Seronegative Neuromyelitis Optica Specturum Disorder with CSF Finding Mimicking Bacterial Meningitis: Case Report
Received: 01-Mar-2022 / Manuscript No. JNID-22-55864 / Editor assigned: 03-Mar-2022 / PreQC No. JNID-22-55864(PQ) / Reviewed: 17-Mar-2022 / QC No. JNID-22-55864 / Revised: 22-Mar-2022 / Manuscript No. JNID-22-55864(R) / Published Date: 29-Mar-2022 DOI: 10.4172/2314-7326.1000382
Abstract
Neuromyelitis optica (NMO) is a disabling, rare, inflammatory, autoimmune, demyelinating disease of the disorder of the central nervous system (CNS) that affects mainly his spinal cord and optic nerves results in severe attacks of optic neuritis and myelitis. The serum autoantibody against aquaporin-4 water channels, NMO-Immunoglobulin (NMO-IgG), is involoved in the disease and is found in up to 75% of patients with an NMO-spectrum disorder but if negative doesn’t exclude the disease. However, the cerebrospinal fluid (CSF) profile in NMO relapses is not well known and there is great variability in CSF profile in those patients, so here we describe one case of seronegative neuromyelitis optica spectrum disorder with CSF findings similar to bacterial meningitis.
Keywords: Neuromyelitis optica spectrum disorder; Aquaporin 4 antibodies; Optic neuritis; Myelitis; Bacterial meningitis
Keywords
Neuromyelitis optica spectrum disorder; Aquaporin 4 antibodies; Optic neuritis; Myelitis; Bacterial meningitis
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is autoimmune disease causes severe demyelination especially in optic nerves with typical clinical manifestations in the form of acute optic neuritis and transverse myelitis occurring simultaneously or separated by a variable period [1-3]. It is more common in the form of polyphasic. New diagnostic criteria which included serological testing of serum aquaporin-4 immunoglobulin G antibodies (AQP4-IgG). Diagnostic criteria of NMOSD with AQP4-IgG requires at least 1 core clinical characteristic or magnetic resonance imaging (MRI) finding related to optic neuritis, acute myelitis, area postrema syndrome, acute brainstem syndrome, diencephalic, or cerebral syndromes. For the diagnosis of NMOSD without AQP4-IgG, more comprehensive clinical requirements, two or more core clinical crieteria with additional neuroimaging findings are needed [4]. The literature supports that AQP4-Ab testing is important in establishing the diagnosis of NMOSD; however, results showing negative antibodies will group the patients in a seronegative subgroup [5].
Informed Consent
Written informed consent was obtained from the case. A copy of the written consent is available for review by the Editor of this journal.
Case Presentation
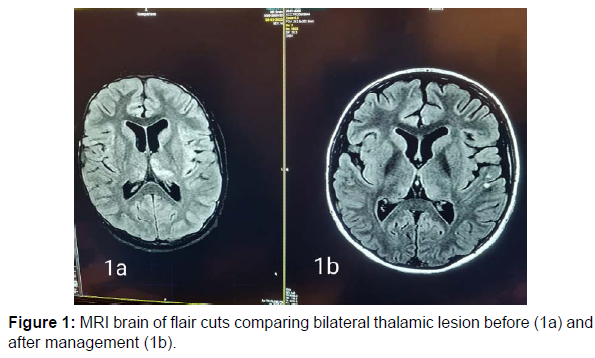
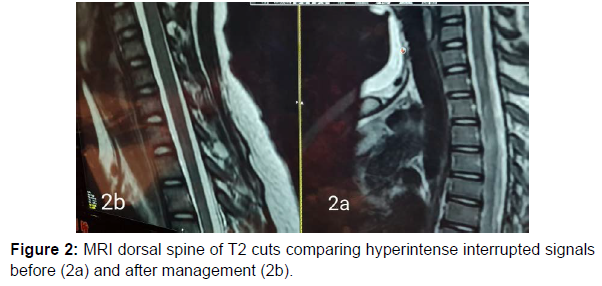
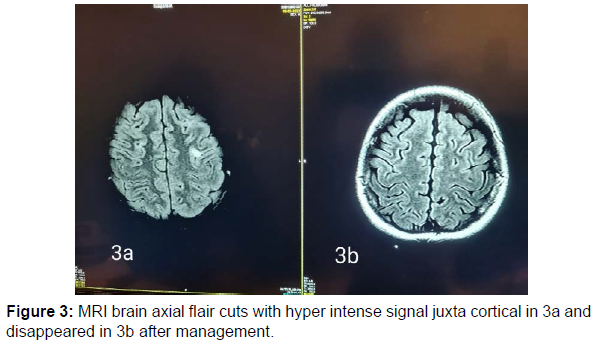
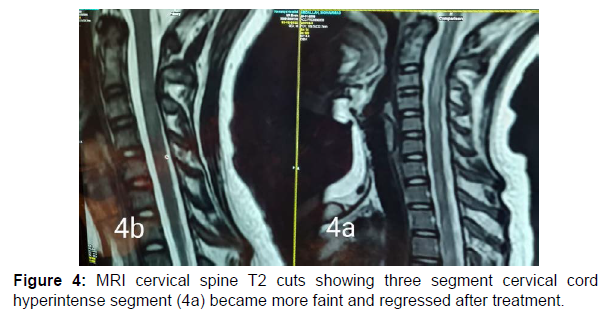
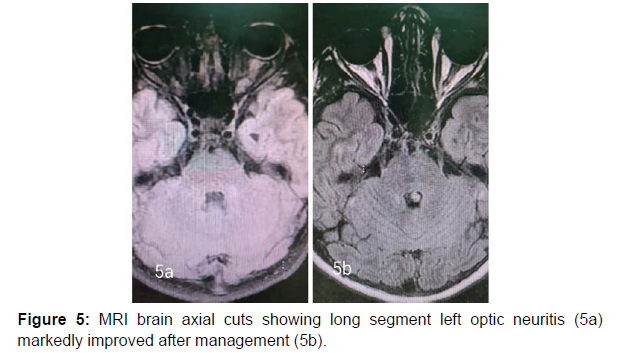
13 years old male patient previously healthy was presented at February 2022 to our hospital with subacute onset stationary course of left sided painful diminution of vision, bilateral flaccid paraplegia, and complete sensory loss till level just above umbilicus and urine retention. By detailed history his father mentioned that his condition was preceded by low grade fever, recurrent unexplained vomiting, excessive sleepiness and narcolepsy. He developed 2 attacks of generalized tonic clonic convulsions 1 week before his symptoms for which he was not properly investigated. By examination, he had left typical painful optic neuritis with disc edema, color desaturation and relative afferent pupillary defect. He had also bilateral flaccid lower limb weakness grade 0, hypotonia, hyporefelxia, mute planter response, sensory level till thoracic segment 10 and urine retention. Urgent MRI brain and whole spine (Figure 1 a-5a) with contrast was done and showed typical MRI charchteristic picture of NMOSD in the form of left long length optic neuritis mainly anterior, juxta cortical, deep cortical, bilateral diencephalic , periepydymal plaques and three segment anterior cervical plaque and multiple interrupted dorsal plaques which showed hyperintense flair signals with moderate post contrast enhancement. Lumbar puncture was done which showed neurtrophilic pleocytosis with total cell count of 1500 cell/mm3 mainly PMNLs with CSF proteins of 1400 mg/dl, normal glucose and lactate levels. Negative CSF PCR for EBV, HSV1, 2, CMV, enteroviruses. Negative CSF culture, sensitivity and CSF T.B PCR. All septic biomarkers including CRP, procalcitonin blood culture, urine culture and serial CBC were negative. All vasculitic screen was negative including ANA, ANCA B,C, lupus anticoagulant antibodies , anticardilipin antibodies, C3, C4 , all serum virology screening including hepatitis B, C, HIV, CMV, EBV, HTLV were negative. Serum angiotensin converting enzyme was negative to rule out sarcoidosis. Brucella antibodies and agglutination tests were negative also. CSF oligoclonal bands done by isoeletric focusing technique was negative.All panel of autoimmune encephalitis including NMDA abs were negative. Anti aquaporin 4 (NMO) antibodies done by cell based assay technique was negative. Anti myelin oligodendrocyte (MOG) antibodies was also negative. Urgent management plan was after ruling out underlying CNS infection was started in the form of pulse methylprednisolone 1gm for 5 days with plasma exchange 7 sessions every other day and then shifted to oral steroids with gradual tapering. He was markedly improved regarding his visual acuity and sensory level including superficial and deep sensations with very mild improvement regarding motor function in lower limbs with early flickering in both feet. MRI brain, orbit and whole spine with contrast were done again (Figure 1b-5b) after management for follow up and showed marked regressive course in all signals including optic, cortical, thalamic and cord lesions with no post contrast enhancement. EMG on both lower limbs showed marked denervation activity more in proximal muscles and nerve conduction showed signs of axonal lower moter neuron lesion suggesting anterior horn cell or radicular injury .Our patient was labeled as double seronegative NMOSD and First dose of Rituximab was given as disease modifying therapy and the next dose was scheduled after 6 months then was discharged on regular physiotherapy. On regular follow up after 1 month from discharge and from his rituximab dose he was markedly improved regarding his motor power that he became ambulant with bilateral support.
Discussion
NMOSD is a rare disease which common to affect female than males with ratio 10-1 [6]. Pathogenesis is not fully understood but there is antibodies against AQP4 water channel mostly expressed at foot processes of astrocytic cell membrane at blood brain barrier [7-8]. The clinical features of NMOSD are severe recurrent attacks of myelitis and bilateral and unilateral optical neuritis that can occur simultaneously. It is more common to be found in the form of polyphasic (90%) than monophasic (10%) [1-2]. Here, we reported a case of 13 year-old boy presenting with optic neuritis, flaccid paraplegia and urinary retention. Cerebrospinal fluid displayed neutrophilic pleocytosis and elevated protein concentration. Without the possibility to rule out an infectious or inflammatory aetiology, antibiotics and corticosteroids were started. Although negative for both aquaporin-4-IgG antibodies and anti MOG abs, the patient fulfilled criteria for seronegative neuromyelitis optica spectrum disorder with the presence of multiple core clinical characteristics (two major in the form of acute transverse myelitis , optic neuritis and two minorin the form of area posterema and diencephalic syndrome symptoms) and highly suggestive MRI finding [1,2,9,10,11]. Serum AQP4-Ig is detected in 60-90% of patients who met NMOSD clinical and radiological criteria [4]. In seronegative AQP4- IgG patients who met the clinical and radiological criteria of NMOSD, serum myelin oligodendrocyte glycoprotein (MOG) antibodies can be detected [2]. In this patient, AQP4-IgG examination was negative, and serum MOG antibody examination was also negative .Cerebrospinal fluid examination in positive NMOSD patients with AQP4-Ab can be found moderate with normal pleocytosis in about 40% of cases during acute recurrence. Oligoclonal bands (OCB) are usually not found, the intrathecal polyspecific antiviral immune response against-Measles, Rubella and Varicella-Zoster viruses (MRZ reaction) are negative, and increased glial fibrillary acidic protein and neurofilament heavy chain (nfH) are commonly found [6]. In this patient, the cerebrospinal fluid examination was surprising with neutrophilic pleocytosis demanded extensive diagnostic workup Neutrophilic pleocytosis with increased total protein level reflected the severity of myelitis. Similar CSF findings with neutrophilic pleocytosis and increased protein level were already described during the acute phase of NMO [12-14]. The MRI examination on the spinal cord in NMOSD have typical features of longitudinal extensive transversal myelitis (LETM), a lesion that extends over 3 or more segments of the adjacent spinal cord [1,4,13,15]. MRI of the optic nerve can be seen as hyperintensity in optic neuritis and tends to have more posterior involvement of the optic nerve involoving optic chiasma but more anterior in MOGAD. Brain MRI features can vary, such as normal or periependimal lesions surrounding the ventricular system, dorsal brain stem lesions bordering the fourth ventricle, periependymal lesions that surround the lateral ventricles, white matter hemispheres, lesions involving the corticospinal tract, non-specific lesions and enhancing lesions [14,16]. In this patient, an MRI examination of the spinal cord showed with the features of myelitis involving C2-4 and Th2-6 while a brain MRI examination showed deep cortical plaques, thalamic, hypothalamin, periepindymal plaques and long segment left sided optic nerve hyperintense signal mainly anterior. Corticosteroids are the main choice in the acute phase. Intravenous methylprednisolone is administered with a dose of 1-1.5 grams in 3-5 days [1-2]. Therapeutic plasma exchange (TPE) can be considered early and combined with steroids for better and rapid outcome. Therapeutic plasma exchange dosage is carried out by giving 5-7 cycles in a period of 2 weeks with a dose of 1-1.5 plasma per time TPE [17] and this regimen was done in our patient. Rituximab a monoclonal antibody to CD20, has been found to be effective in several reports and small uncontrolled studies as a disease modifying therapy for prevention of recurrent relapses and was started to our patient with marked improvement in motor power in both lower limbs.
Conclusion
We report acase with crieteria of douple seronegative NMOSD fullfilling both clinical and radiological crieteria with unusual CSF finding in the form of neutrophilic pleocytosis and high CSF proteins mimicking picture of CNS infection, So, in cases with CSF findings similar to ours, the diagnosis, could be doubtful and frequently require comprehensive diagnostic workup to exclude other diseases, particularly in patients with meningomyelitis and uncertain history of optic neuritis. Because of that, the correct diagnosis and timely initiation of appropriate treatment could be missed. It should be stressed that established neurological damage due to myelitis and optic neuritis in untreated patients is permanent. Accordingly, it is of outstanding importance to commence adequate (immunosuppressive) treatment without any delay in such patients.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Funding sources
No fund was needed for this study
Author contribution: Dr Diana khedr responsible for data collection, analysis, interpretation of results and drafted the article. Dr Sulaiman elkhashan revised, approved the version to be published and was directly approving management plan.
References
- Optik ERN (2017) In: Aninditha T, Wiratman W, editors. Buku Ajar Departemen Neurologi. Jakarta: Penerbit Kedokteran Indonesia. pp. 258-264.
- Jasiak-Zatonska M, Kalinowska-Lyszcarz A, Michalak S, Kozubski W (2016) Review: The Immunology of Neuromyelitis Optica - Current Knowledge, Clinical Implications, Controversies, and Future Perspective. Int J Mol Sci 17(3): 273.
- Broadley S, Khalili E, Heshmat S (2017) Review Article: Neuromyelitis Optica Spectrum Disorder. ACNR 17(1):11-14.
- Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, et al. (2015) International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85(2): 177-189.
- Da Rosa GP, Costa F, Guimarães J, Friões F (2019) Seronegative neuromyelitis optica spectrum disorder: Severe polysymptomatic presentation with successful treatment response. BMJ Case Rep 12(3):e228553.
- Trebst C, Jarius S, Berthele A, Paul F, Schippling S, et al. (2014) Update on The Diagnosis and Treatment of Neuromyelitis Optica:Recommendations of The Neuromyelitis Optica Studi Group (NEMOS). J Neurol 261(1): 1-16.
- Waszczuk A, Wieteska A, Brodko K, Kopera O, Magdalena, et al. (2010) Devic's Disease-The Pathogenesis, Diagnosis, and Treatment. Wellness and Health Condition175-188.
- Bukhari W, Barnett MH, Prain K, Broadley SA (2012) Molecular Pathogenesis of Neuromyelitis Optica. Int J Mol Sci 13(10): 12970-12993.
- Sellner J, Bogglid M, Clanet M, Hintzen RQ, IllesZ, et al. (2010) ENFS Guidelines on Diagnosis and Management of Neuromyelitis Optica. Eur J Neurol 17(8): 1019-1032
- Saji E, Arakawa M, Yanagawa K, Toyoshima Y, Yokoseki A, et al. (2013) Cognitive Impairment and Cortisol Degeneration in Neuromyelitis Optica. Ann Neurol 73(1):65-76.
- Woolley J, Douglas VC, Cree BAC (2010) Neuromyelitis optica, psychiatric symptoms and primary polydipsia: a case report. General hospital psychiatry. 32(6): 648.e5-648.e8.
- Ghezzi A, Bergamaschi R, Martinelli V, Trojano M, Tola M, et al. (2004) Clinical characteristics, course and prognosis of relapsing Devic’s neuromyelitis optica. J Neurol 251(1): 47-52.
- Zaffaroni M (2004) Cerebrospinal fluid findings in Devic’s neuromyelitis optica. Neurol Sci 25 Suppl 4: 368-370.
- Kim W, Kim S, Huh S, Kim HJ (2012) Review Article: Brain Abnormalities In Neuromyelitis Optica Spectrum Disorder. Mult Scler Int 2012:735486
- Lepur D, Peterkovic V, Kalabric N (2009) Neuromyelitis optica with CSF examination mimicking bacterial meningomyelitis. Neurol Sci 30(1): 51-54
- Barnett Y, Sutton IJ, Ghadri M, Masters L, Zivadinov R, et al. (2014) Conventional and Advanced Imaging in Neuromyelitis Optica. AJNR Am J Neuroradiol 35(8): 1458-1466
- Indonesian Neuroscientist Association. Guidelines for the Diagnosis and Management of Multiple Sclerosis in Indonesia. University of Indonesia Faculty Publishing Agency. 2016:1-33.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Khedr D, Alkhashan S (2022) Seronegative Neuromyelitis Optica Specturum Disorder with CSF Finding Mimicking Bacterial Meningitis: Case Report. J Neuroinfect Dis 13: 382. DOI: 10.4172/2314-7326.1000382
Copyright: © 2022 Khedr D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2790
- [From(publication date): 0-2022 - Sep 28, 2025]
- Breakdown by view type
- HTML page views: 2229
- PDF downloads: 561