Sphingosine 1 Phosphate in Cell Signaling with Emphasis in Protozoan Infections
Received: 24-Feb-2015 / Accepted Date: 03-Apr-2015 / Published Date: 07-Apr-2015 DOI: 10.4172/2161-0681.1000222
Abstract
Problem: Protozoan infections represent a serious public health problem requiring novel approaches from the basic science perspective. Sphingosine 1 phosphate (S1P), is an important component of plasma membrane and, in protozoan infections, a role in infection persistence has been noted. The aim of this review is to summarize the current knowledge about sphingolipids (SL), specifically S1P, and their involvement in protozoan infections such as malaria. Methodology: A non-systematic review in the databases Pubmed, Embase, Free Medical Journals, and Lilacs databases was performed using the following keywords: sphingosine 1 phosphate, malaria, protozoa infections, sphingolipids, S1P receptor (S1PR), immunity, receptors, signaling. The search was limited to articles published between January 1995 and December 2014. Selection of articles to be included was based on relevance to the field of interest, regardless of the language. Results: The number of articles retrieved and those which fulfilled the inclusion criteria were, respectively, 4455 and 143. Conclusions: The role of sphingolipids in protozoan infections is poorly understood, especially in plasmodial infection. S1P might act as immune modulator. SL might be promoters of cell invasion and pathology. They also exhibit potential as antimalarials and biomarkers of infection.
Keywords: Sphingosine 1 phosphate; Malaria; Protozoan infections; Sphingolipids; S1P receptor; Immunity; Signaling.
312347List of abbreviations
CD11b: Cluster of Differentiation 11b; CD45: Cluster of Differentiation 45; Cer: Ceramide; CNS: Central Nervous System; COX-2: Cyclooxygenase 2; DC: Dendritic Cell; EDG: Endotelian Differentiation Gene; ERK-1: Extracellular Regulated Kinases 1; ERK-2: Extracellular Regulated Kinases 2; GPCR: G-Protein Coupled Receptors; HDL: High Density Lipoprotein; HER2: Human Epidermal Growth Factor 2 Receptors; IL-6: Interleukin-6; NK: Natural Killer; PGE2: Prostaglandin E2; PV: Parasitophorous Vacuole; S1P: Sphingosine 1 Phosphate; S1PR: Sphingosine 1 Phosphate Receptor; SGPP 1: Sphingosine 1 Phosphate Phosphatases 1; SGPP 2: Sphingosine 1 Phosphate Phosphatases 2; SL: Sphingolipids; SphK 1: Sphingosine Kinase 1; SphK 2: Sphingosine Kinase 2; SphK: Sphingosine Kinase; TNF: Tumor Necrosis Factor; TVM: Tubovesicular Membranes.
Introduction
Malaria is the most lethal protozoan infection observed worldwide and highly endemic in 97 countries [1], where an estimated 3200 million people are exposed to malaria [1]. Several basic mechanisms used by the parasite to induce disease or protection, remain to be elucidated, including the role of sphingolipids.
Sphingolipids (SL) are plasma membrane components in eukaryotic animal and plant cells involved in various signal transduction pathways [2,3]. Based on studies of their metabolism in animal models, some metabolites, particularly ceramide (Cer) and sphingosine 1 phosphate (S1P), are important signaling molecules in immunity and inflammation [4]. Knowledge of the particular characteristics of some of these metabolites may contribute to understanding the inflammatory processes that occur during several diseases and infections.
Among SL, S1P, a membrane phospholipid derived from the metabolism of sphingomyelin, plays an important role in inflammatory processes and is an important participant in cellular signalling of immune cells. [5-9].
Production of SL exerts different effects during protozoan infections. In some cases SL were reported to contribute to parasite´s survival, whereas in others they strengthen defense mechanisms by the host. [10,11]. In Plasmodium spp. SL are involved in the invasion process of the parasite into the host cell. [12]. However, little is known about the role of SL during plasmodial infection, including the mechanism of action and their effects on the host immune response. Furthermore, the function of some SL receptors in different cells of the immune system remains to be elucidated [13-15].
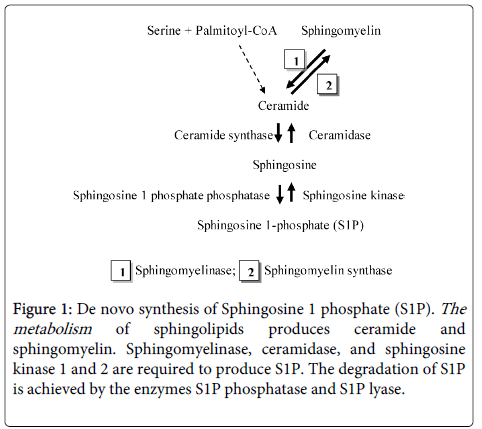
Inflammation is a crucial process in cell migration during protozoan infection and invasion. [2,16]. This process may involve de novo synthesis of various SL by the parasite [2] to promote survival and reproduction of the microorganism (Figure 1) [2].
Figure 1: De novo synthesis of Sphingosine 1 phosphate (S1P). The metabolism of sphingolipids produces ceramide and sphingomyelin. Sphingomyelinase, ceramidase, and sphingosine kinase 1 and 2 are required to produce S1P. The degradation of S1P is achieved by the enzymes S1P phosphatase and S1P lyase.
During plasmodial infection, inflammation is part of both the physiological and pathological responses, where hypoxia and tissue damage dominate [4,8,17,18]. Recent and ongoing studies are underway to expand and redirect research towards discovery of new molecules or metabolites which allow better characterization of the plasmodial infection [19].
Despite evidence of the participation of S1P in inflammatory responses, their specific role during invasion remains elusive. The aim of this review is to summarize the current knowledge about SL, specifically of S1P and their involvement in protozoan infections such as malaria.
Methods
A non-systematic review of the biomedical literature in the databases Pubmed, Embase, Free Medical Journals and Lilacs, was performed using the following keywords and combinations: sphingosine 1 phosphate (S1P), malaria, protozoa infections, sphingolipids (SL), S1P receptor (S1PR), immunity, receptors, signaling. The search was performed by one of the authors (CL-G) and limited to articles published between January 1995 and December 2014. Selection of articles was based on relevance to the field of interest, regardless of the language and included reports from studies in humans and animal models. The topics covered by this review are the following: Characteristics of sphingolipids, biosynthesis of S1P, characterization of S1P in the laboratory, expression of S1P in peripheral blood, activation and effects of the receptors for S1P (S1PR) in different cell populations, general actions of S1P, role of sphingolipids in protozoan infections, sphingolipids and plasmodial infections, role of S1P in plasmodial infection.
Results
The number of articles retrieved and those which fulfilled the inclusion criteria were, respectively: in Pubmed 4268 and 119, in Embase 37 and 2, in Free Medical Journals 100 and 18, in Lilacs 50 and 4. In total, 4455 articles were retrieved and 143 were selected.
Characteristics and biosynthesis of sphingosine 1 phosphate
SL is a complex lipid derived from sphingosine, an 18 carbon non-saturated amino-alcohol. Some SL possess a phosphate, known as phospho-sphingolipids (sphingomyelin), and some have a carbohydrate instead of a phosphate, and are known as glycosphingolipids (gangliosides, cerebrosides) [20,21]. All SL have three basic characteristics: a) a long-chain amino-alcohol called sphingosine (1, 3-dihydroxy-2-amino-4-octadecene); b) functional groups (- OH, NH2, - OH) are observed at carbons 1, 2 and 3; and, c) possess a ceramide residue. Ceramide is the fundamental structural unit of all SL and sphingosine is observed in all S1P [2,3].
Production of S1P is mediated by a sphingosine kinase (SphK), which has two isoforms, SphK 1 and SphK 2, with different physiological functions and cellular localizations. Each isoform is produced depending on the type of cell and the process that is required. For instance, SphK 1 is mainly localized to the cytosol, while SphK 2 can be detected in different intracellular compartments, including the nucleus and mitochondria [22,23]. Once S1P is phosphorylated by SphK 2, this acts as an endogenous histone deacetylase (HDAC) inhibitor and induces gene transcription [22].
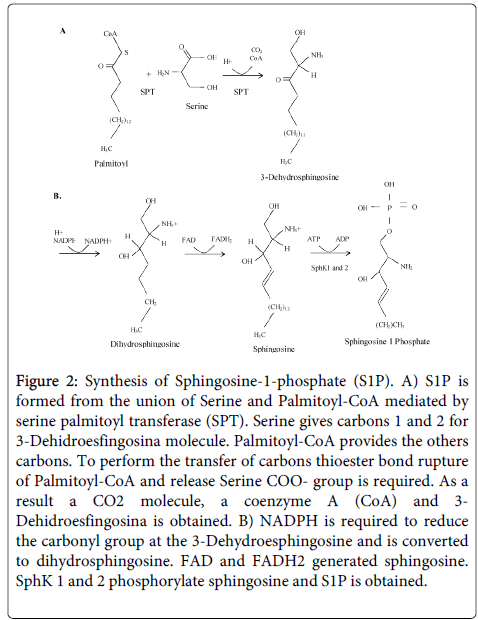
SphK 1 agonists include growth factors, hormones, and pro-inflammatory cytokines [4]. Phosphorylation of S1P via SphK 1 is associated with autoimmune diseases and cancer [4]. Therefore, inhibitors of SphK 1 are of great interest for development of novel therapeutic approaches based on modulation of cell proliferation and induction of apoptosis [4,22,24,25]. In contrast to SphK 1, SphK 2 suppresses cell growth and appears to increase apoptosis [24-28]. The basic aspects of the biosynthesis of S1P are presented in Figure 2 [20].
Figure 2: Synthesis of Sphingosine-1-phosphate (S1P). A) S1P is formed from the union of Serine and Palmitoyl-CoA mediated by serine palmitoyl transferase (SPT). Serine gives carbons 1 and 2 for 3-Dehidroesfingosina molecule. Palmitoyl-CoA provides the others carbons. To perform the transfer of carbons thioester bond rupture of Palmitoyl-CoA and release Serine COO- group is required. As a result a CO2 molecule, a coenzyme A (CoA) and 3- Dehidroesfingosina is obtained. B) NADPH is required to reduce the carbonyl group at the 3-Dehydroesphingosine and is converted to dihydrosphingosine. FAD and FADH2 generated sphingosine. SphK 1 and 2 phosphorylate sphingosine and S1P is obtained.
Characterization of S1P in the laboratory
In general, S1P is measured in human plasma [29,30] using different ELISA or EIA test [18]. In addition to the detection of free S1P [31], specific S1P receptors (S1PR1 - S1PR5) present in tissue, can be evaluated [32]. Analysis of SL in human samples requires assay standardization and validation in each laboratory in order to guarantee precision and reproducibility. The stability and concentration of these molecules can be easily affected if several variables are not controlled, including adequate selection of the technique, the experience of the personnel and precise control of the laboratory temperature [33]. Particularly in the case of S1P, levels increase after repeated cycles of freezing and thawing of the sample [33]. S1P values remain stable if plasma is obtained immediately after blood withdrawal [2, 29]. If plasma separation is delayed, S1P values might be above to 200 nM [2, 28, 29, 33]. Furthermore, red blood cells are rich in S1P (>2000 nM in 1 x 1010 cells, resembling a hematocrit of 100%), levels significantly rise if hemolysis occurs [3,30]. S1P and other SL can also be measured using hydrophilic interaction liquid chromatography (HILIC, SeQuant ™ ZIC ®-HILIC column) [34] and mass spectrometry [35-38].
Expression of S1P in peripheral blood
Control of S1P levels is carried out by enzymes such as S1P lyase and sphingosine 1 phosphate phosphatases 1 and 2 (SGPP 1 and 2) [3,39]. The former degrades S1P in an irreversible manner, while the latter pair result in a reversible reaction [40,41]. Under normal physiological conditions, the highest levels of S1P are detected in the circulation [42,43] and they range from 200 nM in humans to 700 nM in mice [3,42,44]. This high content of S1P probably results from the absence of S1P lyase and the expression of SphK in these cells [3,4,44]. Accordingly, one can expect a rise of plasma S1P in conditions such as malaria, where hemolysis is significant. Other cell populations such as platelets and mast cells also produce and secrete large quantities of S1P but only when activated, therefore, they might not strongly affect basal levels of this SL [5].
In the circulation, S1P attaches to high density lipoproteins (HDL) and albumin and it recognizes five types of cell membranes receptors, initially named Endotelial differentiation gene (EDG), but currently known as Sphingosine-1-phosphate Receptors (S1PR) [45-47]. S1PR exhibit differential affinity for diverse subunits of G-protein coupled receptors (GPCR) [48] and this is required for the multiple actions of S1P [46,49,50]. Endothelial cells mainly express S1PR1 and S1PR3, while lymphocytes and smooth muscle cells of vessels express higher proportions of S1PR1, S1PR2 and S1PR3 [51]. S1PR3, but not S1PR4 and S1PR5, is also expressed in cardiovascular tissue [52]. S1PR4 is expressed in hematopoietic and lymphoid tissues, while S1PR5 is mainly expressed in white matter of the central nervous system (CNS) and the spleen [5]. In addition, researchers reported in 2005 the presence of S1PR3 and S1PR5 in placental tissue [53], and, in 2008, the presence of S1PR1, S1PR2 and S1PR3, was confirmed in this tissue [54].
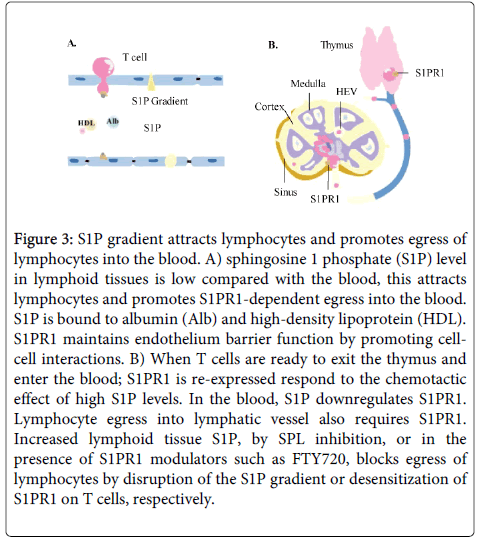
S1P effects vary according to the receptor activated at the cellular level. Accordingly, S1PR1 appears to play an important role in lymphocyte egress from the thymus and secondary lymphoid tissues [55] (Figure 3). Meanwhile, S1PR2 induces expression of COX-2, which in turn increases production of prostaglandin E2 (PGE2). Therefore, the inhibition of S1P/S1PR2 axis-dependent signalling pathways might represent a novel approach in the treatment of chronic inflammatory diseases [9,56].
Figure 3: S1P gradient attracts lymphocytes and promotes egress of lymphocytes into the blood. A) sphingosine 1 phosphate (S1P) level in lymphoid tissues is low compared with the blood, this attracts lymphocytes and promotes S1PR1-dependent egress into the blood. S1P is bound to albumin (Alb) and high-density lipoprotein (HDL). S1PR1 maintains endothelium barrier function by promoting cellcell interactions. B) When T cells are ready to exit the thymus and enter the blood; S1PR1 is re-expressed respond to the chemotactic effect of high S1P levels. In the blood, S1P downregulates S1PR1. Lymphocyte egress into lymphatic vessel also requires S1PR1. Increased lymphoid tissue S1P, by SPL inhibition, or in the presence of S1PR1 modulators such as FTY720, blocks egress of lymphocytes by disruption of the S1P gradient or desensitization of S1PR1 on T cells, respectively.
In the placenta, in vitro tests using BEWO cells (trophoblast lineage), confirmed the importance of the interaction between S1P and S1PR2 to regulate secretion of pro-inflammatory cytokines such as IL-6 [57]. In other tissues, S1PR3 reduces production and secretion of pro-inflammatory cytokines by CD45 (+), CD11b (+), Gr1 (-) and Ly6C (-) cells [58]. This receptor is involved in regeneration of damaged tissues and arteriogenesis without impairment of macrophage phagocytic activity [58]. In some monocytes exhibiting an anti-inflammatory subtype profile, the expression of S1PR3 is predominant [59].
High levels of S1PR4 are associated with malignancy. For instance, in breast cancer, the receptor can be used as a biomarker to establish prognosis [60]. In addition, S1P binding to S1PR4 stimulates activation of extracellular regulated kinases 1 and 2 (ERK-1/2). ERK-1/2 act on human epidermal growth factor 2 receptors (HER2) [61]. The functional interaction of S1PR4 with an oncogene (HER2) provides evidence that S1PR4 might have an important role in breast cancer progression [60-63].
Finally, S1PR5 was implicated in NK cells and monocytes migration [64], but controversial results were reported in mice in which S1P was not involved in chemotaxis of monocytes [65]. Table 1 describes the association between S1PR and the actions on cells of the immune system. Collectively, these reports confirm the importance of S1P on cell migration and inflammation in the immune system and the ubiquity of their receptors present in diverse cell types and in several tissues.
| Cell Type | Receptor | Functions | |||||
|---|---|---|---|---|---|---|---|
| Chemotaxis | Differentiation | Effector Responses | References | ||||
| Innate immune cells | |||||||
| Dendritic cells | S1PR1 | ↑/↔ | ↑ | ↑ | [13,99,100] | ||
| S1PR2 | ND | ND | ND | [13] | |||
| S1PR3 | ↑ | ↑ | ↑ | [14,101] | |||
| S1PR4 | ND | ND | ND | [13] | |||
| S1PR5 | ND | ND | ND | [13] | |||
| Eosinophils | S1PR1 | ↑ | ND | ND | [102] | ||
| S1PR2 | ND | ND | ND | [102] | |||
| S1PR3 | ND | ND | ND | [102] | |||
| Macrophages | S1PR1 | ND | ND | ↑ | [103,104] | ||
| S1PR2 | ↑ | ND | ↑ | [104] | |||
| Mast cells | S1PR1 | ↑ | ND | ND | [15] | ||
| S1PR2 | ↓ | ND | ↑ | [15,105] | |||
| NK cells | S1PR5 | ↑ | ND | ND | [64,106,107] | ||
| Neutrophils | S1PR1 | ↑ | ND | ND | [108,109] | ||
| Adaptive immune cells | |||||||
| T cells | S1PR1 | ↑ | ↑ | ↑/↓ | [76,110] | ||
| S1PR4 | ↑/↔ | ↓ | ↓ | [111,112] | |||
| B cells | S1PR1 | ↑ | ND | ND | [113] | ||
| S1PR3 | ND | ND | ND | [113] | |||
| NKT cells | S1PR1 | ↑ | ND | ND | [107] | ||
| S1PR2 | ND | ND | ND | [107] | |||
| S1PR4 | ND | ND | ND | [107] | |||
Table 1: S1P receptors and effects on immune cells. (↑) denotes a stimulatory effect on the indicated immune function; (↓) inhibition; (↔) no effect on function; ND indicates that this was not determined. Table adapted from [5].
General actions of S1P
S1P exerts intracellular (as a second messenger) and extracellular (binding to GPCR) functions [7,66,67]. S1P is highly bioactive and is involved in diverse physiological processes with several patho-physiological effects at the cellular level including growth regulation, death, senescence, apoptosis, differentiation, proliferation, migration, adhesion, inflammation, cytoskeleton re-organization, angiogenesis, [60,68,69], and interleukin secretion [57,70], among others. In addition, roles in multiple sclerosis [7], cancer [62,71,72], atherosclerosis [73] and osteoporosis [74,75], are reported. Characteristically, S1P can act in an autocrine or paracrine fashion [4].
Taking into account the above, the SphK/S1P/S1PR axis is of interest for researchers exploring diverse aspects of inflammation. Activation of this axis results in emergence of T cells from the thymus [76] (Figure 3), exit of lymphocyte from lymphoid organs [77] and in turn, promotes the release of mature NK cells from bone marrow sinusoids [64,65]. Furthermore, expression of diverse pro-inflammation interleukins [57] can be induced by activation of this axis. In organs such as the placenta, S1P exhibits dual actions during diverse conditions. On one hand, S1P regulates endothelial permeability and vascular tone [78] and participates in proliferation, growth, and formation of the syncytiotrophoblast [79]. On the other hand, S1P inhibits placental trophoblast differentiation during pre-eclampsia [53].
Several S1P agonists and antagonists have been used to simulate and study its biological effects; some of these are listed in Table 2. Among the agonists widely studied is FTY720, a pro-drug that is endogenously phosphorylated (FTY720-P) [80] which inhibits egress of lymphocytes from lymphoid organs by promoting internalization and degradation of S1PR1 in these cells [76]. This effect results in immune-suppression, preventing transplant rejection and inducing clinical improvement in cases of multiple sclerosis [80].
| Name | Mechanism | Disease | References |
|---|---|---|---|
| FTY720 (Fingolimod,Gilenya) | -Prodrug; phosphorylated by SphK2. -S1PR1, S1PR3-5 agonist - Downregulates S1P1 | -FDA approved for multiple sclerosis* -Dermatitis, Arthritis, Allergy | [13,80] |
| SK1-1 (BML-258) | -SphK1 inhibitor | -Glioblastoma -Leukemia | [114,115] |
| Safingol L-threo-dihydrosphingosine | -pan SphK inhibitor | -Solid tumors(Phase 1)* | [116] |
| SK1-2 | SphK1 inhibitor Induces proteasomal and lysosomal degradation of SphK1 | -Pancreatic cancer -Leukemia | [117,118] |
| ABC294640 | SphK2 inhibitor Estrogen receptor agonist | -Cancer -Inflammatory bowel disease | [119,120] |
| ABC294735 | pan SphK inhibitor | -Cancer | [121] |
| SKI-II (SKI-2, SPHK I2) | SphK1 inhibitor | -Asthma | [122] |
| THI 2-acetyl-4(5)-[1(R) | S1P Lyase inhibitor | -Ischemia/reperfusion injury | [123] |
| LX2931 | S1P Lyase inhibitor | -Rheumatoid arthritis(Phase II) * | [124] |
| SKI 5C | SphK1 inhibitor | -Sepsis | [125] |
| SEW2871 | S1PR1 agonist | -Diabetic nephropathy -Renal protection | [126,127] |
| JTE013 | S1PR2 antagonist | -Anaphylaxis -Cancer | [128] [71] |
| AAL® | Prodrug; phosphorylated by SphK2 S1PR1/S1PR3 agonist | -Influenza | [129] |
| AUY954 | S1PR1 agonist | -Experimental autoimmune neuritis | [130] |
Table 2: Small molecules targeting S1P and applications in health. *Effects in humans. All other compounds have only been tested in animal models.
Role of sphingolipids in protozoan infections
SL are involved in the interactions between the parasite and their host cells, and potentially contribute to survival of the parasite and the host´s defense [10]. The following section summarizes the current knowledge on SL, their synthesis and their role in several of the major groups of parasitic protozoan.
Trypanosomatids are a group of protozoa of which Trypanosoma cruzi, Trypanosoma brucei and Leishmania spp. are notorious in causing disease in human populations. These parasites infect 20-30 million people worldwide and tropism for different tissues is reflected in the wide range of conditions: from self-limited skin lesions to life threating brain, intestine and heart damage [81,82]. Other protozoa such as Toxoplasma gondii, Giardia spp. and Entameoba histolytica, are also pathogens with a wide geographic distribution and can result in severe disease in humans [10,83].
SL can present as intracellular lipids or transport proteins [84,85], and controversy remains as to the ability of parasites to uptake and process ceramide and gangliosides already present in the host cells [86]. More clear is the fact that SL are important for protozoa as they are involved in invasion, and parasite survival and multiplication under adverse conditions [2]. It was reported by Pratt, et al. that de novo synthesis of inositolphosphoryl ceramide is involved in T. gondii multiplication, particularly when the parasite undergoes stress [2]. Table 3 details the different types of SL observed in the life cycle of some protozoa.
| Species | Sphingolipids identified | References |
|---|---|---|
| Leishmania major | SM, IPC | [131,132] |
| Trypanosoma brucei | IPC | [133] |
| EPC (in peripheral blood) | [134] | |
| SM | [135] | |
| Glycosyl-cer | [136] | |
| Trypanosoma cruzi | SM, IPC | [137,138] |
| Toxoplasma gondii | Glycosyl-cer, SM, IPC | [2,139] |
| Trichomonas vaginalis | SM, IPC | [140] |
| Giardia lamblia | SM | [83] |
| Plasmodium falciparum | Glycosyl-cer, SM | [88,141,142] |
Table 3: Sphingolipids in parasitic protozoa. SM: Sphingomyelin; IPC: Inositolphosphoryl Ceramide; EPC: Ethanolamine Phosphorylceramide.
Sphingolipids and plasmodial infections
Many facts about Plamodium spp. metabolism and other biological processes such as invasion remain unknown. Therefore, studies aimed at understanding crucial survival paths of the parasite might result in development of novel therapeutic approaches.
As discussed earlier, the location of SL in the cell membrane is highly strategic in several vital processes of parasites. Particularly in Plasmodium spp, cell invasion can be strongly affected by the presence and characteristics of SL. This was known experimentally with measures of viscosity and thermal fluctuations of the RBC membrane [12]. Plasmodium spp have the potential to invade and colonize erythrocytes of any age, depending on the species. Once inside the erythrocyte, the parasite cytoplasm and cell membrane undergo major changes [87] in order to modify the erythrocyte cytoskeleton and the environment and to evade specific immune responses [87]. One of the features of Plasmodium spp infected erythrocytes is an increase in sphingolipid synthesis [88]. While uninfected, erythrocytes membranes are impermeable to sphingomyelin, but the membrane of infected cells allows two-way passage of SL and nutrients [88,89].
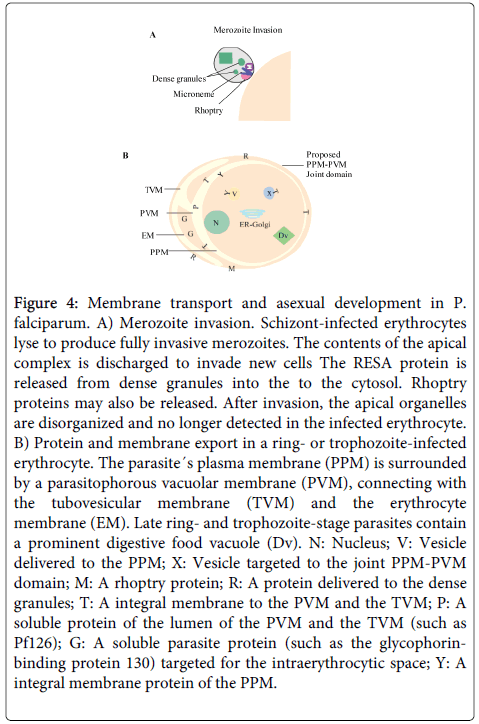
Intracellular development of P. falciparum depends on the formation of a cytosol system consisting of a novel network of tubovesicular membranes (TVM) within the host cell [90-92]. The parasitized cell membrane (PM) and an inner parasitophorous vacuole (PV) originate in the parasite. The TVM network is in charge of repairing the gap caused by the parasite after invasion and is induced during the intra-vacuolar development of the parasite [90-92]. The sphingomyelin formation by the parasite is an essential requirement for construction of the TVM [90,91,93] (Figure 4). SL are important constituents during the process of cell invasion by the parasite, and are major determinants of the immune response elicited by the invasive micro-organism.
Figure 4: Membrane transport and asexual development in P. falciparum. A) Merozoite invasion. Schizont-infected erythrocytes lyse to produce fully invasive merozoites. The contents of the apical complex is discharged to invade new cells The RESA protein is released from dense granules into the to the cytosol. Rhoptry proteins may also be released. After invasion, the apical organelles are disorganized and no longer detected in the infected erythrocyte. B) Protein and membrane export in a ring- or trophozoite-infected erythrocyte. The parasite´s plasma membrane (PPM) is surrounded by a parasitophorous vacuolar membrane (PVM), connecting with the tubovesicular membrane (TVM) and the erythrocyte membrane (EM). Late ring- and trophozoite-stage parasites contain a prominent digestive food vacuole (Dv). N: Nucleus; V: Vesicle delivered to the PPM; X: Vesicle targeted to the joint PPM-PVM domain; M: A rhoptry protein; R: A protein delivered to the dense granules; T: A integral membrane to the PVM and the TVM; P: A soluble protein of the lumen of the PVM and the TVM (such as Pf126); G: A soluble parasite protein (such as the glycophorinbinding protein 130) targeted for the intraerythrocytic space; Y: A integral membrane protein of the PPM.
Role of S1P in plasmodial infection
During infection, a group of SL facilitates cellular infection and damage, but another group contributes to damage attenuation [18]. Sphingosine 1 phosphate is part of the later and has a protective effect, particularly during cerebral malaria [18]. However, the role of S1P has not been explored in other forms of malaria. Further involvement of S1P in other severe manifestations of the disease might be expected as additional studies are performed.
One of the mechanisms which might be involved in the protection observed during severe malaria might include reduced activity of S1P lyase. Studies on an experimental model of Plasmodium berghei ANKA (PbA) confirmed that S1P lyase deficiency led to high S1P bioavailability and reduction in severity, with higher survival rates [18]. Compounds with a proven effect on the regulation of S1P in mice have been tested in humans in the context of modulation of the immune system [18].
Plasmodium spp obtain SL from endogenous and exogenous sources [86,90,94]. Therefore, therapeutic agents able to limit the exogenous supply of SL are anti-malarial candidates. For this, chemicals and structure-related compounds found in humans have been tested for their ability to limit intracellular growth and replication of the parasite since they limit membrane formation and TVM network establishment once within the red cell and reduce formation of transport networks [94]. Further studies aimed at understanding the origin and route of lipid compounds required to maintain integrity of the red cell membrane during the intracellular phase of the parasite, can contribute to understanding the immune mechanisms of evasion used by Plasmodium spp [95].
Conclusions and future trends
SL play an important role both as intracellular or extracellular constituent lipids [84,85]. They perform important activities as signalling molecules during cell differentiation, proliferation, apoptosis, and inflammation [24,76,96]. Information on their importance during plasmodial infection is scarce, but S1P may be involved in immunomodulation during cerebral malaria, with significant protection from disease and death observed in animal models [18]. In addition, sphingomyelin allows formation of a TVM network in the infected cell [89,90,97], which is crucial for parasites survival [92,93].
An important aspect worth highlighting is the ability of protozoan parasites to regulate production of some SL, such as ceramide [86,98], resulting in potential auto-regulation of cell signaling by the parasite.
In order to understand the mechanisms of action and potential use of SL as antimalarials or as biomarkers of infection, further studies in animal models and human populations are required. Finally, insight into the metabolism of SL as mediators and promoters of cell invasion and their requirement by the parasite to induce development of the TVM network should be pursued.
Acknowledgments
The authors are grateful to Dr Stephanie Yanow for proof reading the manuscript. Funding was received from CODI Proyecto 2014-969 and Estrategia Sostenibilidad 2014-2015, Universidad de Antioquia.
References
- World Health Organization, (WHO) (2013) Factsheet on the World Malaria Report 2013. (Eds): 284.
- Pratt S, Wansadhipathi-Kannangara NK, Bruce CR, Mina JG, Shams-Eldin H, et al. (2013) Sphingolipid synthesis and scavenging in the intracellular apicomplexan parasite, Toxoplasma gondii. Molecular Biochemical Parasitology 187: 43-51.
- Thuy AV, Reimann CM, Hemdan NY, Gräler MH (2014) Sphingosine 1-phosphate in blood: function, metabolism, and fate. Cellular Physiology & Biochemistry 34: 158-171.
- Maceyka M, Harikumar KB, Milstien S, Spiegel S (2012) Sphingosine-1-phosphate signaling and its role in disease. Trends in Cell Biology 22: 50-60.
- Rivera J, Proia RL, Olivera A (2008) The alliance of sphingosine-1-phosphate and its receptors in immunity. Nature Reviews. Immunology 8: 753-763.
- Nixon GF (2009) Sphingolipids in inflammation: pathological implications and potential therapeutic targets. British Journal of Pharmacology 158: 982-993.
- Spiegel S, Milstien S (2011) The outs and the ins of sphingosine-1-phosphate in immunity. Nature Reviews. Immunology 11: 403-415.
- Fernández-Pisonero I, Dueñas AI, Barreiro O, Montero O, Sánchez-Madrid F, et al. (2012) Lipopolysaccharide and sphingosine-1-phosphate cooperate to induce inflammatory molecules and leukocyte adhesion in endothelial cells J Immunol 189: 5402-5410.
- Hsu CK, Lee IT, Lin CC, Hsiao LD, Yang CM (2015) Sphingosine-1-phosphate mediates COX-2 expression and PGE2 /IL-6 secretion via c-Src-dependent AP-1 activation. J Cell Physiol 230: 702-715.
- Zhang K, Bangs JD, Beverley SM (2010) Sphingolipids in parasitic protozoa. Adv Exp Med Biol 688: 238-248.
- Pankova-Kholmyansky I, Dagan A, Gold D, Zaslavsky Z, Skutelsky E, et al. (2003) Ceramide mediates growth inhibition of the Plasmodium falciparum parasite. Cell Mol Life Sci 60: 577-587.
- Li H, Lykotrafitis G2 (2014) Erythrocyte membrane model with explicit description of the lipid bilayer and the spectrin network. Biophys J 107: 642-653.
- Idzko M, Hammad H, van Nimwegen M, Kool M, Müller T, et al. (2006) Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J Clin Invest 116: 2935-2944.
- Niessen F1, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, et al. (2008) Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature 452: 654-658.
- Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, et al. (2004) Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med 199: 959-970.
- Agudelo OM, Aristizabal BH, Yanow SK, Arango E, Carmona-Fonseca J, et al. (2014) Submicroscopic infection of placenta by Plasmodium produces Th1/Th2 cytokine imbalance, inflammation and hypoxia in women from north-west Colombia. Malar J 13: 122.
- . Agudelo OM (2012) Citoquinas y apoptosis en placentas a término con y sin infección por Plasmodium, Colombia, Universidad de Antioquia.
- Finney CA, Hawkes CA, Kain DC, Dhabangi A, Musoke C, et al. (2011) S1P is associated with protection in human and experimental cerebral malaria. Mol Med 17: 717-725.
- Mu AK, Bee PC, Lau YL, Chen Y (2014) Identification of protein markers in patients infected with Plasmodium knowlesi, Plasmodium falciparum and Plasmodium vivax. Int J Mol Sci 15: 19952-19961.
- Carter H, Glick F, Norris W, Phillips G (1947) Biochemistry of the sphingolipides: III . Structure of sphingosine. Journal of Biological Chemistry 170: 285-294.
- Brady RN, Di Mari SJ, Snell EE (1969) Biosynthesis of sphingolipid bases. 3. Isolation and characterization of ketonic intermediates in the synthesis of sphingosine and dihydrosphingosine by cell-free extracts of Hansenula ciferri. J Biol Chem 244: 491-496.
- Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, et al. (2009) Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325: 1254-1257.
- Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, et al. (2011) Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J 25: 600-612.
- Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S (2006) Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta 1758: 2016-2026.
- Alemany R, van Koppen CJ, Danneberg K, Ter Braak M, Meyer Zu Heringdorf D (2007) Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch Pharmacol 374: 413-428.
- Snider AJ (2013) Sphingosine kinase and sphingosine-1-phosphate: regulators in autoimmune and inflammatory disease. Int J Clin Rheumtol 8.
- Oskeritzian CA, Alvarez SE, Hait NC, Price MM, Milstien S, et al. (2008) Distinct roles of sphingosine kinases 1 and 2 in human mast-cell functions. Blood 111: 4193-4200.
- Sankala HM, Hait NC, Paugh SW, Shida D, Lépine S, et al. (2007) Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res 67: 10466-10474.
- Hammad SM, Al Gadban MM, Semler AJ, Klein RL (2012) Sphingosine 1-phosphate distribution in human plasma: associations with lipid profiles. J Lipids 2012: 180705.
- Hänel P, Andréani P, Gräler MH (2007) Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J 21: 1202-1209.
- CEDARLANE C, Court P, Burlington O (2014) ELISA Kit for Sphingosine 1 Phosphate Receptor 1 (S1PR1).
- Ceglarek U, Dittrich J, Helmschrodt C et al. (2014) Preanalytical standardization of sphingosine-1-phosphate, sphinganine-1-phosphate and sphingosine analysis in human plasma by liquid chromatography-tandem mass spectrometry. Clinica Chimca Acta 435: 1-6.
- Andréani P, MH. G (2006) Comparative quantification of sphingolipids and analogs in biological samples by high-performance liquid chromatography after chloroform extraction. Analytical Biochemistry 358: 239-246.
- Lan T, Bi H, Liu W, Xie X, Xu S, et al. (2011) Simultaneous determination of sphingosine and sphingosine 1-phosphate in biological samples by liquid chromatography-tandem mass spectrometry. Journal of Chromatography B, Analytical Technologies in the Biomedical Life Sciences 879: 520-526.
- Schmidt H, Schmidt R, Geisslinger G (2006) LC-MS/MS-analysis of sphingosine-1-phosphate and related compounds in plasma samples. Prostaglandins Other Lipid Mediat 81: 162-170.
- Cutignano A, Chiuminatto U, Petruzziello F, Vella FM, Fontana A (2010) UPLC-MS/MS method for analysis of sphingosine 1-phosphate in biological samples. Prostaglandins Other Lipid Mediat 93: 25-29.
- Scherer M, Böttcher A, Schmitz G, Liebisch G (2011) Sphingolipid profiling of human plasma and FPLC-separated lipoprotein fractions by hydrophilic interaction chromatography tandem mass spectrometry. Biochimca et Biophysica Acta 1811: 68-75.
- Johnson KR, Johnson KY, Becker KP, Bielawski J, Mao C, et al. (2003) Role of human sphingosine-1-phosphate phosphatase 1 in the regulation of intra- and extracellular sphingosine-1-phosphate levels and cell viability. Journal of Biological Chemistry 278: 34541-34547.
- Brindley DN (2004) Lipid phosphate phosphatases and related proteins: signaling functions in development, cell division, and cancer. Journal of Cellular Biochemistry 92: 900-912.
- Kumar A, Saba JD (2009) Lyase to live by: sphingosine phosphate lyase as a therapeutic target. Expert Opinion on Therapuetic Targets 13: 1013-1025.
- Sensken SC, Bode C, Nagarajan M, Peest U, Pabst O, et al. (2010) Redistribution of sphingosine 1-phosphate by sphingosine kinase 2 contributes to lymphopenia. Journal of Immunology 184: 4133-4142.
- Murata N, Sato K, Kon J, Tomura H, Okajima F (2000) Quantitative measurement of sphingosine 1-phosphate by radioreceptor-binding assay. Analytical Biochemistry 282: 115-120.
- Bode C, Sensken SC, Peest U, Beutel G, Thol F, et al. (2010) Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. Journal of Cellular Biochemistry 109: 1232-1243.
- van Doorn R, Lopes Pinheiro MA, Kooij G, Lakeman K, van het Hof B, et al. (2012) Sphingosine 1-phosphate receptor 5 mediates the immune quiescence of the human brain endothelial barrier. Journal of Neuroinflammation 9: 133.
- Anliker B, Chun J (2004) Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol 15: 457-465.
- Zondag GC, Postma FR, Etten IV, Verlaan I, Moolenaar WH (1998) Sphingosine 1-phosphate signalling through the G-protein-coupled receptor Edg-1. Biochem J 330 : 605-609.
- Kluk MJ, Hla T (2002) Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochimica et Biophysica Acta 1582: 72-80.
- An S, Goetzl EJ, Lee H (1998) Signaling mechanisms and molecular characteristics of G protein-coupled receptors for lysophosphatidic acid and sphingosine 1-phosphate. Journal of Cellular Biochemistry, Supplement 30-31: 147-57.
- Blaho VA, Hla T1 (2014) An update on the biology of sphingosine 1-phosphate receptors. J Lipid Res 55: 1596-1608.
- Skoura A, Hla T (2009) Regulation of vascular physiology and pathology by the S1P2 receptor subtype. Cardiovasc Res 82: 221-228.
- Alewijnse AE, Peters SL, Michel MC (2004) Cardiovascular effects of sphingosine-1-phosphate and other sphingomyelin metabolites. Br J Pharmacol 143: 666-684.
- Johnstone ED, Chan G, Sibley CP, Davidge ST, Lowen B, et al. (2005) Sphingosine-1-phosphate inhibition of placental trophoblast differentiation through a G(i)-coupled receptor response. Journal of Lipid Research 46: 1833-1839.
- Ye X (2008) Lysophospholipid signaling in the function and pathology of the reproductive system. Human Reproduction Update 14: 519-536.
- Chiba K, Matsuyuki H, Maeda Y, Sugahara K (2006) Role of sphingosine 1-phosphate receptor type 1 in lymphocyte egress from secondary lymphoid tissues and thymus. Cellular & Molecular Immunology 3: 11-19.
- Völzke A, Koch A, Meyer Zu Heringdorf D, Huwiler A, Pfeilschifter J (2014) Sphingosine 1-phosphate (S1P) induces COX-2 expression and PGE2 formation via S1P receptor 2 in renal mesangial cells. Biochimica Biophysica Acta 1841: 11-21.
- Goyal P, Brünnert D, Ehrhardt J, Bredow M, Piccenini S, et al. (2013) Cytokine IL-6 secretion by trophoblasts regulated via sphingosine-1-phosphate receptor 2 involving Rho/Rho-kinase and Rac1 signaling pathways. Molecular Human Reproduction 19: 528-538.
- Awojoodu AO, Ogle ME, Sefcik LS, Bowers DT, Martin K, et al. (2013) Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proceedings of the National Academy of Sciences of the U S A 110: 13785-13790.
- Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Gräler M, et al. (2011) Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circulation Research 108: 314-323.
- Pyne NJ, Long JS, Lee SC, Loveridge C, Gillies L, et al. (2009) New aspects of sphingosine 1-phosphate signaling in mammalian cells. Advances in Enzyme Regulation 49: 214-221.
- Ohotski J, Long JS, Orange C, Elsberger B, Mallon E, et al. (2012) Expression of sphingosine 1-phosphate receptor 4 and sphingosine kinase 1 is associated with outcome in oestrogen receptor-negative breast cancer. British Journal of Cancer 106: 1453-1459.
- Pyne NJ, Pyne S (2010) Sphingosine 1-phosphate and cancer. Nature Reviews. Cancer 10: 489-503.
- Pyne S1, Pyne NJ (2000) Sphingosine 1-phosphate signalling in mammalian cells. The Biochemical Journal 349: 385-402.
- Zhang J1, Dunk CE, Lye SJ (2013) Sphingosine signalling regulates decidual NK cell angiogenic phenotype and trophoblast migration. Human Reproduction 28: 3026-3037.
- Mayol K, Biajoux V, Marvel J, Balabanian K, Walzer T (2011) Sequential desensitization of CXCR4 and S1P5 controls natural killer cell trafficking. Blood 118: 4863-4871.
- Windh RT, Lee MJ, Hla T, An S, Barr AJ, et al. (1999) Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the G(i), G(q), and G(12) families of heterotrimeric G proteins. The Journal of Biological Chemistry 274: 27351-27358.
- Rosen H, Stevens RC, Hanson M, Roberts E, Oldstone MB (2013) Sphingosine-1-phosphate and its receptors: structure, signaling, and influence. Annual Reviews of Biochemistry 82: 637-662.
- Simmons S, Ishii M (2014) Sphingosine-1-phosphate: a master regulator of lymphocyte egress and immunity. Arch Immunol Ther Exp (Warsz) 62: 103-115.
- Hannun YA, Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nature Reviews. Molecular Cell Biology 9: 139-150.
- Nishino E, Matsuzaki N, Masuhiro K et al. (1990) Trophoblast-derived interleukin-6 (IL-6) regulates human chorionic gonadotropin release through IL-6 receptor on human trophoblasts. The Journal of Clinical Endocrinology & Metabology 71: 436-441.
- Salas A, Ponnusamy S, Senkal CE, Meyers-Needham M, Selvam SP, et al. (2011) Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood 117: 5941-5952.
- Kim ES, Kim JS, Kim SG, Hwang S, Lee CH, et al. (2011) Sphingosine 1-phosphate regulates matrix metalloproteinase-9 expression and breast cell invasion through S1P3-Gαq coupling. Journal of Cell Science 124: 2220-2230.
- Daum G, Grabski A, Reidy MA (2009) Sphingosine 1-phosphate: a regulator of arterial lesions. Arterioscler Thromb Vasc Biol 29: 1439-1443.
- Ryu J, Kim HJ, Chang EJ, Huang H, Banno Y, et al. (2006) Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EM
- Ishii M, Kikuta J, Shimazu Y, Meier-Schellersheim M, Germain RN (2010) Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med 207: 2793-2798.
- Garris CS, Blaho VA, Hla T, Han MH (2014) Sphingosine-1-phosphate receptor 1 signalling in T cells: trafficking and beyond. Immunology 142: 347-353.
- Arnon TI, Cyster JG (2014) Blood, sphingosine-1-phosphate and lymphocyte migration dynamics in the spleen. Curr Top Microbiol Immunol 378: 107-128.
- Hemmings DG, Hudson NK, Halliday D, O'Hara M, Baker PN, et al. (2006) Sphingosine-1-phosphate acts via rho-associated kinase and nitric oxide to regulate human placental vascular tone. Biol Reprod 74: 88-94.
- Singh AT, Dharmarajan A, Aye IL, Keelan JA (2012) Sphingosine-sphingosine-1-phosphate pathway regulates trophoblast differentiation and syncytialization. Reprod Biomed Online 24: 224-234.
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, et al. (2010) Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 9: 883-897.
- Schofield CJ, Jannin J, Salvatella R (2006) The future of Chagas disease control. Trends Parasitol 22: 583-588.
- Cunningham AC1 (2002) Parasitic adaptive mechanisms in infection by leishmania. Exp Mol Pathol 72: 132-141.
- Hernandez Y, Shpak M, Duarte TT, Mendez TL, Maldonado RA, et al. (2008) Novel role of sphingolipid synthesis genes in regulating giardial encystation. Infect Immun 76: 2939-2949.
- Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387: 569-572.
- Muñiz M1, Riezman H (2000) Intracellular transport of GPI-anchored proteins. EMBO J 19: 10-15.
- de Melo EJ, de Souza W (1996) Pathway of C6-NBD-Ceramide on the host cell infected with Toxoplasma gondii. Cell Struct Funct 21: 47-52.
- Orjih AU (2014) Maturation of Plasmodium falciparum in multiply infected erythrocytes and the potential role in malaria pathogenesis. Parasitol Res 113: 4045-4056.
- Ansorge I, Jeckel D, Wieland F, Lingelbach K (1995) Plasmodium falciparum-infected erythrocytes utilize a synthetic truncated ceramide precursor for synthesis and secretion of truncated sphingomyelin. The Biochemical Journal 308: 335-341.
- Lauer SA, Rathod PK, Ghori N, Haldar K (1997) A membrane network for nutrient import in red cells infected with the malaria parasite. Science 276: 1122-1125.
- Haldar K (1996) Sphingolipid synthesis and membrane formation by Plasmodium. Trends in Cell Biology 6: 398-405.
- Haldar K (1998) Intracellular trafficking in Plasmodium-infected erythrocytes. Current Opinion in Microbiology 1: 466-471.
- Deitsch KW, Wellems TE (1996) Membrane modifications in erythrocytes parasitized by Plasmodium falciparum. Molecular & Biochemical Parasitology 76: 1-10.
- Haldar K (1992) Lipid transport in Plasmodium. Infectious Agents and Disease 1: 254-262.
- Meyer EV, Holt JJ2, Girard KR1, Ballie MT3, Bushnev AS3, et al. (2011) Sphingolipid analogues inhibit development of malaria parasites. ACS Medicinal Chemical Letters 3: 43-47.
- Lauer SA, Ghori N, Haldar K (1995) Sphingolipid synthesis as a target for chemotherapy against malaria parasites. Proc Natl Acad Sci U S A 92: 9181-9185.
- Gandy KA, Obeid LM (2013) Regulation of the sphingosine kinase/sphingosine 1-phosphate pathway. Handb Exp Pharmacol : 275-303.
- Lauer SA, Chatterjee S, Haldar K (2001) Uptake and hydrolysis of sphingomyelin analogues in Plasmodium falciparum-infected red cells. Mol Biochem Parasitol 115: 275-281.
- Haldar K, Uyetake L, Ghori N, Elmendorf HG, Li WL (1991) The accumulation and metabolism of a fluorescent ceramide derivative in Plasmodium falciparum-infected erythrocytes. Mol Biochem Parasitol 49: 143-156.
- Czeloth N, Schippers A, Wagner N, Müller W, Küster B, et al. (2007) Sphingosine-1 phosphate signaling regulates positioning of dendritic cells within the spleen. J Immunol 179: 5855-5863.
- Idzko M, Panther E, Corinti S, Morelli A, Ferrari D, et al. (2002) Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. FASEB J 16: 625-627.
- Maeda Y, Matsuyuki H, Shimano K, Kataoka H, Sugahara K, et al. (2007) Migration of CD4 T cells and dendritic cells toward sphingosine 1-phosphate (S1P) is mediated by different receptor subtypes: S1P regulates the functions of murine mature dendritic cells via S1P receptor type 3. Journal of Immunology 178: 3437-3446.
- Roviezzo F, Del Galdo F, Abbate G, Bucci M, D'Agostino B, et al. (2004) Human eosinophil chemotaxis and selective in vivo recruitment by sphingosine 1-phosphate. Proc Natl Acad Sci U S A 101: 11170-11175.
- Singer II, Tian M, Wickham LA, Lin J, Matheravidathu SS et al. (2005) Sphingosine-1-phosphate agonists increase macrophage homing, lymphocyte contacts, and endothelial junctional complex formation in murine lymph nodes. Journal of Immunology 175: 7151-7161.
- Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, et al. (2008) Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circulation Research 102: 950-958.
- Yokoo E, Yatomi Y, Takafuta T, Osada M, Okamoto Y, et al. (2004) Sphingosine 1-phosphate inhibits migration of RBL-2H3 cells via S1P2: cross-talk between platelets and mast cells. The Journal of Biochemistry 135: 673-681.
- Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, et al. (2007) Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nature Immunology 8: 1337-1344.
- Allende ML, Zhou D, Kalkofen DN, Benhamed S, Tuymetova G, et al. (2008) S1P1 receptor expression regulates emergence of NKT cells in peripheral tissues. FASEB Journal 22: 307-315.
- Rahman MM, Alkhouri H, Tang F, Che W, Ge Q, et al. (2014) Sphingosine 1-phosphate induces neutrophil chemoattractant IL-8: repression by steroids. PLoS One 9
- Finley A, Chen Z, Esposito E, Cuzzocrea S, Sabbadini R, et al. (2013) Sphingosine 1-phosphate mediates hyperalgesia via a neutrophil-dependent mechanism. PLoS One 8
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, et al. (2004) Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427: 355-360.
- Wang W, Graeler MH, Goetzl EJ (2005) Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. FASEB Journal, 19 1731-1733.
- Matsuyuki H, Maeda Y, Yano K, Sugahara K, Chiba K, et al. (2006) Involvement of sphingosine 1-phosphate (S1P) receptor type 1 and type 4 in migratory response of mouse T cells toward S1P. Cellular & Molecular Immunology 3: 429-437.
- Mariño E, Batten M, Groom J, Walters S, Liuwantara D, et al. (2008) Marginal-zone B-cells of nonobese diabetic mice expand with diabetes onset, invade the pancreatic lymph nodes, and present autoantigen to diabetogenic T-cells. Diabetes 57: 395-404.
- Paugh SW, Paugh BS, Rahmani M, Kapitonov D, et al. (2008) A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood, 112: 1382-1391.
- Kapitonov D, Allegood JC, Mitchell C, Hait NC, Almenara JA, et al. (2009) Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Research 69: 6915-6923.
- Dickson MA, Carvajal RD, Merrill AH Jr, Gonen M, Cane LM, et al. (2011) A phase I clinical trial of safingol in combination with cisplatin in advanced solid tumors. Clin Cancer Res 17: 2484-2492.
- Guillermet-Guibert J, Davenne L, Pchejetski D, Saint-Laurent N, Brizuela L, et al. (2009) Targeting the sphingolipid metabolism to defeat pancreatic cancer cell resistance to the chemotherapeutic gemcitabine drug. Mol Cancer Ther 8: 809-820.
- Ricci C, Onida F, Servida F, Radaelli F, Saporiti G, et al. (2009) In vitro anti-leukaemia activity of sphingosine kinase inhibitor. Br J Haematol 144: 350-357.
- Antoon JW, White MD, Meacham WD, Slaughter EM, Muir SE, et al. (2010) Antiestrogenic effects of the novel sphingosine kinase-2 inhibitor ABC294640. Endocrinology 151: 5124-5135.
- Chumanevich AA, Poudyal D, Cui X, Davis T, Wood PA, et al. (2010) Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of sphingosine kinase. Carcinogenesis 31: 1787-1793.
- Beljanski V, Knaak C, Zhuang Y, Smith CD (2011) Combined anticancer effects of sphingosine kinase inhibitors and sorafenib. Invest New Drugs 29: 1132-1142.
- Chiba Y, Takeuchi H, Sakai H, Misawa M (2010) SKI-II, an inhibitor of sphingosine kinase, ameliorates antigen-induced bronchial smooth muscle hyperresponsiveness, but not airway inflammation, in mice. Journal of Pharmacological Sciences 114: 304-310.
- Bandhuvula P, Honbo N, Wang GY, Jin ZQ, Fyrst H, et al. (2011) S1P lyase: a novel therapeutic target for ischemia-reperfusion injury of the heart. American Journal of Physiology. Heart and Circulatory Physiology 300: H1753-1761.
- Bagdanoff JT, Donoviel MS, Nouraldeen A, Carlsen M, Jessop TC, et al. (2010) Inhibition of sphingosine 1-phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone oxime (LX2931) and (1R,2S,3R)-1-(2-(isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932). Journal of Medicinal Chemistry 53: 8650-8662.
- Puneet P, Yap CT, Wong L, Lam Y, Koh DR, et al. (2010) SphK1 regulates proinflammatory responses associated with endotoxin and polymicrobial sepsis. Science 328: 1290-1294.
- Awad AS, Rouse MD, Khutsishvili K, Huang L, Bolton WK, et al. (2011) Chronic sphingosine 1-phosphate 1 receptor activation attenuates early-stage diabetic nephropathy independent of lymphocytes. Kidney International 79: 1090-1098.
- Bajwa A, Jo SK, Ye H, Huang L, Dondeti KR, et al. (2010) Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol 21: 955-965.
- Oskeritzian CA, Price MM, Hait NC, Kapitonov D, Falanga YT, et al. (2010) Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. Journal of Experimental Medicine 207: 465-474.
- Marsolais D, Hahm B, Walsh KB, Edelmann KH, McGavern D, et al. (2009) A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proceedings of the National Academy Sciences of the U S A 106: 1560-1565.
- Zhang ZY, Zhang Z, Zug C, Nuesslein-Hildesheim B, Leppert D, et al. (2009) AUY954, a selective S1P(1) modulator, prevents experimental autoimmune neuritis. Journal of Neuroimmunology 216: 59-65.
- Zhang O, Wilson MC, Xu W, Hsu FF, Turk J, et al. (2009) Degradation of host sphingomyelin is essential for Leishmania virulence. PLoS Pathogensis
- Hsu FF, Turk J, Zhang K, Beverley SM (2007) Characterization of inositol phosphorylceramides from Leishmania major by tandem mass spectrometry with electrospray ionization. Journal of the American Society for Mass Spectrometry 18: 1591-1604.
- Fridberg A, Olson CL, Nakayasu ES, Tyler KM, Almeida IC, et al. (2008) Sphingolipid synthesis is necessary for kinetoplast segregation and cytokinesis in Trypanosoma brucei. Journal of Cell Sciences 121: 522-535.
- Sutterwala SS, Hsu FF, Sevova ES, Schwartz KJ, Zhang K, et al. (2008) Developmentally regulated sphingolipid synthesis in African trypanosomes. Mol Microbiol 70: 281-296.
- Patnaik PK, Field MC, Menon AK, Cross GA, Yee MC, et al. (1993) Molecular species analysis of phospholipids from Trypanosoma brucei bloodstream and procyclic forms. Mol Biochem Parasitol 58: 97-105.
- Uemura A, Watarai S, Kushi Y, Kasama T, Ohnishi Y, et al. (2006) Analysis of neutral glycosphingolipids from Trypanosoma brucei. Vet Parasitol 140: 264-272.
- Bertello LE, Gonçalvez MF, Colli W, de Lederkremer RM (1995) Structural analysis of inositol phospholipids from Trypanosoma cruzi epimastigote forms. Biochem J 310 : 255-261.
- Uhrig ML, Couto AS, Colli W, de Lederkremer RM (1996) Characterization of inositolphospholipids in Trypanosoma cruzi trypomastigote forms. Biochim Biophys Acta 1300: 233-239.
- Sonda S, Sala G, Ghidoni R, Hemphill A, Pieters J (2005) Inhibitory effect of aureobasidin A on Toxoplasma gondii. Antimicrob Agents Chemother 49: 1794-1801.
- Costello CE, Glushka J, van Halbeek H, Singh BN (1993) Structural characterization of novel inositol phosphosphingolipids of Tritrichomonas foetus and Trichomonas vaginalis. Glycobiology 3: 261-269.
- Landoni M, Duschak VG, Peres VJ, Nonami H, Erra-Balsells R, et al. (2007) Plasmodium falciparum biosynthesizes sulfoglycosphingolipids. Mol Biochem Parasitol 154: 22-29.
- Gerold P, Schwarz RT (2001) Biosynthesis of glycosphingolipids de-novo by the human malaria parasite Plasmodium falciparum. Mol Biochem Parasitol 112: 29-37.
- Maceyka M, Spiegel S1 (2014) Sphingolipid metabolites in inflammatory disease. Nature 510: 58-67.
Citation: López Guzmán C, Carmona Fonseca J, Maestre A (2015) Sphingosine 1 Phosphate in Cell Signaling with Emphasis in Protozoan Infections . J Clin Exp Pathol 5:222. DOI: 10.4172/2161-0681.1000222
Copyright: ©2015 Zhou W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16661
- [From(publication date): 6-2015 - Jul 01, 2025]
- Breakdown by view type
- HTML page views: 11988
- PDF downloads: 4673