Signaling Pathways Supporting Tumor Invasion in Head and Neck Squamous Cell Carcinoma
Biology Medical Director, Morphologic and Molecular Core Research Laboratory, 500 University Drive, Hershey, Pennsylvania 17033, USA, Tel: 7175316725, Email: dbs18@psu.edu
Received: 25-Mar-2015 / Accepted Date: 18-May-2015 / Published Date: 21-May-2015 DOI: 10.4172/2161-0681.1000227
Abstract
Head and neck squamous cell carcinoma (HNSCC) is a highly invasive cancer. A number of signaling pathways like PI3K, Rho and TGFβ/SMAD drive the invasive nature of HNSCC. The PI3K pathway is the most altered pathway in HNSCC. Both upstream and downstream members of this pathway have been found to be mutated or overexpressed leading to an increase in cell invasion. The Rho pathway is also commonly activated; however, only overexpression or downregulation of Rho’s upstream regulators are found in HNSCC. Finally, TGFβ/SMAD pathway activation leads to epithelial mesenchymal transition in HNSCC and subsequently invasion, though loss of TGFβ/ SMAD signaling has also been shown to increase cell invasion.
Keywords: Invasion; HNSCC; PI3K; EGFR; Rho; SMAD; TGFβ
312678Abbreviations
HNSCC: Head and Neck Squamous Cell Carcinoma; PI3K: Phosphoinositide 3-Kinase; Rho: Ras Homologue; RTK: Receptor Tyrosine Kinase; EGFR: Epidermal Growth Factor Receptor; FGFR: Fibroblast Growth Factor Receptor; GTPase: small Guanosine Triphosphatase; HRAS: Harvey Rat Sarcoma Viral Oncogene Homolog; PIP2: Phosphatidylinositol 4,5-Bisphosphate; PIP3: Phosphatidylinositol (3,4,5)-Trisphosphate; PTEN: Phosphatase And Tensin Homolog; PDK-1: Phosphoinositide-Dependent Kinase-1; AKT: Protein Kinase B; TSC1: Tuberous Sclerosis 1; TSC2: Tuberous Sclerosis 2; mTORC1: Mechanistic Target Of Rapamycin Complex 1; mTORC2: Mechanistic Target Of Rapamycin Complex 2; EIF4E: Eukaryotic Translation Initiation Factor 4e; EGF: Epidermal Growth Factor; SphK1: Sphingosine Kinase-1; TGFα: Transforming Growth Factor Α; HER2: Human Epidermal Growth Factor Receptor 2; c-MET: Hepatocyte Growth Factor Receptor; MMP9: matrix metalloprotease 9; SGK-1: Serum/Glucocorticoid Regulated Kinase 1; GSK3β: Glycogen Synthase Kinase-3 Β; EMT: Epithelial-Mesenchymal Transition; ADAM12: Disintegrin And Metalloproteinase Domain-Containing Protein 12; EMPPRIN: Extracellular Matrix Metalloproteinase Inducer; COX2: Prostaglandin-Endoperoxide Synthase 2; PKC: Protein Kinase C Epsilon; p120ctn: p120-Catenin; S100A4: S100 Calcium Binding Protein A4; TGFβ: Transforming Growth Factor Β; ROCK1: Rho Associated Protein Kinase 1; ROCK2: Rho-Associated Protein Kinase 2; PI4P5K: Phosphatidylinositol-4-Phosphate 5-Kinase; DIAPH1: Protein Diaphanous Homolog 1; MLCK: Myosin Light Chain Kinase; Rac1: Ras-Related C3 Botulinum Toxin Substrate 1; PAK1: p21 Activated Kinase; Arp2/3: Actin-Related Proteins 2 and 3; FAK: Focal Adhesion Kinase 1; src: V-Src Avian Sarcoma (Schmidt-Ruppin A-2) Viral Oncogene Homolog; cdc42: Cell Division Control Protein 42 Homolog; CCR7: Chemokine Receptor Type 7; VEGF: Vascular Endothelial Growth Factor; LEF1: Lymphoid-Enhancer Binding Factor 1; TAK1: TGFβ-Activated Kinase 1; IL-8: Interleukin-8; IL-1: Interleukin-1; TNFα: Tumor Necrosis Factor α.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a term covering a group of cancers arising from the squamous epithelia in the head and neck region. The public health burden of HNSCC is profound, with ~300,000 people living with the disease in the United States. Approximately 40% of patients die within 5 years of diagnosis [1]. The treatment of HNSCC frequently entails large, disfiguring surgical excisions. Thus, of those who survive the disease, a large fraction has substantial functional and cosmetic morbidity. Precise characterization of the pathways involved in HNSCC invasion is very useful, as this understanding may allow for earlier diagnosis and better treatment.
Multiple risk factors have been found to contribute to the development of HNSCC. An estimated 73% of all HNSCCs are due to the combined effects of tobacco smoking and alcohol consumption [2]. The most prominent risk factor is tobacco smoking, which multiplies the risk of developing HNSCC by 6 to 13 times [3-5]. In addition to tobacco and alcohol, many other risk factors like viral infections (Epstein Barr Virus and Human Papillomavirus), radiation, chemical exposures due to occupational hazards and diet can contribute to the development of this deadly disease [6-10]. Some of these risk factors directly affect the signaling pathways that are involved in HNSCC invasion.
The majority of HNSCCs appear to have modifications in the same signaling pathways despite the variety of risk factors and anatomical locations of origin. The three pathways that appear to be most consistently modified are the Phosphoinositide 3-kinase (PI3K), Ras homologue (Rho) and TGFβ/SMAD pathways [11-13]. These pathways have been found to be responsible for neoplastic transformation. They have also been implicated in cancer invasion, a prerequisite to the development of metastatic disease [14,15]. Much work has focused on changes to the PI3K, Rho and TGFβ/SMAD pathways in HNSCC, but only a portion of it has focused on the invasive capacity of HNSCC.
PI3K Pathway
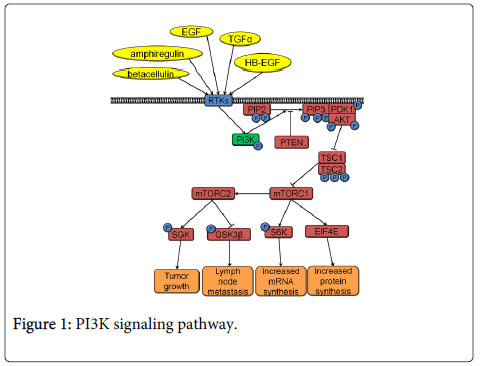
The PI3K pathway is the most commonly activated pathway in HNSCC [12]. PI3K signaling occurs downstream of the cell surface receptor tyrosine kinases (RTKs), such as epidermal growth factor receptor (EGFR) or fibroblast growth factor receptor (FGFR) (Figure 1). Small Guanosine Triphosphatase (GTPases) like Harvey rat sarcoma viral oncogene homolog (HRAS) can also activate the PI3K pathway. PI3K is a protein kinase that phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) into its active form, phosphatidylinositol (3,4,5) trisphosphate (PIP3). The opposite activity to PI3K is performed by phosphatase and tensin homolog (PTEN), which works by dephosphorylating PIP3 to deactivate it. Conversion of PIP2 to PIP3 leads to recruitment of phosphoinositide-dependent kinase-1 (PDK-1) and protein kinase B (AKT) to the plasma membrane. PDK-1 phosphorylates threonine 308 of AKT resulting in AKT activation. Activated AKT phosphorylates tuberous sclerosis 1 (TSC1) and tuberous sclerosis 2 (TSC2) resulting in the activation of the mechanistic target of rapamycin complex 1 (mTORC1) and mechanistic target of rapamycin complex 2 (mTORC2) that signal further downstream (Figure 1). mTORC1 signaling through eukaryotic translation initiation factor 4e (EIF4E) results in increased protein synthesis while mTORC1 signaling through S6K has been associated with increased mRNA synthesis, cap dependent translation and elongation, and translation of ribosomal proteins. mTORC2 signaling has been less studied than mTORC1 and so large gaps of knowledge still exist regarding its function. However, mTORC2 is thought to affect cell survival, proliferation and cytoskeletal organization [16]. Interestingly, PI3K pathway members are activated by HNSCC’s primary risk factor, tobacco smoking. Nicotine contained in tobacco smoke binds nicotinic acetylcholine receptors leading to activation of AKT and mTORC1 [17].
PI3K pathway activation leads to neoplastic transformation and tumor formation in many tissues. Thus far, research regarding tumor initiation in HNSCC is still limited. Increased PI3K pathway activity immortalizes oral keratinocytes by AKT-dependent phosphorylation and activation of hTERT [18]. A carcinogen-induced oral cancer model implicates the PI3K pathway in HNSCC tumor initiation. In this animal model, RTKs that signal through the PI3K pathway are frequently mutated and overexpressed in the developing tumors [19]. In tissues such as skin, mammary and lymphoid (T-cells), PTEN deletion triggers tumor development [20-22]. Moreover, AKT phosphorylation is known to be one of the first steps in chemical-induced carcinogenesis of epidermal keratinocytes [23]. The activated PI3K pathway promotes tumor initiation by increasing cell proliferation and inhibiting apoptosis. AKT phosphorylates CDKN1A (p21), which is then exported from the nucleus where it is unable to inhibit Cyclin/CDK complexes [24]. AKT also decreases the transcription of a cell proliferation inhibitor, CDKN1B (p27) [25]. Additionally, Cyclin D1 is up-regulated upon activation of the PI3K pathway [26]. Finally, activated AKT can phosphorylate BAD resulting in an inhibition of apoptosis [27]. Interestingly, data from cutaneous squamous cell carcinoma, indicates that PI3K signaling can phosphorylate and activate FOXO3 to regulate the expression of proteins involved in cell cycle and death such as ΔNp63, β-catenin, lymphoid-enhancer binding factor 1 (LEF1), C-MYC, and Cyclin D1 [28,29]. Breast cancer has a similar spectrum of PI3K pathway mutations as HNSCC. Mammary cells expressing mutant PIK3CA show increased growth and anchorage-independent survival due to increased NFκB activity [30,31]. Many of these pathway alterations leading to transformation still need to be investigated in HNSCC.
PI3K pathway activation plays a prominent role not only in HNSCC initiation, but also in HNSCC invasion. A number of RTKs and activation mechanisms lead to PI3K pathway activity and increased invasion in HNSCC. HNSCC tumors often secrete ligands, which lead to an autocrine activation of the PI3K pathway. Ninety one percent of HNSCC tumors have increased levels of transforming growth factor α (TGFα), an EGFR ligand [32,33]. In addition, treatment of HNSCC cell lines with EGFR ligands (e.g., epidermal growth factor (EGF), betacellulin, TGFα, heparin binding EGF and amphiregulin) increased cell invasion [34,35].
An additional mechanism of PI3K pathway activation is RTK overexpression. An overexpression of RTKs results in spontaneous receptor dimerization events. These consequently lead to ligand-independent receptor activation. Overexpression of RTKs like FGFR1, FGFR2, and FGFR3 on the HNSCC cell surface correlates with increased invasion and metastasis [36-38]. RTKs such as human epidermal growth factor receptor 2 (HER2) and hepatocyte growth factor receptor (c-MET), when overexpressed, increased cell invasion in vitro [39-41]. Besides RTK overexpression, PI3K pathway activation can also occur due to an RTK mutation. EGFRvIII, a constitutively active EGFR mutant, increased motility and cell invasion [42]. Finally, the PI3K signaling pathway can be activated due to the overexpression of sphingosine kinase 1 (SphK1). SphK1 transactivates EGFR and increases invasion [43].
PI3K pathway activation can occur due to activating mutations or overexpression of PI3K. The PI3K heterodimer is composed of an 85 kDa regulatory and a 110 kDa catalytic subunit. The PIK3CA gene codes for the 110 kDa subunit. This gene is overexpressed and mutated in 56% and 21% of HNSCC [12]. PIK3CA overexpression results in increased activation of the PI3K pathway and correlates with increased lymph node metastases in HNSCC [44]. Seventy three percent of all PIK3CA mutations are localized to three hotspots - residues 542, 545, and 1047 and cause constitutive activation of PI3K [12]. Mutations E542K and E545K are in the helical domain, while the H1047R mutation is in the kinase domain of the 110 kDa catalytic subunit. The gain-of-function helical domain mutations lead to PI3K activation by binding RAS and are not affected by the regulatory 85 kDa subunit. The mutations in the kinase domain activate PI3K independently of RAS via interaction with the regulatory 85 kDa subunit [45]. E542K, E545K, and H1047R mutants increase the invasive phenotype of HNSCC cell lines [46]. However, E545K and H1047R induced different rates of invasion in a breast cancer model; therefore, additional work is needed to characterize these mutants [47].
The PI3K pathway’s involvement in invasive properties of HNSCC comes from the pathway’s effects on proteins involved in basement membrane breakdown and actin mobilization. PI3K pathway activation can increase the expression of MMP 1, 2, 3, 9, 12 and ADAM12 by increasing the expression of prostaglandin-endoperoxide synthase 2 (COX2) and extracellular matrix metalloproteinase inducer (EMPPRIN) [39,41,48,49]. MMP9 is also induced due to mTORC1 activity through EIF4E [17,50]. MMP2 and MMP9 degrade collagen IV, the main component of the basement membrane, facilitating cell invasion [51]. Interestingly, ADAM12 cleaves stromal proteins akin to metalloproteases, but can also increase HER2 expression, further activating the PI3K pathway [39]. In addition, the activity of SGK-1 and glycogen synthase kinase-3β (GSK3β), proteins downstream of mTORC1, led to invasion or EMT in other cell types [52,53]. Further studies are required to evaluate the role of SGK-1 and GSK3β in HNSCC invasion.
PI3K pathway activity has been shown to result in activation or increased expression of proteins regulating cytoskeleton rearrangement like RhoC, Vav2, and fibronectin [48,54]. Increased activity of RhoC, Vav2 and fibronectin correlates with increased cell invasion [35,48,55]. Activated RhoC increased invasion through E-cadherin downregulation in HNSCC [55]. Vav2 functions are still largely unknown. Vav2 interacts with both PI3K and EGFR to become activated, and acts as a guanine nucleotide exchange factor to activate GTPases. Some of the Vav2 regulated GTPases belong to the Rho family [56,57]. Finally, work in HONE1 cells, an HNSCC cell line, indicates that PI3K signaling enhanced fibronectin expression which activated the cell division control protein 42 homolog (cdc42) and Rac1, increasing cell invasion [48].
Rho Pathway
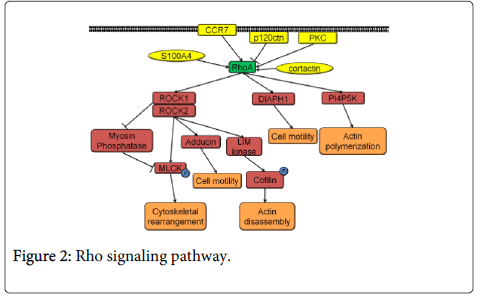
The Rho family of GTPases consists of signaling proteins that have been shown to regulate actin cytoskeleton rearrangement [58]. There are many members belonging to this protein family (e.g., RhoA, RhoC, ras-related c3 botulinum toxin substrate 1 (Rac1), cdc42). RhoA and RhoC are homologous proteins with differing affinity towards their effector proteins. The effects of RhoA and RhoC on invasion and migration differ in the cancer setting. RhoC activation always leads to increased migration and invasion, while RhoA activation appears to either increase or decrease invasion depending on the experimental system [55,59,60].
RhoA pathway activation occurs as a result of a guanosine diphosphate being replaced by a guanosine triphosphate in the RhoA by a guanine nucleotide exchange factor. The signal propagates through its effectors Rho associated protein kinase 1 (ROCK1), Rho-associated protein kinase 2 (ROCK2), phosphatidylinositol-4-phosphate 5-kinase (PI4P5K), and protein diaphanous homolog 1 (DIAPH1) (Figure 2). ROCK phosphorylates myosin light chain kinase (MLCK) and inhibits myosin phosphatase that de-phosphorylates MLCK. MLCK is responsible for cytoskeletal rearrangement. ROCK also activates adducin which binds F-actin filaments, leading to an increase in cell motility. LIM kinase, another target of ROCK, phosphorylates cofilin leading to actin filament cleavage which can contribute to increased cell motility. RhoA signaling via PI4P5K and DIAPH1 increases actin polymerization and cell motility (Figure 2) [61].
There are multiple regulators of RhoA such as chemokine receptor type 7 (CCR7), cortactin, S100 calcium binding protein A4 (S100A4), protein kinase C epsilon (PKC), and p120ctn. Expression of CCR7, cortactin, and S100A4 is increased in HNSCC. Direct RhoA activation by CCR7 or cortactin, indirect RhoA activation by S100A4 via rhotekin, results in increased cell invasion [62-66]. PKC, also overexpressed in HNSCC, can activate RhoC in addition to RhoA. Even when PKC is inhibited, either RhoA or RhoC activity alone is enough to rescue the increase in motility [67]. Another very likely candidate controlling RhoA activity is p120ctn. This protein is frequently downregulated or lost in HNSCC [68]. In addition, p120ctn downregulation or loss in HNSCC has been correlated with poor prognosis [68]. p120ctn normally suppresses RhoA activity, and upon p120ctn loss, RhoA activity increases [69]. The effects of p120ctn are even more significant considering that tobacco smoking and alcohol consumption lead to a decrease in p120ctn expression [70-72]. Further studies are needed to explore the role of p120ctn in HNSCC since p120ctn deregulation can either decrease or increase the invasive capabilities of cells in vitro depending on the cell type [73,74].
Multiple factors lying downstream of RhoA have been shown to be activated in HNSCC and their activation has been linked to the process of invasion. p21 activated kinase (PAK1) is activated in HNSCC. PAK1 phosphorylates actin-related proteins 2 and 3 (Arp2/3) leading to actin mobilization [75]. FAK is overexpressed in HNSCC, though its expression has been shown not to correlate with increased gene copy number found in tumors [76]. RhoA signaling activates FAK, which colocalizes at the focal adhesions and promotes invasion by increasing MMP2 production in HNSCC cell lines [77]. Avian sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (src) and fascin, proteins directly controlling actin localization and invadopodia formation, are also activated in HNSCC downstream of Rho. Src and fascin activity was correlated with HNSCC invasion and lymphatic metastasis, respectively [78,79].
TGFβ/SMAD Pathway
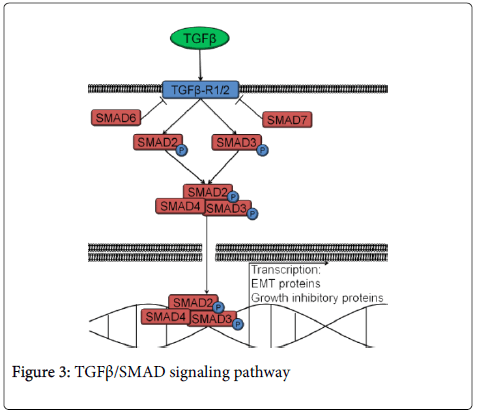
TGFβ/SMAD signaling can either suppress or support cancer progression [80, 81]. However, the exact role it plays in HNSCC is yet to be determined. The pathway is activated by transforming growth factor β (TGFβ) binding its cell surface receptor and phosphorylating either SMAD2 or SMAD3, which form a heterotrimeric complex with SMAD4 (Figure 3). The complex translocates to the nucleus where it leads to the transcription of target genes. These genes result in increased invasion (e.g., MMP2, MMP9, SNAIL) or growth suppression (e.g., p21, p12CDK2-AP1, p15-INK4B) [82-87]. SMAD6 and SMAD7 are inhibitory SMADs that prevent SMAD2/3 from getting phosphorylated (Figure 3).
The effects of TGFβ/SMAD signaling in HNSCC are still a matter of debate. TGFβ/SMAD pathway activation results in EMT, a process commonly associated with invasion and metastasis. TGFβ expression is increased in tumor samples from HNSCC patients [88]. In addition, work in HNSCC cell lines revealed that activation of the TGFβ/SMAD pathway by TGFβ drives increased cell motility. TGFβ treatment of HNSCC cell lines also results in increased expression of EMT proteins such as SLUG, leading to increased invasion [87,89]. Additionally, TGFβ treatment increases MMPs and extracellular matrix protein expression in an HNSCC cell line, SCC9 [90]. TGFβ/SMAD signaling activation in HNSCC increased cellular motility, expression of EMT proteins such as SNAIL and increased MMPs, leading to increased invasion [91-93]. Moreover, TGFβ-activated inhibitory proteins such as p21, p12CDK2-AP1 and p15-INK4B are often mutated in HNSCC, which allows increased cell invasion due to TGFβ/SMAD signaling without undergoing cell cycle arrest [82-86].
In addition to the autocrine effects TGFβ has on tumors, TGFβ also supports tumor invasion by its effects on the tumor microenvironment. Immune cells, such as macrophages, are recruited to the tumor site by TGFβ secreted by tumor cells. In the tumor stroma, the macrophages undergo activation and secrete Vascular Endothelial Growth Factor (VEGF) and Interleukin-8 (IL-8) leading to neo-vascularization. Macrophages also secrete Tumor Necrosis Factor α (TNFα) and Interleukin-1 (IL-1) which stimulate the tumor cells themselves to also secrete VEGF and IL-8 further potentiating neo-vascularization [94]. Increased levels of VEGF in HNSCC have been correlated with increased metastasis [95]. In addition, stromal fibroblasts also promote HNSCC invasion. Tumor-secreted TGFβ induces fibroblasts to secrete Hepatocyte Growth Factor, which can bind c-Met in tumor cells and increase their invasive capabilities [96]. Moreover, HNSCC-produced reactive oxygen species induce senescence in the stromal fibroblasts. These senescent fibroblasts secrete an increased amount of TGFβ thus promoting keratinocyte invasion [97].
While the activation of the TGFβ/SMAD signaling pathway can increase invasion in HNSCC, inhibition of TGFβ/SMAD signaling in HNSCC has also been shown to increase cell invasion. Multiple reports indicate that decreased levels of TGFβ1 and TGFβ2 receptors in HNSCC are the result of mutations or promoter hypermethylation [98-101]. TGFβ/SMAD signaling is disrupted in close to 50% of human HNSCCs. Mutations in TGFβ/SMAD pathway have been associated with a poor prognosis in HNSCC [102]. TGFβ1 receptor loss with concomitant loss of PTEN resulted in invasive HNSCC in mice [13]. Also, when SMAD4 is deleted in mice it leads to HNSCC development with evidence of cellular invasion [103]. In addition, SMAD2 and SMAD4 are frequently mutated in HNSCC cell lines due to nonsense and missense mutations [104]. Deletion of SMAD4, which leads to inhibition of TGFβ/SMAD signaling, increased cell invasion in the HNSCC cell line FaDu-Hyg-R [105]. It is still unclear what mechanisms are responsible for increased invasion in HNSCC due to TGFβ/SMAD signaling pathway inhibition. Work in esophageal keratinocytes, a cell type closely related to squamous epithelium of the head and neck region, implicates the secretion of proteases and change in integrin expression as the way in which TGFβ/SMAD signaling pathway inhibition leads to invasion [106]. Alternatively, SMAD4 loss in colorectal carcinoma leads to invasion by activating the Rho pathway [107]. Further investigation is necessary to identify the exact mechanism used to drive cell invasion in HNSCC due to TGFβ/SMAD signaling pathway inhibition.
Pathway Crosstalk
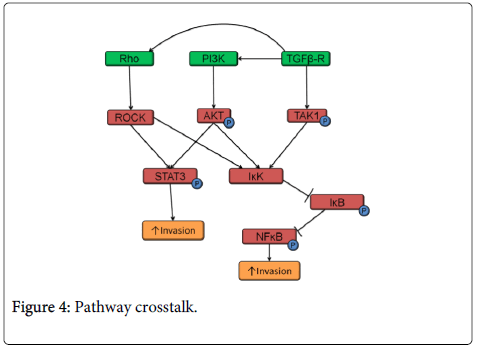
As with most signaling pathways, the PI3K, TGFβ and Rho pathways have common protein targets and therefore crosstalk is common among these three pathways in HNSCC (Figure 4). PI3K-AKT and Rho-ROCK signaling both phosphorylate STAT3 and increase HNSCC invasion [42,108,109]. All three pathways: Rho, PI3K and TGFβ lead to NFκB activation. IκK can be activated by ROCK (a Rho target), TGFβ-activated kinase 1 (TAK1), and AKT (a PI3K target).
In turn, IκK phosphorylates IκB (which undergoes proteosomal degradation) and NFκB resulting in its activation and translocation to the nucleus [110-113]. Activated NFKB in HNSCC correlates with increased invasion [114]. In addition, TGFβ/SMAD signaling can also activate Rho and PI3K signaling pathways resulting in EMT (Figure 4) [115,116]. In TGFβ1 receptor knockout mice, PI3K pathway activation was critical for tumor development [117]. Loss of TGFβ signaling led to the development of invasive HNSCC only when coupled with increased PI3K pathway signaling [13].
Summary
In conclusion, the PI3K pathway becomes activated in HNSCC, leading to invasion due to multiple members of the pathway being mutated or overexpressed. Even though the PI3K pathway has been studied intensely in the last few decades, it is still unclear which members of the pathway are essential to the process of invasion. The Rho pathway, which directly deals with cytoskeletal rearrangement, is also commonly activated in HNSCC. Upon activation, this pathway leads to increased invasion in HNSCC. Moreover, HNSCC risk factors like tobacco smoking and alcohol consumption activate Rho signaling. Additionally, it is not clear whether activated or repressed TGFβ/SMAD signaling leads to increased invasion in HNSCC. It is possible that there are two subtypes of HNSCC, one with increased and one with decreased TGFβ/SMAD signaling. Further studies are required to validate the role of TGFβ/SMAD signaling in HNSCC. Finally, the complexity is also increased by the cross-activation between the PI3K, Rho and TGFβ/SMAD signaling pathways. It is possible that each of these pathways further increases HNSCC invasion through their activation of each other.
References
- Day GL, Blot WJ, Austin DF, Bernstein L, Greenberg RS, et al. (1993) Racial differences in risk of oral and pharyngeal cancer: alcohol, tobacco, and other determinants. J Natl Cancer Inst 85: 465-473.
- Andre K, Schraub S, Mercier M, Bontemps P (1995) Role of alcohol and tobacco in the aetiology of head and neck cancer: a case-control study in the Doubs region of France. Eur J Cancer B Oral Oncol 31B: 301-309.
- Lewin F, Norell SE, Johansson H, Gustavsson P, Wennerberg J, et al. (1998) Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck: a population-based case-referent study in Sweden. Cancer 82: 1367-1375.
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, et al. (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363: 24-35.
- Saku T, Hayashi Y, Takahara O, Matsuura H, Tokunaga M, et al. (1997) Salivary gland tumors among atomic bomb survivors, 1950-1987. Cancer 79: 1465-1475.
- Luce D, Leclerc A, Bégin D, Demers PA, Gérin M, et al. (2002) Sinonasal cancer and occupational exposures: a pooled analysis of 12 case-control studies. ancer Causes Control 13: 147-157.
- Chien YC, Chen JY, Liu MY, Yang HI, Hsu MM, et al. (2001) Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med 345: 1877-1882.
- Maden C, Beckmann AM, Thomas DB, McKnight B, Sherman KJ, et al. (1992) Human papillomaviruses, herpes simplex viruses, and the risk of oral cancer in men. Am J Epidemiol 135: 1093-1102.
- Bravi F, Bosetti C, Filomeno M, Levi F, Garavello W, et al. (2013) Foods, nutrients and the risk of oral and pharyngeal cancer. Br J Cancer 109: 2904-2910.
- Abraham MT, Kuriakose MA, Sacks PG, Yee H, Chiriboga L, et al. (2001) Motility-related proteins as markers for head and neck squamous cell cancer. Laryngoscope 111: 1285-1289.
- Cancer Genome Atlas Network (2015) Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517: 576-582.
- Bian Y, Hall B, Sun ZJ, Molinolo A, Chen W, et al. (2012) Loss of TGF-β signaling and PTEN promotes head and neck squamous cell carcinoma through cellular senescence evasion and cancer-related inflammation. Oncogene 31: 3322-3332.
- Xiaoming Li BD, Qi Song, YupengShen. (2012) Metastasis of Head and Neck Squamous Cell Carcinoma. INTECH Open Access Publisher.
- Myers JN, Greenberg JS, Mo V, Roberts D (2001) Extracapsular spread. A significant predictor of treatment failure in patients with squamous cell carcinoma of the tongue. Cancer 92: 3030-3036.
- Laplante M, Sabatini DM (2009) mTOR signaling at a glance. J Cell Sci 122: 3589-3594.
- Clark CA, McEachern MD, Shah SH, Rong Y, Rong X, et al. (2010) Curcumin inhibits carcinogen and nicotine-induced Mammalian target of rapamycin pathway activation in head and neck squamous cell carcinoma. Cancer Prev Res (Phila) 3: 1586-1595.
- Heeg S, Hirt N, Queisser A, Schmieg H, Thaler M et al. (2011) EGFR overexpression induces activation of telomerase via PI3K/AKT-mediated phosphorylation and transcriptional regulation through Hif1-alpha in a cellular model of oral-esophageal carcinogenesis. Cancer science 102: 351-360.
- Vairaktaris E, Spyridonidou S, Papakosta V, Vylliotis A, Lazaris A, et al. (2008) The hamster model of sequential oral oncogenesis. Oral Oncol 44: 315-324.
- Burns JM Jr, Shreffler WG, Rosman DE, Sleath PR, March CJ, et al. (1992) Identification and synthesis of a major conserved antigenic epitope of Trypanosomacruzi. ProcNatlAcadSci U S A 89: 1239-1243.
- Li G, Robinson GW, Lesche R, Martinez-Diaz H, Jiang Z, et al. (2002) Conditional loss of PTEN leads to precocious development and neoplasia in the mammary gland. Development 129: 4159-4170.
- Suzuki A, Sasaki T, Mak TW, Nakano T (2004) Functional analysis of the tumour suppressor gene PTEN in murine B cells and keratinocytes. BiochemSoc Trans 32: 362-365.
- Segrelles C, Ruiz S, Perez P, Murga C, Santos M, et al. (2002) Functional roles of Akt signaling in mouse skin tumorigenesis. Oncogene 21: 53-64.
- Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, et al. (2001) Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol 3: 245-252.
- Gesbert F, Sellers WR, Signoretti S, Loda M, Griffin JD (2000) BCR/ABL regulates expression of the cyclin-dependent kinase inhibitor p27Kip1 through the phosphatidylinositol 3-Kinase/AKT pathway. J BiolChem 275: 39223-39230.
- Takuwa N, Fukui Y, Takuwa Y (1999) Cyclin D1 expression mediated by phosphatidylinositol 3-kinase through mTOR-p70(S6K)-independent signaling in growth factor-stimulated NIH 3T3 fibroblasts. Mol Cell Biol 19: 1346-1358.
- Downward J (1999) How BAD phosphorylation is good for survival. Nat Cell Biol 1: E33-35.
- Segrelles C, Moral M, Lara MF, Ruiz S, Santos M, et al. (2006) Molecular determinants of Akt-induced keratinocyte transformation. Oncogene 25: 1174-1185.
- Burgering BM, Kops GJ (2002) Cell cycle and death control: long live Forkheads. Trends BiochemSci 27: 352-360.
- Isakoff SJ, Engelman JA, Irie HY, Luo J, Brachmann SM, et al. (2005) Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res 65: 10992-11000.
- Hutti JE, Pfefferle AD, Russell SC, Sircar M, Perou CM et al. (2012) Oncogenic PI3K mutations lead to NF-kappaB-dependent cytokine expression following growth factor deprivation. Cancer research 72: 3260-3269.
- Rubin Grandis J, Melhem MF, Gooding WE, Day R, Holst VA, et al. (1998) Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst 90: 824-832.
- Grandis JR, Tweardy DJ (1993) Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res 53: 3579-3584.
- O-charoenrat P, Modjtahedi H, Rhys-Evans P, Court WJ, Box GM, et al. (2000) Epidermal growth factor-like ligands differentially up-regulate matrix metalloproteinase 9 in head and neck squamous carcinoma cells. Cancer Res 60: 1121-1128.
- Patel V, Rosenfeldt HM, Lyons R, Servitja JM, Bustelo XR, et al. (2007) Persistent activation of Rac1 in squamous carcinomas of the head and neck: evidence for an EGFR/Vav2 signaling axis involved in cell invasion. Carcinogenesis 28: 1145-1152.
- Nguyen PT, Tsunematsu T, Yanagisawa S, Kudo Y, Miyauchi M, et al. (2013) The FGFR1 inhibitor PD173074 induces mesenchymal-epithelial transition through the transcription factor AP-1. Br J Cancer 109: 2248-2258.
- Hase T1, Kawashiri S, Tanaka A, Nozaki S, Noguchi N, et al. (2006) Correlation of basic fibroblast growth factor expression with the invasion and the prognosis of oral squamous cell carcinoma. J Oral Pathol Med 35: 136-139.
- Vairaktaris E, Ragos V, Yapijakis C, Derka S, Vassiliou S, et al. (2006) FGFR-2 and -3 play an important role in initial stages of oral oncogenesis. Anticancer Res 26: 4217-4221.
- Rao VH, Kandel A, Lynch D, Pena Z, Marwaha N, et al. (2012) A positive feedback loop between HER2 and ADAM12 in human head and neck cancer cells increases migration and invasion. Oncogene 31: 2888-2898.
- Zhang Y, Zhao J, Zhang Q (2014) [The expression and clinical significance of OPN and C-met in laryngeal carcinoma]. Lin Chung Er Bi Yan HouTou Jing WaiKeZaZhi 28: 256-258.
- Lim YC, Han JH, Kang HJ, Kim YS, Lee BH, et al. (2012) Overexpression of c-Met promotes invasion and metastasis of small oral tongue carcinoma. Oral Oncol 48: 1114-1119.
- Wheeler SE, Suzuki S, Thomas SM, Sen M, Leeman-Neill RJ, et al. (2010) Epidermal growth factor receptor variant III mediates head and neck cancer cell invasion via STAT3 activation. Oncogene 29: 5135-5145.
- Tamashiro PM, Furuya H2, Shimizu Y3, Kawamori T4 (2014) Sphingosine kinase 1 mediates head & neck squamous cell carcinoma invasion through sphingosine 1-phosphate receptor 1. Cancer Cell Int 14: 76.
- Fenic I, Steger K, Gruber C, Arens C, Woenckhaus J (2007) Analysis of PIK3CA and Akt/protein kinase B in head and neck squamous cell carcinoma. Oncol Rep 18: 253-259.
- Zhao L, Vogt PK (2008) Helical domain and kinase domain mutations in p110alpha of phosphatidylinositol 3-kinase induce gain of function by different mechanisms. ProcNatlAcadSci U S A 105: 2652-2657.
- Murugan AK, Hong NT, Fukui Y, Munirajan AK, Tsuchida N (2008) Oncogenic mutations of the PIK3CA gene in head and neck squamous cell carcinomas. Int J Oncol 32: 101-111.
- Meyer DS, Koren S, Leroy C, Brinkhaus H, Müller U, et al. (2013) Expression of PIK3CA mutant E545K in the mammary gland induces heterogeneous tumors but is less potent than mutant H1047R. Oncogenesis 2: e74.
- Hsu JY, Chang KY, Chen SH, Lee CT, Chang ST et al. (2015) Epidermal growth factor-induced cyclooxygenase-2 enhances head and neck squamous cell carcinoma metastasis through fibronectin up-regulation. Oncotarget 6: 1723-1739.
- Suzuki S, Ishikawa K1 (2014) Combined inhibition of EMMPRIN and epidermal growth factor receptor prevents the growth and migration of head and neck squamous cell carcinoma cells. Int J Oncol 44: 912-917.
- Das G1, Shiras A, Shanmuganandam K, Shastry P (2011) Rictor regulates MMP-9 activity and invasion through Raf-1-MEK-ERK signaling pathway in glioma cells. MolCarcinog 50: 412-423.
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, et al. (1993) Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 4: 197-250.
- Schmidt EM, Gu S, Anagnostopoulou V, Alevizopoulos K, Föller M, et al. (2012) Serum- and glucocorticoid-dependent kinase-1-induced cell migration is dependent on vinculin and regulated by the membrane androgen receptor. FEBS J 279: 1231-1242.
- Zhou BP, Deng J, Xia W, Xu J, Li YM, et al. (2004) Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 6: 931-940.
- Hornstein I, Alcover A, Katzav S (2004) Vav proteins, masters of the world of cytoskeleton organization. Cell Signal 16: 1-11.
- Tumur Z, Katebzadeh S1, Guerra C1, Bhushan L2, Alkam T2, et al. (2015) RhoC mediates epidermal growth factor-stimulated migration and invasion in head and neck squamous cell carcinoma. Neoplasia 17: 141-151.
- Tamás P, Solti Z, Bauer P, Illés A, Sipeki S, et al. (2003) Mechanism of epidermal growth factor regulation of Vav2, a guanine nucleotide exchange factor for Rac. J BiolChem 278: 5163-5171.
- Yang HW, Shin MG, Lee S, Kim JR, Park WS, et al. (2012) Cooperative activation of PI3K by Ras and Rho family small GTPases.Mol Cell 47: 281-290.
- Sadok A, Marshall CJ1 (2014) Rho GTPases: masters of cell migration. Small GTPases 5: e29710.
- Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM (2004) p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev 18: 862-876.
- Worthylake RA, Burridge K (2003) RhoA and ROCK promote migration by limiting membrane protrusions. J BiolChem 278: 13578-13584.
- O'Connor K, Chen M (2013) Dynamic functions of RhoA in tumor cell migration and invasion. Small GTPases 4: 141-147.
- Oliveira MV, Fraga CA, Barros LO, Pereira CS, Santos SH, et al. (2014) High expression of S100A4 and endoglin is associated with metastatic disease in head and neck squamous cell carcinoma. ClinExp Metastasis 31: 639-649.
- Chen M, Bresnick AR, O'Connor KL (2013) Coupling S100A4 to Rhotekin alters Rho signaling output in breast cancer cells. Oncogene 32: 3754-3764.
- Xu Z, Zheng X, Yang L, Liu F, Zhang E, et al. (2015) Chemokine receptor 7 promotes tumor migration and invasiveness via the RhoA/ROCK pathway in metastatic squamous cell carcinoma of the head and neck. Oncol Rep 33: 849-855.
- Rothschild BL, Shim AH, Ammer AG, Kelley LC, Irby KB et al. (2006) Cortactin overexpression regulates actin-related protein 2/3 complex activity, motility, and invasion in carcinomas with chromosome 11q13 amplification. Cancer Res 66: 8017-8025.
- Croucher DR, Rickwood D, Tactacan CM, Musgrove EA, Daly RJ (2010) Cortactin modulates RhoA activation and expression of Cip/Kip cyclin-dependent kinase inhibitors to promote cell cycle progression in 11q13-amplified head and neck squamous cell carcinoma cells. Mol Cell Biol 30: 5057-5070.
- Pan Q, Bao LW, Teknos TN, Merajver SD (2006) Targeted disruption of protein kinase C epsilon reduces cell invasion and motility through inactivation of RhoA and RhoCGTPases in head and neck squamous cell carcinoma. Cancer Res 66: 9379-9384.
- Lo Muzio L, Pannone G, Santarelli A, Bambini F, Mascitti M, et al. (2013) Is expression of p120ctn in oral squamous cell carcinomas a prognostic factor? Oral Surg Oral Med Oral Pathol Oral Radiol 115: 789-798.
- Epifano C, Megias D, Perez-Moreno M (2014) p120-catenin differentially regulates cell migration by Rho-dependent intracellular and secreted signals. EMBO Rep 15: 592-600.
- Iwata T, Nozu F, Imawari M (2011) Ethanol impairs the assembly and disassembly of actin cytoskeleton and cell adhesion via the RhoA signaling pathway, catenin p120 and E-cadherin in CCK-stimulated pancreatic acini. BiochemBiophys Res Commun 405: 558-563.
- Zhang L, Gallup M, Zlock L, Chen YT, Finkbeiner WE, et al. (2014) Pivotal role of MUC1 glycosylation by cigarette smoke in modulating disruption of airway adherens junctions in vitro. J Pathol 234: 60-73.
- Krais AM, Hautefeuille AH, Cros MP, Krutovskikh V, Tournier JM et al. (2011) CHRNA5 as negative regulator of nicotine signaling in normal and cancer bronchial cells: effects on motility, migration and p63 expression. Carcinogenesis 32: 1388-1395.
- Cheng Z, Assfag V, Shi X, Lin S, Xia J, et al. (2012) Effect of p120 catenin silencing on biological behaviors of PANC-1 cells. J HuazhongUnivSciTechnolog Med Sci 32: 707-712.
- Schackmann RC, Klarenbeek S, Vlug EJ, Stelloo S, van Amersfoort M et al. (2013) Loss of p120-catenin induces metastatic progression of breast cancer by inducing anoikis resistance and augmenting growth factor receptor signaling. Cancer Res 73: 4937-4949.
- Vadlamudi RK, Li F, Barnes CJ, Bagheri-Yarmand R, Kumar R (2004) p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep 5: 154-160.
- Canel M, Secades P, Rodrigo JP, Cabanillas R, Herrero A, et al. (2006) Overexpression of focal adhesion kinase in head and neck squamous cell carcinoma is independent of fak gene copy number. Clin Cancer Res 12: 3272-3279.
- Canel M, Secades P, Garzón-Arango M, Allonca E, Suarez C, et al. (2008) Involvement of focal adhesion kinase in cellular invasion of head and neck squamous cell carcinomas via regulation of MMP-2 expression. Br J Cancer 98: 1274-1284.
- Koppikar P, Choi SH, Egloff AM, Cai Q, Suzuki S, et al. (2008) Combined inhibition of c-Src and epidermal growth factor receptor abrogates growth and invasion of head and neck squamous cell carcinoma. Clin Cancer Res 14: 4284-4291.
- Papaspyrou K, Brochhausen C, Schmidtmann I3, Fruth K, Gouveris H, et al. (2014) Fascinupregulation in primary head and neck squamous cell carcinoma is associated with lymphatic metastasis. OncolLett 7: 2041-2046.
- Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S (2012) TGF-β - an excellent servant but a bad master. J Transl Med 10: 183.
- Derynck R, Akhurst RJ, Balmain A (2001) TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 29: 117-129.
- Peng H, Shintani S, Kim Y, Wong DT (2006) Loss of p12CDK2-AP1 expression in human oral squamous cell carcinoma with disrupted transforming growth factor-beta-Smad signaling pathway. Neoplasia 8: 1028-1036.
- Coffey RJ Jr, Bascom CC, Sipes NJ, Graves-Deal R, Weissman BE, et al. (1988) Selective inhibition of growth-related gene expression in murine keratinocytes by transforming growth factor beta. Mol Cell Biol 8: 3088-3093.
- Hannon GJ, Beach D (1994) p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature 371: 257-261.
- Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, et al. (1995) Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. ProcNatlAcadSci U S A 92: 5545-5549.
- Laiho M, DeCaprio JA, Ludlow JW, Livingston DM, Massagué J (1990) Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell 62: 175-185.
- Takayama S, Hatori M, Kurihara Y, Kinugasa Y, Shirota T, et al. (2009) Inhibition of TGF-beta1 suppresses motility and invasiveness of oral squamous cell carcinoma cell lines via modulation of integrins and down-regulation of matrix-metalloproteinases. Oncol Rep 21: 205-210.
- Pasini FS, Brentani MM, Kowalski LP, Federico MH. (2001) Transforming growth factor beta1, urokinase-type plasminogen activator and plasminogen activator inhibitor-1 mRNA expression in head and neck squamous carcinoma and normal adjacent mucosa. Head Neck 23: 725-732.
- Joseph MJ, Dangi-Garimella S, Shields MA, Diamond ME, Sun L, et al. (2009) Slug is a downstream mediator of transforming growth factor-beta1-induced matrix metalloproteinase-9 expression and invasion of oral cancer cells. J Cell Biochem 108: 726-736.
- Dang D, Yang Y, Li X, Atakilit A, Regezi J, et al. (2004) Matrix metalloproteinases and TGFbeta1 modulate oral tumor cell matrix. BiochemBiophys Res Commun 316: 937-942.
- Sinpitaksakul SN, Pimkhaokham A, Sanchavanakit N, Pavasant P (2008) TGF-beta1 induced MMP-9 expression in HNSCC cell lines via Smad/MLCK pathway. BiochemBiophys Res Commun 371: 713-718.
- Sun L, Diamond ME, Ottaviano AJ, Joseph MJ, Ananthanarayan V, et al. (2008) Transforming growth factor-beta 1 promotes matrix metalloproteinase-9-mediated oral cancer invasion through snail expression. Mol Cancer Res 6: 10-20.
- Leivonen SK, Ala-Aho R, Koli K, Grénman R, Peltonen J, et al. (2006) Activation of Smad signaling enhances collagenase-3 (MMP-13) expression and invasion of head and neck squamous carcinoma cells. Oncogene 25: 2588-2600.
- Liss C, Fekete MJ, Hasina R, Lam CD, Lingen MW (2001) Paracrine angiogenic loop between head-and-neck squamous-cell carcinomas and macrophages. Int J Cancer 93: 781-785.
- Sedivy R, Beck-Mannagetta J, Haverkampf C, Battistutti W, Honigschnabl S. (2003) Expression of vascular endothelial growth factor-C correlates with the lymphatic microvessel density and the nodal status in oral squamous cell cancer. J Oral Pathol Med 32: 455-460.
- Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, et al. (2004) Tumour-derived TGF-beta1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer 90: 822-832.
- Hassona Y, Cirillo N, Lim KP, Herman A, Mellone M, et al. (2013) Progression of genotype-specific oral cancer leads to senescence of cancer-associated fibroblasts and is mediated by oxidative stress and TGF-β. Carcinogenesis 34: 1286-1295.
- Xie W, Bharathy S, Kim D, Haffty BG, Rimm DL, et al. (2003) Frequent alterations of Smad signaling in human head and neck squamous cell carcinomas: a tissue microarray analysis. Oncol Res 14: 61-73.
- Wang D, Song H, Evans JA, Lang JC, Schuller DE, et al. (1997) Mutation and downregulation of the transforming growth factor beta type II receptor gene in primary squamous cell carcinomas of the head and neck. Carcinogenesis 18: 2285-2290.
- Huntley SP, Davies M, Matthews JB, Thomas G, Marshall J et al. (2004) Attenuated type II TGF-beta receptor signalling in human malignant oral keratinocytes induces a less differentiated and more aggressive phenotype that is associated with metastatic dissemination. Int J Cancer 110: 170-176
- Munoz-Antonia T, Torrellas-Ruiz M, Clavell J, Mathews LA, Muro-Cacho CA et al. (2009) Aberrant methylation inactivates transforming growth factor Beta receptor I in head and neck squamous cell carcinoma. Int J Otolaryngol 2009: 848695
- Xie W, Aisner S, Baredes S, Sreepada G, Shah R, et al. (2013) Alterations of Smad expression and activation in defining 2 subtypes of human head and neck squamous cell carcinoma. Head Neck 35: 76-85.
- Bornstein S, White R, Malkoski S, Oka M, Han G, et al. (2009) Smad4 loss in mice causes spontaneous head and neck cancer with increased genomic instability and inflammation. J Clin Invest 119: 3408-3419.
- Qiu W, Schönleben F, Li X, Su GH (2007) Disruption of transforming growth factor beta-Smad signaling pathway in head and neck squamous cell carcinoma as evidenced by mutations of SMAD2 and SMAD4. Cancer Lett 245: 163-170.
- Reiss M, Santoro V, de Jonge RR, Vellucci VF (1997) Transfer of chromosome 18 into human head and neck squamous carcinoma cells: evidence for tumor suppression by Smad4/DPC4. Cell Growth Differ 8: 407-415.
- Le Bras GF, Taylor C, Koumangoye RB, Revetta F, Loomans HA, et al. (2015) TGFβ loss activates ADAMTS-1-mediated EGF-dependent invasion in a model of esophageal cell invasion. Exp Cell Res 330: 29-42.
- Voorneveld PW, Kodach LL, Jacobs RJ, Liv N, Zonnevylle AC, et al. (2014) Loss of SMAD4 alters BMP signaling to promote colorectal cancer cell metastasis via activation of Rho and ROCK. Gastroenterology 147: 196-208.
- Li L, Xu M, Li X, Lv C, Zhang X et al. (2015) Platelet-derived growth factor-B (PDGF-B) induced by hypoxia promotes the survival of pulmonary arterial endothelial cells through the PI3K/Akt/Stat3 pathway. Cell PhysiolBiochem 35: 441-451.
- Debidda M, Wang L, Zang H, Poli V, Zheng Y (2005) A role of STAT3 in Rho GTPase-regulated cell migration and proliferation. J BiolChem 280: 17275-17285.
- Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, et al. (2006) p120-catenin mediates inflammatory responses in the skin. Cell 124: 631-644.
- Perona R, Montaner S, Saniger L, Sanchez-Perez I, Bravo R et al. (1997) Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 11: 463-475.
- Bai D, Ueno L, Vogt PK (2009) Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int J Cancer 125: 2863-2870.
- Freudlsperger C, Bian Y, Contag Wise S, Burnett J, Coupar J, et al. (2013) TGF-β and NF-κB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene 32: 1549-1559.
- Zhang W, Liu Y, Wang CW (2014) S100A4 promotes squamous cell laryngeal cancer Hep-2 cell invasion via NF-kB/MMP-9 signal. Eur Rev Med PharmacolSci 18: 1361-1367.
- Zhang K, Zhang H, Xiang H, Liu J, Liu Y, et al. (2013) TGF-β1 induces the dissolution of tight junctions in human renal proximal tubular cells: role of the RhoA/ROCK signaling pathway. Int J Mol Med 32: 464-468.
- Zhang HY, Wang ZQ, Li YY, Wang F, Zeng QR et al. (2014) Transforming growth factor-beta1-induced epithelial-mesenchymal transition in human esophageal squamous cell carcinoma via the PTEN/PI3K signaling pathway. Oncol Rep 32: 2134-2142.
- Bian Y1, Terse A, Du J, Hall B, Molinolo A, et al. (2009) Progressive tumor formation in mice with conditional deletion of TGF-beta signaling in head and neck epithelia is associated with activation of the PI3K/Akt pathway. Cancer Res 69: 5918-5926.
Citation: Kidacki M, Lehman HL, Warrick JI, Stairs DB (2015) Signaling Pathways Supporting Tumor Invasion in Head and Neck Squamous Cell Carcinoma. J Clin Exp Pathol 5:227. DOI: 10.4172/2161-0681.1000227
Copyright: ©2015 Kidacki M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 21191
- [From(publication date): 6-2015 - Aug 29, 2025]
- Breakdown by view type
- HTML page views: 16269
- PDF downloads: 4922