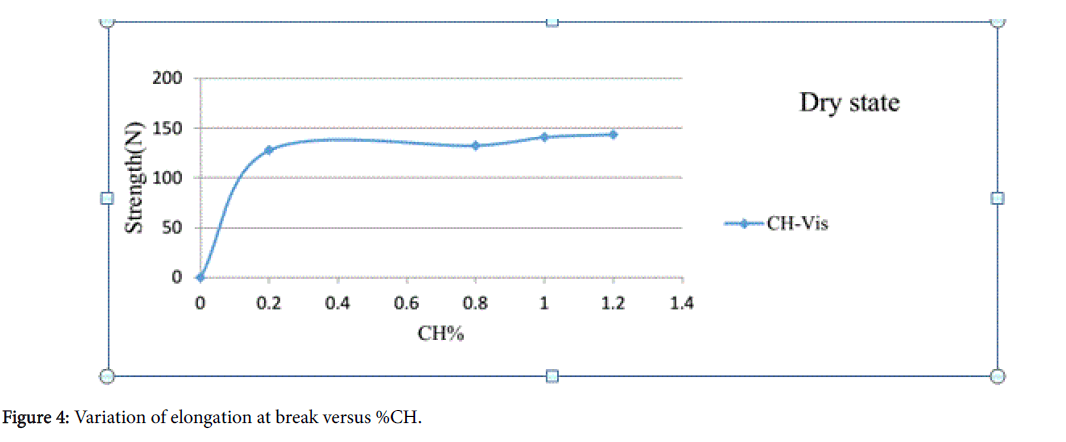
The dry test specimen’s size of 125 mm2 were taken and laid between the jaws of the tensile testing machine (TESTOMETRIC, UK). A standard method ASTM-3787 (Ball Bursting) was followed to conduct these tests which were performed with a traverse speed of 305 mm/min at a pretension of 0.5 N. Figure 4 displays the outcome of the elongation (mm) at break where the experimental data showed that the tensile strength values increase due to the increase of %CH. A gradual increase of tensile strength was observed with the %CH increasing in the order 0.8%, 1% and 1.2% and so on. The reason of such increase may be the cross-linking action along with the amount of CH linked to viscose polymer through the formation of hydrogen bonding and/or ion dipole interaction.
Non-dyed CH-viscose hybrid material
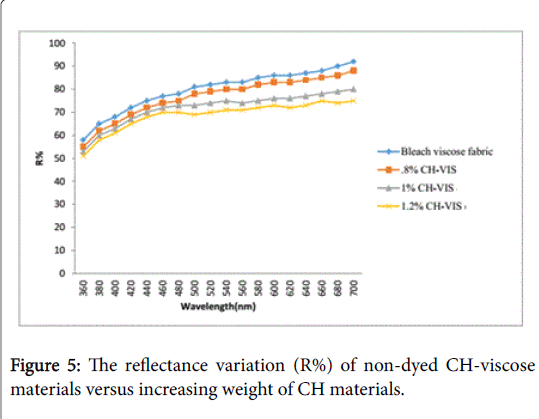
By means of L*, a*, b*coordinates of the CIE color space, the color changing of CH-viscose hybrid material against increased weight of chitosan (in %CH) were measured. The reflectance curve was taken by spectrophotometer where the experimental data shows that, different color properties can be shown by CH-viscose hybrid material with different %CH. Table 3 demonstrated the variation of the three color coordinate parameters that bleached viscose lost its whiteness relying on higher %CH.
In addition, Figure 5 showed that the reflectance (R%) decreased on increasing %CH, in particular, for wavelength value higher than 550 nm indicating that the CH-viscose hybrid material had a yellow appearance (higher Db* value).
Dyed CH-viscose hybrid material
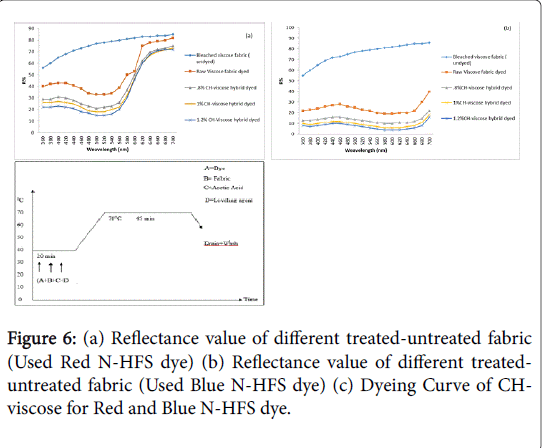
In Figures 6a and 6b, the variation of reflectance against %CH has been compared where same dyeing procedure were followed according to Figure 6c with the help of two dyes like Red N-HFS and Blue NHFS. Comparison has shown that, reflectance values of dyed CHviscose are a function of %CH for the two dyes where with the increase of %CH, the reflectance values decrease gradually. The mechanism was followed by the dye adsorption process as the cationic site increased along with the increase of maximum solid-phase dye concentration where electrostatic interaction between cationic form (-NH3 +) of CHviscose and anionic dye ions took place [11]. The registered minimum reflectance was observed at the wavelength 420 nm and 520 nm, respectively for Red N-HFS and Blue N-HFS.
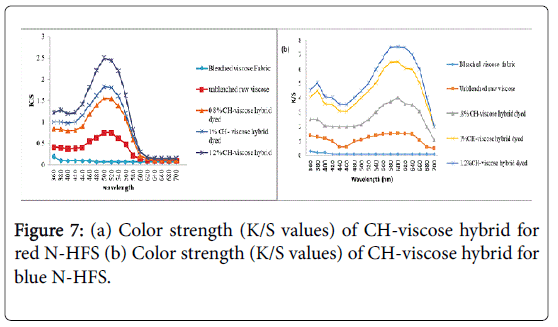
Measurement of color strength in case of dyed CH-viscose hybrid material
The Kubelka-Munk equation was used for measuring color strength (K/S). Spectrophotometer based on D65 light source was used to assess the dyed fabric color strength at wavelength of 420 nm and 590 nm for different %CH treated viscose fabrics which were graphically displayed at Figures 7a and 7b. In both the mentioned graphs, the K/S values were calculated at the wavelength of maximum absorption (λmax) for each dye of (a) Red N-HFS and (b) Blue N-HFS, where K/S values of unbleached viscose (raw) and dyed CH-viscose hybrid materials were compared against undyed bleached viscose fabric. In both dye classes, illustration shows the increased color strength values of each dye with increasing %CH. Besides, the highest K/S value was achieved for 1.2% CH-viscose hybrid material in comparison to other samples, indicate more dye interaction with cationized viscose.
Total color difference measurements
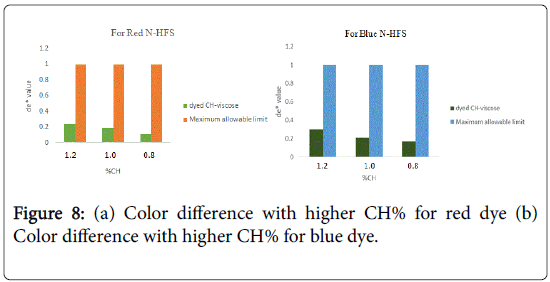
The total color difference (ΔE*) was calculated as per the following equation (2), where the corresponding untreated material (undyed bleached CH-viscose) was taken as a reference.
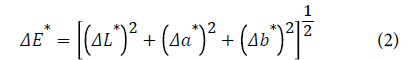
Figures 8a and 8b illustrated strong evidence of higher total color difference value for two mentioned dyes with the increase the amount of CH% cross linked to viscose fabric. More precisely, the growth in CH% is carried out by the increase in (-NH3 +) sites responsible for the adsorption process of dyes made greater color difference in comparison to standard sample.
Conclusion
From this study we observed a new idea of cellulose dyeing with acid dye apart from the conventional reactive dye process. To sum up, this method may be considered as an easy and economical way to prepare CH-viscose hybrid material. Evidence of interaction between viscose and CH is confirmed by FTIR spectroscopy analysis. Changes or improvements including increase of tensile strength, dye adsorption, color pick up% and GSM with the increased %CH are the direct consequences of cross-linking of cellulosic chain molecules with viscose fiber through CH segments formed during the pad-dry-cure process .
Spectrophotometer analysis illustrated that high adsorption capacities are thus found for two types of acid dyes. Moreover, it can be revealed that the lack of affinity for acid dyes is widely overcome by the use of low %CH content ranging from 0.4 to 1.0.
Application of CH onto viscose is thus an alternative way to create cationic sites. We have observed that some of the special characteristics and uses of CH-viscose hybrid materials, such as its use in adsorption, are clearly worthy of exploration in this context. This method is also useful in preparing cellulosic materials containing immobilized amino groups. Such materials should have both the strength of cellulose and the functionality of CH. Further research work needs to investigate the barrier like good dye sorption, wash fastness and crocking tests that could significantly impact on proper application process for bulk.
References
- Deepti G, Adane H (2006) Multifunctional properties of cotton fabric treated with chitosan and carboxymethyl chitosan. Carbohydr Polym 9: 23.
- El-Shishtawy RM, Nassar SH (2000) Cationic pretreatment of cotton fabric for anionic dye and pigment printing with better fastness properties. Color Technol 118: 115-120.
- Hauser PJ, Tabba AH (2001) Improving the environmental and economic aspects of cotton dyeing using a cationised cotton. Color Technol 117: 282-288.
- Rastogi D, Gulrajani ML, Gupta P (2000) Application of lac dye on cationised cotton. Colourage 47: 36-40.
- Janhom S, Griffiths P, Watanesk R, Watanesk S (2004) Enhancement of lac dye adsorption on cotton fibers by poly (ethyleneimine). Dyes Pigm 63: 231-237.
- Rattanaphani S, Chairat M, Bremner J, Rattanaphani V (2007) An adsorption and thermodynamic study of lac dyeing on cotton pretreated with chitosan. Dyes and Pigm 72: 88-96.
- Bairagi N, Gulrajani ML, Deopuraa BL, Shrivastava A (2005) Dyeing of N-modiï¬ed viscose rayon ï¬bres with acid and metal-complex dyes. Color Technol 121: 320-324.
- Bairagi N, Gulrajani ML, Deopuraa BL, Shrivastava A (2006) Dyeing of N-modiï¬ed viscose rayon ï¬bres with reactive dyes. Color Technol 121: 113-120.
- Mahjoub J, Mohamed HVB, Mohamed SR, Aghleb B (2011) Adsorption of acid dyes from aqueous solution on a chitosan-cotton composite material prepared by a new pad-dry process. J Eng Fiber Fabr 6: 1-12.
- Chunxiu L, Renbi B (2005) Preparation of chitosan/cellulose acetate blend hollow fibers for adsorptive performance. J Membr Sci Technol 267: 68-77.
- Sakkayawong N, Thiravetyan P, Nakbanpote W (2005) Adsorption mechanism of synthetic reactive dye wastewater by chitosan. J Colloid Interface Sci 286: 36-42.
Citation: Bhuiyan AH, Sakila F, Khan AM, Md. Salauddin SK (2018) Scope of Adsorption of Acid Dyes by Chitosan-Viscose Hybrid Material from an Aqueous Solution. J Mater Sci Nanomater 2: 105.
Copyright: © 2018 Bhuiyan AH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 6358
- [From(publication date): 0-2018 - Dec 18, 2025]
- Breakdown by view type
- HTML page views: 5330
- PDF downloads: 1028