Screening of Tobacco Genotypes for Tolerance/Resistance to Striga gesnerioides infestation in Zimbabwe
Received: 06-Feb-2018 / Accepted Date: 13-Feb-2018 / Published Date: 19-Feb-2018 DOI: 10.4172/2329-8863.1000340
Abstract
Witch weed (Striga gesnerioides) is a root parasitic weed of tobacco and cowpea in Sub- Saharan Africa. It is very difficult to control and host plant resistance is the most promising effective method affordable to small-scale farmers. This study was done to screen local tobacco genotypes for tolerance and resistance to Striga gesnerioides infection. Laboratory screening experiments were conducted during the 2016/17 season using fifteen local tobacco genotypes. The experiments were done using an Agar gel technique at the University of Zimbabwe, with treatments replicated four times in a Randomised Complete Block Design (RCBD). All tobacco genotypes significantly (p<0.05) stimulated the germination percentage and there were significant differences (p<0.05) in the germination distance of Striga seeds. The tobacco genotypes K RK66 and T74 had the closest Striga germination distance and the lowest germination percentages of Striga seeds, indicating their ability to produce low germination stimulants (strigolactones). This study revealed that all the tobacco genotypes were susceptible to Striga infection except the genotypes K RK66 and T74 which showed some level of tolerance and could be adopted by farmers in Striga infested areas. Additional evaluations, targeting the parental lines of the tolerant cultivars and is recommended as further studies.
Keywords: Striga gesnerioides; Tobacco; Strigolactones
Introduction
Witch weed (Striga gesnerioides ) is one of the most devastating parasitic weed of broad-leaved crops in most parts of the world. It is an obligate root parasitic flowering weed that belongs to the Orobanchaceae family [1]. The germination of Striga is induced by strigolactones which are Striga germination stimulants found in root exudates of both host and non-host plants [2]. In Zimbabwe S. gesnerioides was reported on tobacco in the Mvuma and Chegutu growing areas, by both small and large-scale growers [3]. However, there is little information on tobacco leaf yield losses caused by this parasite although, 100% yield losses were reported in the Mvuma area under severe infestations [4]. Notably, the biotype of S. gesnerioides affecting tobacco in the Mvuma area did not affect cowpea, another known host of the parasitic weed. Striga gesnerioides is a problem on cowpea in African countries such as Nigeria, Kenya and Mali [5]. In Botswana, it was reported on sweet potato [6]. In cowpea yield losses resulting from S. gesnerioides infestations in the Northern Nigeria ranged from about 83 to 100%. For the control of S. gesnerioides , several strategies, including the use of nitrogen (N) fertilizer, host plant resistance, intercropping, crop rotation, suicidal germination in a trap crop system and herbicide control have been proposed [4,5]. However, for the control these measures have not been widely adopted by farmers in Zimbabwe, probably due to lack of resources, especially the smallholder farmers.
Host plant resistance is an effective and affordable option for the control of Striga . Cowpea varieties (B301, IT97K-499-35, IT84S-2049 and IT98K-205-8) have shown complete resistance to S. gesnerioides [5]. The commercial tobacco cultivars (K RK66, and K RK26) were reported to be tolerant to Striga under field trials in the Mvuma area compared to landraces [3]. The commercial tobacco varieties cultivated by farmers were not bred to be Striga resistant and were not evaluated for Striga resistance/tolerance. Currently, there is no breeding work being conducted at Kutsaga with the aim to improve tolerance to Striga . This research was thus targeted at identifying resistant/tolerant genotypes which can be used in breeding programmes and to elucidate their mechanisms of resistance to Striga .
Methodology
Study site and plant materials
The experiment was conducted in the Plant Pathology Laboratory of the Crop Science Department at the University of Zimbabwe. Fifteen local tobacco genotypes from the Tobacco Research Board were used in this experiment. These comprised of eight open release varieties (KM10, K RK22, K RK26, K RK28, K RK29, K RK60, K RK64, and K RK66) and seven limited release tobacco varieties (T70-T76). The experiment was laid out in a Randomized Complete Block Design (RCBD) with sixteen treatments (15 tobacco genotypes and water as a negative control) in two blocks. The treatments were replicated four times in each block.
Surface disinfection of Striga seeds
Striga gesnerioides seeds that were collected from the infested fields of smallholder farmers in the Mvuma area were surface disinfested in order to destroy microbial contaminants. This was done by immersing the desired amount of Striga seeds in 1% sodium hypochlorite for two minutes. The floating seeds and chaff were discarded and the Striga seeds were washed with sterile water on a filter paper fitted in a funnel.
Pre-conditioning of Striga seeds
Striga seeds were preconditioned following the method [7] described by Berner et al. Surface disinfested S. gesnerioides seeds were placed in a sterile Petri dish with 30 ml of sterile water and stirred until they sunk. The Petri dish was covered with aluminium foil in order to exclude light and incubated at 28ºC for 14 days, changing water after every two days using a pipette.
Selecting for hosts with low stimulant production
The agar gel technique [8] was done following the procedure adapted from Reda et al. The surface disinfected Striga seeds were used in this technique. Host seeds were germinated by placing them on wet filter papers in petri dishes and incubating at room temperature for 14 days. Water agar was prepared by dissolving 6.8 g of bacto agar in 1000 ml of deionised water and the solution autoclaved at 120ºC for 20 minutes to eliminate microbial contaminants. The agar was allowed to cool for an hour before pouring. Fifty micro-millilitres (approximately 150 seeds) of conditioned S. gesnerioides seeds were pipetted into sterile petri dishes with a 9 cm diameter. Thirty millilitres of autoclaved water agar was poured over the Striga seeds allowing random distribution of the Striga seeds on the plate. One tobacco seedling was submerged in the solidifying agar near one edge of the plate with the root tip pointing across the plate. The plates were incubated in the dark at 28ºC for 48 hours
Data collection
Striga germination percentage: The germinating seeds were observed using a dissecting microscope at 100X magnification focusing through the bottom of the petri dish. Four sampling areas were randomly selected in each of the petri dishes and the germinated Striga seeds were counted and germination percentages calculated.
Striga germination distance from the host root: The distance from the tip of the tobacco seedling to the furthest germinated Striga seed was measured using a microscope micrometer calibration ruler on a dissecting microscope at 100X magnification.
Data analysis: Analysis of variance (ANOVA) for the Striga germination data was done using Genstat version 14. Treatment means were separated using 5% LSD (Fisher’s Protected LSD). Graphs were plotted using ggplot2 of R Statistics version 3.1.3.
Results
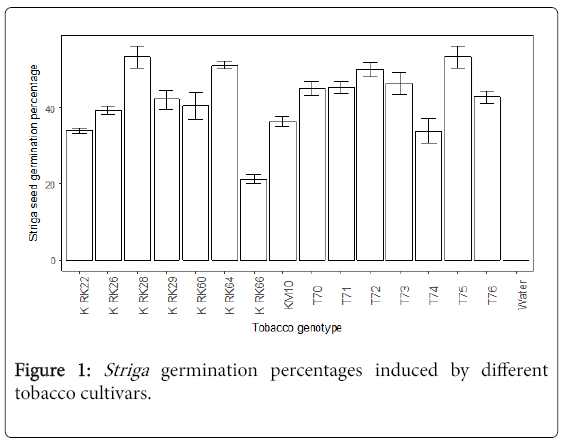
Striga germination percentage influenced by different varieties: The tobacco genotypes released Striga germination stimulants that significantly (P<0.05) stimulated the germination of Striga seeds. An open release tobacco cultivar K RK66 had the lowest Striga germination percentage and the tobacco genotypes T75, K RK28, K RK64, T72 and T73 had the highest Striga germination percentage as shown in Figure 1. In contrast, in the negative control treatment (water), no Striga germination was observed, as expected.
Striga germination distance from the host root
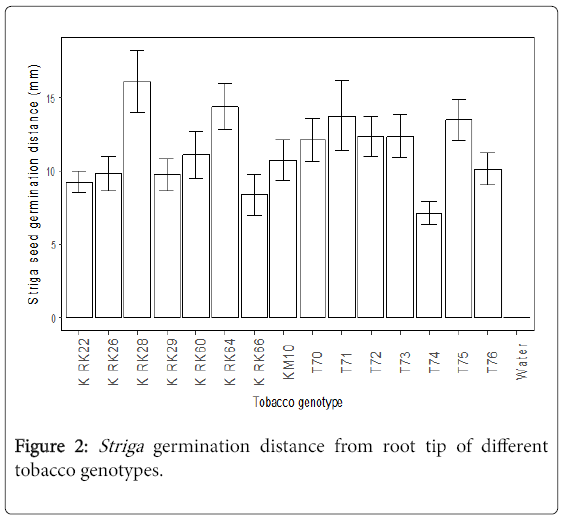
The germination distances of Striga seeds were significantly different (p<0.05) among the cultivars. The tobacco cultivars K RK28 K, RK64 K, RK71 and T75 had the furthest Striga germination distance (Figure 2). These cultivars were not significantly different in terms of Striga germination distances. In contrast, the tobacco genotypes T74 and K RK66 had the shortest Striga germination distance.
Discussion
Striga germination percentage
The results of the Agar gel assays revealed that the tobacco genotypes K RK28, T75 and K RK64 stimulated high Striga seed germination, suggesting that they produced high Striga germination stimulants [9,10]. These tobacco varieties are susceptible genotypes as they stimulate high germination of Striga seeds, thereby, increasing the chances of many Striga plants to be attached to the host roots. In previous laboratory screening trials K RK28 and K RK64 showed above 60% stimulation [3]. The tobacco cultivar K RK66 was associated with low Striga germination, suggesting low release of Striga germination stimulants [11]. The genotype was, thus, the most Striga tolerant of the tested varieties based on Striga seed germination and this confirmed previous observations under field conditions [3]. However, in the previous laboratory screening experiment, this tobacco cultivar was comparable with other cultivars evaluated in Striga germination studies [3]. The differences in outcome observed could be as a result of the fact that the age of the tobacco seedlings and the screening methods used in the two studies were different. Further laboratory studies are needed to investigate the germination of S. gesnerioides seeds stimulated by the host at different ages.
Furthest Striga germination distance
A high stimulation of Striga seed germination and a furthest germination distance of Striga seeds was associated with K RK28, suggesting the host produced high amounts of strigolactones [11]. This implies susceptibility to Striga infection since the host could stimulate Striga seeds that were located far away from the roots to germinate, potentially resulting in many Striga attachments. The least Striga germination distance was recorded from T74 and K RK66. Both stimulated low Striga seed germination suggesting low production of Striga germination stimulants. Tobacco genotypes with low germination stimulant (LGS) produce insufficient amounts of the exudates required for germination of conditioned Striga seeds. Genotypes producing low levels of germination stimulants were found to be resistant to Striga in field tests, and this low germination stimulant (LGS) trait was found to be inherited as a single, nuclear, recessive gene with largely addative gene action [2]. In studies done with sorghum, all highly susceptible genotypes were high producers of the germination stimulant. Most Striga seeds close to the growing tip of the tobacco seedling germinated better when compared to seeds that were close to the older part of the roots. This indicated that root exudation was active in the root tip of the tobacco seedling. This is in line with the report by Okonkwo [12] which states that smaller quantities of strigolactones are produced by the older root cells of the host plant.
Conclusions and Recommendation
The study revealed that the tobacco genotypes K RK66 and T74 were the most tolerant to S. gesnerioides infestation as these produced low Striga germination stimulants. The most susceptible tobacco genotypes were K RK28, T75, K RK64, T72 and T76; and these genotypes produced more germination stimulants. The root exudates from susceptible tobacco genotypes induced enhanced Striga seed germination. The tobacco genotypes K RK66 and T74 can be grown in Striga infested areas since these genotypes have shown to be tolerant to S. gesnerioides infection. Host avoidance of Striga can be implemented as a control measure in Striga infested areas of the country. However, there is need to conduct more laboratory studies to investigate the germination of Striga seeds using the parental lines of the tolerant tobacco genotypes and field evaluation is recommended to validate the results obtained from the laboratory studies.
Acknowledgements
The authors are thankful to the Tobacco Research Board for the provision of the genetic materials and the University of Zimbabwe for the laboratory facilities.
Disclosure Statement
The authors declare no conflict of interest.
References
- Parker C (2012) Parasitic weeds: A world challenge. Weed Science 60: 269–276.
- Xie X, Yoneyama K, Kisugi T, Uchida K, Ito S, et al. (2013) Confirming Stereochemical Structures of Strigo-lactones Produced by Rice and Tobacco. Mol Plant 6: 153–163.
- Koga C, Mwenje E, Garwe D (2011) Germination stimulation of Strigagesnerioides seeds from tobacco plantations by hosts and non-hosts. Journal of Applied Biosciences 37: 2453-2459.
- Koga C (2016) The resurgence of Strigagesnerioides and the discovery of Dodder (Cuscutaspp) parasitic weeds in tobacco in Zimbabwe.
- Omoigui LO, Kamara AY, Ishiyaku, MF, Boukar O (2012) Comparative responses of cowpea breeding lines to Striga and Alectra in the dry savanna of northeast Nigeria. African Journal of Agriculture Res 7: 747-754.
- Parker C, Riches CR (1993) Parasitic Weeds of the World. Biology and Control. CAB International. Wallingford, UK.
- Berner DK, Winslow MD, Awad AE, Cardwell KF, Raj DM, et al. (1997) Striga research methods. International Institute of Tropical Agriculture, Ibadan, Nigeria.
- Reda F, Buttler LG, Ejeta G, Ramson JK (1994) Screening of maize genotypes for low Strigaasiatica stimulant production using the Agar gel technique. African Crop Science Journal 2: 2. 173–177.
- Gbehounou G, Adango E (2003) Trap crops of Strigahermonthica, Invitro identification identification and effectiveness in situ. Crop protection 22: 395-404.
- Pierce S, Mbwaga AM, Press MC, Scholes JD (2003) Xenognosin production and tolerance to Strigaasiatica infection of highâ€yielding maize cultivars. Weed Research 43: 139–145.
- Mohamed AH, Housley TL, Ejeta G (2010) An in vitro technique for studying specific Striga resistance mechanisms in sorghum. African Journal of Agricultural Research 5: 1868-1875.
- Okonkwo SNC (1991) In vitro growth response of cultured and germinated seed of witchweed (Strigaasiatica).
Citation: Koga C, Mabasa S, Mazarura U, Banwa T, Garwe D (2018) Screening of Tobacco Genotypes for Tolerance/Resistance to Striga gesnerioides infestation in Zimbabwe . Adv Crop Sci Tech 6: 340. DOI: 10.4172/2329-8863.1000340
Copyright: © 2018 Koga C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6329
- [From(publication date): 0-2018 - Dec 23, 2025]
- Breakdown by view type
- HTML page views: 5316
- PDF downloads: 1013