Simultaneous Quantification of Drospirenone, Ethinyl Estradiol and Levomefolate by Stability Indicating RP-HPLC Method
Received: 25-Jun-2018 / Accepted Date: 09-Jul-2018 / Published Date: 15-Jul-2018 DOI: 10.4172/2155-9872.1000408
Keywords: Drospirenone; Ethinyl estradiol; Levomefolate; Safyral; HPLC; Quantification
Introduction
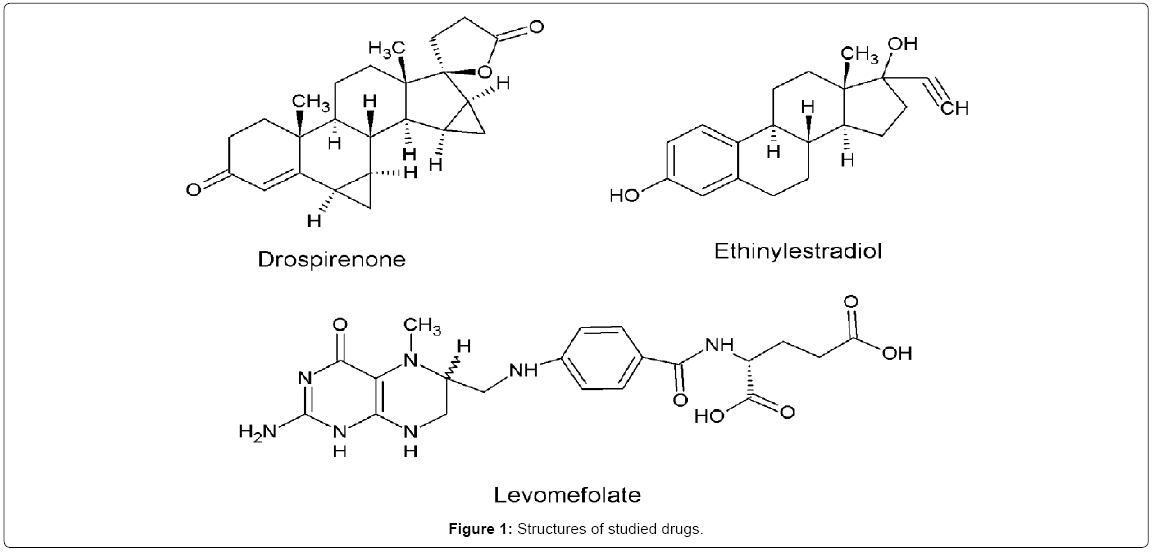
Drospirenone is a synthetic progestogen, progestin, with antimineralocorticoid and progestational activity [1]. Drospirenone is used in menopausal hormone therapy, treatment of premenstrual dysphoric disorder and acne [2-4]. Drospirenone is also an important ingredient in most of the oral contraceptive pills. Chemically, drospirenone is described as (6R,7R,8R,9S,10R,13S,14S,15S,16S,17S)-1,3',4',6,6a,7,8,9 ,10,11,12,13,14,15,15a,16-Hexadecahydro-10,13-dimetylspiro-[17Hdicyclopropa[ 6,7:15,16]cyclopenta[a]phenantrene-17,2'(5'H)-furan]- 3,5'(2H)-dione (Figure 1). Drospirenone exerts its activity through binding strongly and specifically to progesterone receptor [5,6]. Drospirenone-progesterone receptor complex produces an activated complex which binds to specific sites in DNA. As the result, luteinizing hormone activity is suppressed and ovulation is inhibited. This activated complex also changes the cervical membrane and endometrium.
Ethinyl estradiol, semisynthetic estrogen, is an estrogen receptor agonist [7]. Chemically, ethinyl estradiol is known as (8R,9S,13S,14S,17R)-17-ethynyl-13-methyl-7,8,9,11,12,14,15,16- octahydro-6H-cyclopenta[a]phenanthrene-3,17-diol (Figure 1). Ethinyl estradiol, alone is used in post menopausal hormonal replacement therapy, and to treat female hypogonadism, and symptoms of breast cancer and prostate cancer. Ethinyl estradiol is also used as oral contraceptive in combination with progestin [8,9]. The complex formed through the binding of ethinyl estradiol to estrogen receptor increases the transcription of genes which are responsible for estrogenic cellular responses [10]. By inhibiting 5-α-reductase enzyme, ethinyl estradiol lessens testosterone levels and disrupts the prostatic cancer progression [11].
Levomefolate, chemically known as (2S)-2-[[4-[(2-Amino-5- methyl-4-oxo-1,6,7,8-tetrahydropteridin-6-yl)methylamino]benzoyl] amino]pentanedioic acid (Figure 1), is an biologically active of vitamin B9 (folic acid) [12]. Levomefolate plays an important role in synthesis of DNA, cysteine cycle and regulation of metabolism of homocysteine. Levomefolic acid is prescribed for patients with symptoms of vitamin B12 deficiency [13]. Levomefolate is also been used for treating patients with cardiovascular disease and cancers of breast and colorectal [14,15].
Drospirenone, ethinyl estradiol and levomefolate combination is available in oral contraception tablet dosage form with brand names Safyral, Beyaz and Rajani [16-19]. In this combination, drospirenone and ethinyl estradiol prevent pregnancy by repressing ovulation. These two drugs also make changes in cervical mucus and endometrial which inhibits penetration of sperm and lessen the implantation, respectively. Levomefolate in the tablet increases the levels of folate levels in women who opt oral contraceptive [20].
To the best of our knowledge, there is no report for the simultaneous determination of drospirenone, ethinyl estradiol and levomefolate in bulk and combined tablet dosage form by stability indicating reverse phase high performance liquid chromatographic (RP-HPLC) method. Therefore, the main aim of this investigation was to develop and validate a stability indicating RP-HPLC method to determine drospirenone, ethinyl estradiol and levomefolate simultaneously in the presence of their stress degradation products [21-23].
Experimental
Materials
Drospirenone, ethinyl estradiol and levomefolate reference drug standards were provided kindly by Rainbow Pharma Training Labs (Hyderabad, India). Safyral (Bayer Health Care Pharmaceuticals Inc. Whippany, NJ) tablets labeled to contain 3 mg drospirenone, 0.03 mg ethinyl estradiol and 0.451 mg levomefolate were obtained from a local pharmacy market. HPLC grade acetonitrile and methanol were purchased from Merck India Ltd (Mumbai, India). Analytical reagent orthophosphoric acid, hydrogen peroxide, hydrochloric acid and sodium hydroxide were supplied by Sd. Fine Chemicals Ltd (Mumbai, India). HPLC grade water was prepared using Milli-Q system (Millipore, USA).
Instrumentation
Waters Alliance HPLC system 2695 Module with a 2998 PDA detector, degasser, auto sample injector and column oven were used in the present analysis. Data acquisition and processing was done with Empower 2 software. Method development and validation was done using Waters, C18, 5 μm, 250 mm × 4.6 mm analytical column.
Optimized HPLC conditions
Isocratic elution was performed with a mobile phase comprised of filtered (using a 0.45 μm membrane filter) and degassed 0.1% orthophosphoric acid: methanol: acetonitrile (60:20:20 v/v/v) adjusted to pH 4.8 and pumped at a flow rate of 1.0 mL/min. The column temperature was set at 27°C. The samples were injected at 10 μL injection volume and eluted samples were analyzed at a wavelength of 245 nm. The total runtime was 8 min.
Standard stock and working solutions
The standard stock solution (Drospirenone–1200 μg/mL, Ethinyl estradiol–12 μg/mL and Levomefolate–180.4 μg/mL) was prepared by dissolving an accurately weighed 30 mg, 0.30 mg and 4.51 mg of drospirenone, ethinyl estradiol and levomefolate, reference standard respectively in 25 mL of mobile phase in a volumetric flask (25 mL). The working standard solutions in the range 30–240 μg/mL of drospirenone, 0.3–2.4 μg/mL of ethinyl estradiol and 4.51–36.08 μg/mL of levomefolate were obtained by appropriately diluting the standard stock solution with mobile phase.
Construction of calibration curve
Aliquots (10 μL) of working standard solutions were injected into the HPLC system and eluted by the mobile phase under the optimum HPLC conditions. The peak area response of drug versus the final concentration of drug (μg/mL) was plotted. On the other hand, the corresponding regression equations were derived.
Analysis of drospirenone, ethinyl estradiol and levomefolate in tablet sample solution
Ten tablets were crushed into powder. The tablet powder weight equivalent to 30 mg, 0.30 mg and 4.51 mg of drospirenone, ethinyl estradiol and levomefolate, respectively was transferred to 25 mL volumetric flask and sonicated with 10 mL of mobile phase for 20 min. The volume was diluted to 25 mL with mobile phase and filtered through 0.45 μm membrane filter. The stock tablet sample solution was then diluted aptly with mobile phase to get the final concentration 120 μg/mL, 1.2 μg/mL and 18.04 μg/mL of drospirenone, ethinyl estradiol and levomefolate, respectively. 10 μL of working tablet sample solution prepared was injected into the HPLC system and analyzed by the developed method. The nominal content of drospirenone, ethinyl estradiol and levomefolate in the tablet was calculated either using the corresponding calibration graph or corresponding regression equation.
Stress degradation studies
The stress degradation studies were performed through the analysis of tablet sample solution (drospirenone-120 μg/mL, ethinyl estradiol-1.2 μg/mL and levomefolate -18.04 μg/mL), which was exposed to accelerated degradation conditions as per the ICH guidelines [21]. The results are compared to a reference standard solution prepared in the same day.
Acid and alkaline hydrolysis
Volumetric flasks (100 mL) containing 10 mL of tablet sample solution (drospirenone–1200 μg/mL, ethinyl estradiol–12 μg/mL and levomefolate–180.4 μg/mL) were mixed with 10 mL of 0.1 N HCl solution for acidic degradation acid or 10 mL of 0.1 N NaOH solution for alkaline degradation. The solutions were sonicated at room temperature for 30 min. After this period, the acid and alkali degraded solutions were neutralized with apt volume of 0.1 N NaOH and 0.1N HCl, respectively. The resulting solutions were diluted with mobile phase to get a concentration of 120 μg/mL, 1.2 μg/mL and 18.04 μg/ mL drospirenone, ethinyl estradiol and levomefolate, respectively. The solutions were filtered and injected.
Thermal and photo degradation
10 mL of tablet sample solution (drospirenone–1200 μg/mL, ethinyl estradiol–12 μg/mL and levomefolate–180.4 μg/mL) was transferred to volumetric flask (100 mL) and exposed to 105°C for 30 min in oven (for thermal degradation) or exposed to sun light for 24 hr (for photo degradation). After the specified period of degradation, the resulting solution was diluted with mobile phase for a concentration of 120 μg/mL, 1.2 μg/mL and 18.04 μg/mL drospirenone, ethinyl estradiol and levomefolate, respectively. The solutions were filtered and injected.
Oxidative and neutral degradation
10 mL of 30% hydrogen peroxide solution (for oxidative degradation) or 10 mL of deionised water (for neutral degradation) was added into a 100 mL volumetric flask containing 10 mL tablet sample solution (drospirenone–1200 μg/mL, ethinyl estradiol–12 μg/ mL and levomefolate–180.4 μg/mL). After sonication for 30 min at room temperature, the solutions were diluted to 100 mL with mobile phase until a concentration 120 μg/mL, 1.2 μg/mL and 18.04 μg/mL of drospirenone, ethinyl estradiol and levomefolate, respectively was obtained. These solutions were filtered and injected.
Results and Discussion
Optimization of HPLC conditions
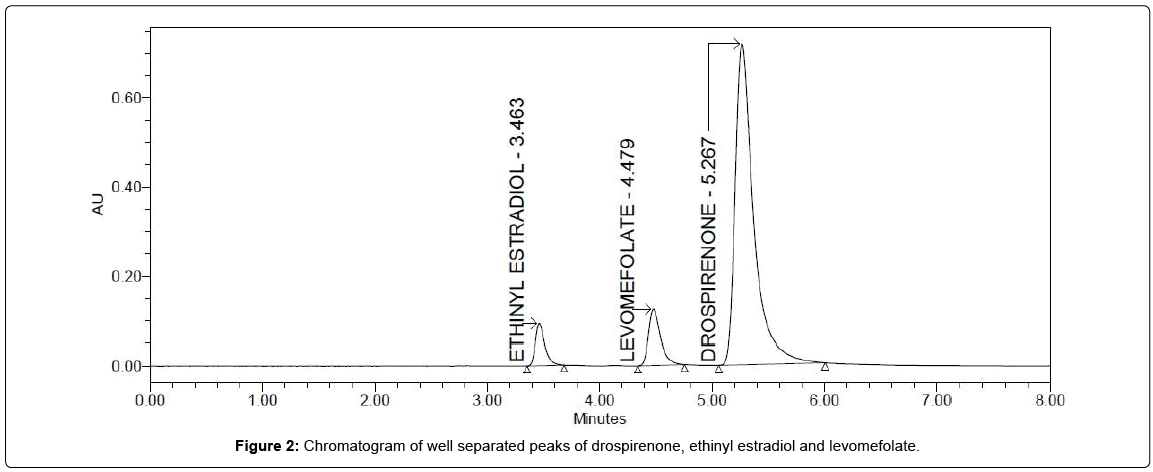
Preliminary studies involved testing different mobile phase compositions, pH, flow rates, temperatures and detection wavelength for the effective separation and simultaneous analysis of drospirenone, ethinyl estradiol and levomefolate. Trial experiments were conducted using a Waters C18 column with a length of 250 mm, internal diameter of 4.6 mm and particle size of 5 μm as the stationary phase. Different proportions of 0.1% orthophosphoric acid, acetonitrile and methanol with different pH units in isocratic elution mode were investigated to obtain optimum resolution, symmetric peak shape and optimal sensitivity in reasonable time. Best results were obtained with a mixture of 0.1% orthophosphoric acid, acetonitrile and methanol in the ratio of 60:20:20 (v/v/v) with pH 4.8 units was employed as the mobile phase. The flow rate of mobile phase, for improved resolution and quick separation, was adjusted to 1.0 mL/min. Room temperature was adequate for the separation and analysis of selected drug combination and so the same was used in the whole separation and analysis. Detection at 245 nm was used as it was observed as the optimum detection wavelength for the three analytes (drospirenone, ethinyl estradiol and levomefolate). At this detection wavelength (245 nm), the peak area response for the three analytes was high. Representative chromatogram of the finalized chromatographic conditions, showing drospirenone, ethinyl estradiol and levomefolate, is illustrated in Figure 2.
Method validation
The method was validated following ICH and FDA guidelines for system suitability, selectivity, specificity, linearity, sensitivity, accuracy, precision and robustness [22,23].
System suitability
System suitability parameters like peak tailing, plate count, resolution, and percent relative standard deviation for retention time and peak area response were calculated to demonstrate that the HPLC system performed well. For this study, standard solution (drospirenone-120 μg/mL, ethinyl estradiol-1.2 μg/mL and levomefolate-18.04 μg/mL) was injected into the HPLC system in five replicates. The obtained values were in the acceptable limits as given in Table 1.
| Drug Parameter |
Ethinyl estradiol | Levomefolate | Drospirenone | Recommended limit | |||
|---|---|---|---|---|---|---|---|
| Value* | RSD (%) | Value* | RSD (%) | Value* | RSD (%) | ||
| RT** (min) | 3.472 | 0.232 | 4.485 | 0.169 | 5.268 | 0.175 | RSD ≤ 2 |
| Peak area (mAU) | 548342 | 0.439 | 924440 | 0.506 | 8225662 | 0.437 | RSD ≤ 2 |
| Plate Count | 8501 | 0.645 | 9101 | 0.353 | 5773 | 0.948 | >2000 |
| Peak Tailing | 1.414 | 0.387 | 1.436 | 0.381 | 1.756 | 0.312 | ≤ 2 |
| Resolution | - | - | 5.794 | 0.262 | 3.238 | 0.594 | >1.5 |
*Average of five determinations; **Retention time
Table 1: System suitability parameters of the method for drospirenone, ethinyl estradiol and levomefolate analysis.
Selectivity
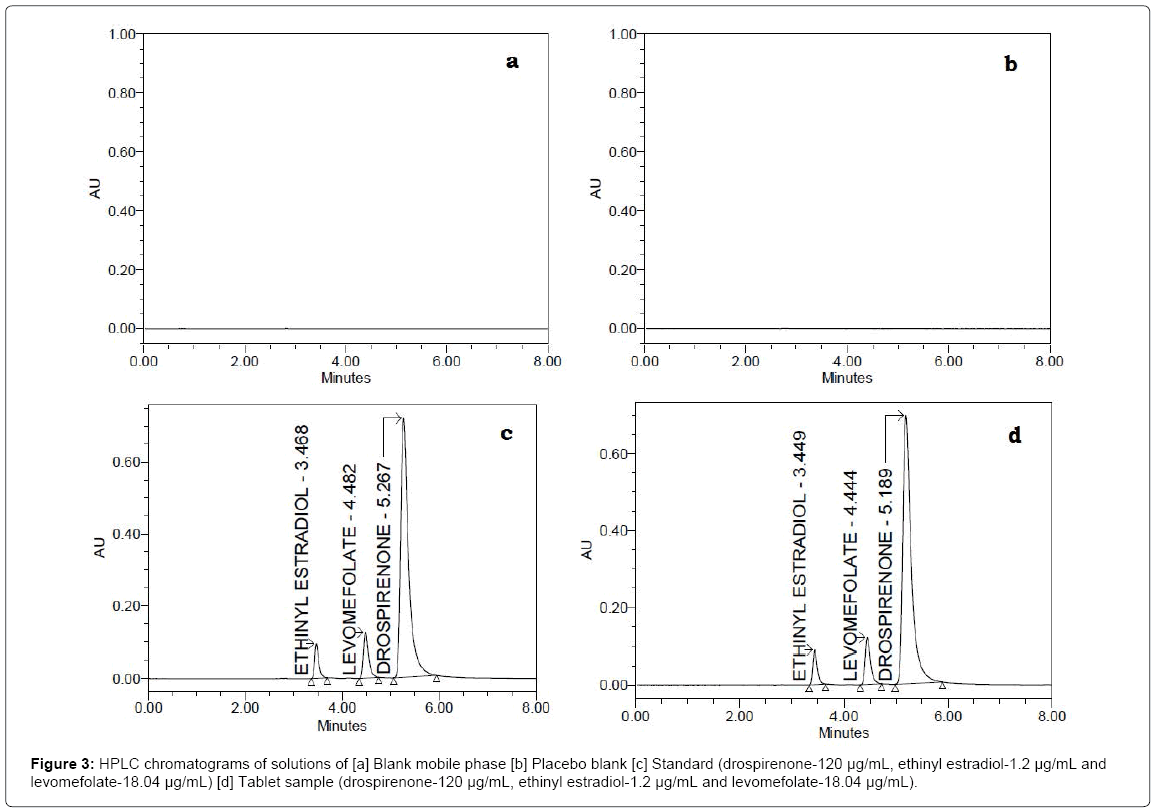
The selectivity of the method was evaluated by comparison of chromatograms of blank mobile phase, placebo blank (mixture of excipients), tablet sample solution with standard solution. The representative chromatograms of the four samples are shown in Figure 3a-3d. The chromatograms of blank mobile phase (Figure 3a) and placebo (Figure 3b) did not show a response at the retention times of three analytes. Interfering peaks are not found in the chromatogram of the tablet sample (Figure 3d), demonstrating that excipients used in the tablets did not interfere with the peaks of drospirenone, ethinyl estradiol and levomefolate. This proved the method selectivity.
Specificity
Stress degradation was done to demonstrate the method specificity, stability of the drugs, detect the possible degradation products and stability indicating properties of the developed method. Stress degradation was carried out by exposing tablet sample solution to stress conditions of hydrolysis (acid, alkali and neutral), oxidation, photo and thermal. Stressed samples were analyzed by the proposed method. The corresponding peaks were checked for the peaks interference, retention times, and peak purity. The percentage of degradation in all the stressed samples was also determined.
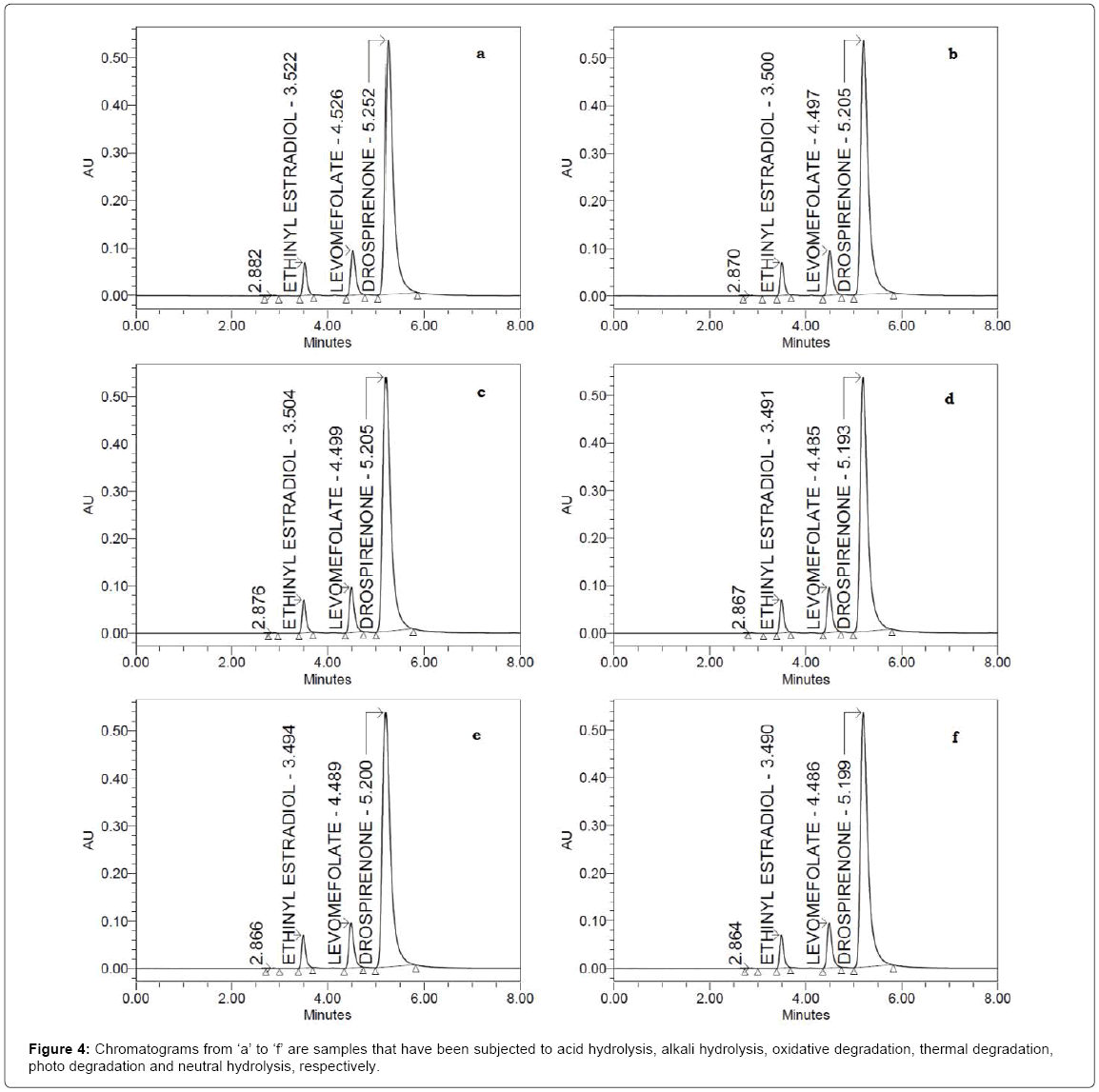
The chromatograms of acid, base, hydrogen peroxide, heat, sun light and water degraded tablet sample solution are shown in Figure 4a-4e. The chromatograms showed no interference between the peaks of studied drugs (levomefolate, ethinyl estradiol, drospirenone) and the degradation product produced in the applied stress conditions. The percentage of recovery and degradation results of the forced degradation studies are summarized in Table 2. The applied stress conditions were enough to degrade the three drugs. The percent degradation value comparison of the three drugs showed that the order of stability is: levomefolate>ethinyl estradiol>drospirenone. The degradation product was observed at retention times of 2.882 min (acid hydrolysis, Figure 4a), 2.870 min (base hydrolysis, Figure 4b), 2.876 min (oxidative degradation, Figure 4c), 2.867 min (thermal degradation, Figure 4d), 2.866 min (photo degradation, Figure 4e) and 2.864 min (neutral hydrolysis, Figure 4f). The peak of degradation product is well resolved from the analytes peaks using the proposed method. The homogeneity of the peaks of studied drugs was checked using photodiode array detector. The results were shown in Table 2. The increased peak threshold value than peak purity angle value (Table 2) confirmed the purity and homogeneity of levomefolate, ethinyl estradiol, drospirenone peaks in all the stress conditions applied. The results of stress degradation studies proved the specificity and stability indicating properties of the developed HPLC method.
| Analyte | Degradation condition | Peak area (mAU) | Percent of drug | Peak purity | ||
|---|---|---|---|---|---|---|
| Recovered (%) | Degraded (%) | Purity angle | Purity threshold | |||
| Ethinyl Estradiol | Undegraded | 548342 | 99.60 | - | - | - |
| Acidic | 477576 | 86.75 | 13.25 | 0.344 | 0.959 | |
| Basic | 472362 | 85.80 | 14.20 | 0.300 | 0.871 | |
| Oxidative | 463459 | 84.18 | 15.82 | 0.355 | 0.860 | |
| Thermal | 482790 | 87.69 | 12.31 | 0.388 | 0.764 | |
| Photo | 494754 | 89.87 | 10.13 | 0.341 | 0.861 | |
| Neutral | 493323 | 89.61 | 10.39 | 0.379 | 0.863 | |
| Levomefolate | Undegraded | 924439.7 | 99.50 | - | - | - |
| Acidic | 829896 | 89.32 | 10.68 | 0.249 | 0.800 | |
| Basic | 836466 | 90.03 | 9.97 | 0.422 | 0.904 | |
| Oxidative | 833293 | 89.69 | 10.31 | 0.382 | 0.693 | |
| Thermal | 824163 | 88.71 | 11.29 | 0.298 | 0.596 | |
| Photo | 822654 | 88.54 | 11.46 | 0.378 | 0.694 | |
| Neutral | 831421 | 89.49 | 10.51 | 0.320 | 0.699 | |
| Drospirenone | Undegraded | 8225662 | 99.60 | - | - | - |
| Acidic | 6980365 | 84.52 | 15.48 | 0.269 | 0.883 | |
| Basic | 7193782 | 87.11 | 12.89 | 0.231 | 0.681 | |
| Oxidative | 6880465 | 83.31 | 16.69 | 0.239 | 0.578 | |
| Thermal | 6730682 | 81.5 | 18.50 | 0.241 | 0.579 | |
| Photo | 7246081 | 87.74 | 12.26 | 0.236 | 0.579 | |
| Neutral | 7323993 | 88.68 | 11.32 | 0.243 | 0.580 | |
Table 2: Stress degradation results of drospirenone, ethinyl estradiol and levomefolate in tablet sample solution.
Linearity
The calibration curve was constructed by plotting the peak area response (mAU) of drug against the concentration (μg/mL). Calibration curve was linear over a range of concentration 0.3-2.4 μg/ mL (ethinyl estradiol), 4.51-36.08 μg/mL (levomefolate) and 30-240 μg/mL (drospirenone). Linear regression equation and regression coefficient (R2) were:
y=45651 x+670.3 and 0.9996, respectively for ethinyl estradiol.
y=51146 x+673.7 and 0.9999, respectively for levomefolate
y=68231 x+10671 and 0.9998, respectively for drospirenone
where ‘y’ is peak area response (mAU) and ‘x’ is concentration of drug (μg/mL). The results showed excellent correlation exists between the peak area response and concentration.
Sensitivity
The method sensitivity was determined with respect to limit of detection (LOD) and limit of quantitation (LOQ). The LOD and LOQ were assessed at a signal-to-noise ratio of 3:1 and 10:1, respectively using the developed method by analyzing different dilute solutions of drospirenone, ethinyl estradiol and levomefolate. The determined LOD values are 0.010 μg/mL, 0.109 μg/mL and 0.126 μg/mL for ethinyl estradiol, levomefolate and drospirenone, respectively. The determined LOQ values are 0.032 μg/mL, 0.363 μg/mL and 0.420 μg/mL for ethinyl estradiol, levomefolate and drospirenone, respectively. The values showed that the sensitivity of the method was good.
Precision
The precision of the HPLC method for drospirenone, ethinyl estradiol and levomefolate was evaluated by analyzing standard solution (drospirenone-120 μg/mL, ethinyl estradiol-1.2 μg/mL and levomefolate-18.04 μg/mL) six times. Percentage relative standard deviation (%RSD) of peak area response of the studied drugs was used to assess the precision. The results of precision exhibited %RSD below 0.5% (Table 3), indicating the excellent precision of the method.
| Injection No. | Peak area response of drug (mAU) | ||
|---|---|---|---|
| Ethinyl estradiol | Levomefolate | Drospirenone | |
| 1 | 548672 | 924625 | 8223258 |
| 2 | 548654 | 924095 | 8227782 |
| 3 | 548533 | 924445 | 8229372 |
| 4 | 548370 | 924665 | 8220001 |
| 5 | 548775 | 924330 | 8222567 |
| 6 | 548357 | 924462 | 8226070 |
| Mean | 548560 | 924437 | 8224842 |
| % RSD | 0.031 | 0.023 | 0.043 |
Table 3: Precision of the method for drospirenone, ethinyl estradiol and levomefolate.
Accuracy
The method accuracy for drospirenone, ethinyl estradiol and levomefolate was determined by analyzing standard solution (drospirenone-120 μg/mL, ethinyl estradiol-1.2 μg/mL and levomefolate-18.04 μg/mL) six times. The accuracy of the results was demonstrated by calculating the percent recovery. The results showed good accuracy performance for the determination of the three analytes (Table 4).
| Ethinyl estradiol | Levomefolate | Drospirenone | ||||||
|---|---|---|---|---|---|---|---|---|
| Concentration (μg/mL) | Recovery (%) | Concentration (μg/mL) | Recovery (%) | Concentration (μg/mL) | Recovery (%) | |||
| Taken | Found | Taken | Found | Taken | Found | |||
| 1.2 | 1.196 | 99.66 | 18.04 | 17.953 | 99.52 | 120 | 119.484 | 99.57 |
| 1.2 | 1.196 | 99.66 | 18.04 | 17.943 | 99.46 | 120 | 119.556 | 99.63 |
| 1.2 | 1.196 | 99.63 | 18.04 | 17.950 | 99.50 | 120 | 119.568 | 99.64 |
| 1.2 | 1.195 | 99.61 | 18.04 | 17.953 | 99.52 | 120 | 119.436 | 99.53 |
| 1.2 | 1.196 | 99.68 | 18.04 | 17.948 | 99.49 | 120 | 119.472 | 99.56 |
| 1.2 | 1.195 | 99.60 | 18.04 | 17.950 | 99.50 | 120 | 119.520 | 99.60 |
| Mean | 1.196 | 99.64 | Mean | 17.949 | 99.50 | Mean | 119.506 | 99.59 |
| RSD (%) | 0.032 | 0.031 | RSD (%) | 0.022 | 0.023 | RSD (%) | 0.043 | 0.043 |
Table 4: Accuracy of the method for drospirenone, ethinyl estradiol and levomefolate.
Recovery
The newly developed HPLC method was further evaluated for its accuracy by the analysis of the placebo spiked with pure drospirenone, ethinyl estradiol and levomefolate at three different concentration levels. Recovery of the spiked drospirenone, ethinyl estradiol and levomefolate was determined by the proposed method three times. The recovery values (Table 5), indicating that the developed method ensure the acquisition of reliable accurate data for drospirenone, ethinyl estradiol and levomefolate at different concentrations.
| Spiked level (%) | Concentration of drug (µg/mL) | Recovered (%) | RSD (%) | |
|---|---|---|---|---|
| Spiked | Found* | |||
| Ethinyl estradiol | ||||
| 50 | 0.60 | 0.599 | 99.803 | 0.067 |
| 100 | 1.20 | 1.196 | 99.66 | 0.067 |
| 150 | 1.80 | 1.792 | 99.57 | 0.038 |
| Levomefolate | ||||
| 50 | 8.93 | 8.893 | 100.61 | 0.109 |
| 100 | 17.86 | 17.949 | 100.50 | 0.010 |
| 150 | 26.79 | 26.903 | 100.56 | 0.119 |
| Drospirenone | ||||
| 50 | 60 | 59.767 | 99.62 | 0.075 |
| 100 | 120 | 119.477 | 99.56 | 0.041 |
| 150 | 180 | 179.873 | 99.93 | 0.061 |
*Average of three determinations
Table 5: Recovery of drospirenone, ethinyl estradiol and levomefolate by the proposed method.
Robustness
Method robustness was established by deliberately varying the experimental conditions such as flow rate (± 0.1 mL/min), column oven temperature (± 2°C), mobile phase components ratio (± 5%), pH of mobile phase (±0.2 units) and detection wavelength (±2 nm). The study was carried out on the same day with standard solution of concentration 120 μg/mL of drospirenone, 1.2 μg/mL of ethinyl estradiol and 18.04 μg/mL of levomefolate. In each case, resolution, plate count and peak tailing were calculated. The calculated values were within the acceptance limits (Table 6). Therefore, the method is considered as robust.
| Parameter Investigated | Ethinyl estradiol | Levomefolate | Drospirenone | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Plate count | Peak Tailing | Resolution | Plate count | Peak Tailing | Resolution | Plate count | Peak Tailing | Resolution | |
| Flow rate-0.9 mL/min | 7966 | 1.38 | - | 8722 | 1.37 | 5.54 | 5564 | 1.70 | 2.89 |
| Flow rate-1.1 mL/min | 9778 | 1.39 | - | 10273 | 1.38 | 5.99 | 6344 | 1.79 | 3.00 |
| Column temperature-25°C | 8054 | 1.37 | - | 8658 | 1.38 | 5.58 | 5480 | 1.69 | 2.93 |
| Column temperature-29°C | 9653 | 1.39 | - | 10143 | 1.38 | 5.98 | 6426 | 1.77 | 3.00 |
| Mobile phase ratio (0.1% H3PO4: methanol: acetonitrile) –60:25:15 v/v/v | 8547 | 1.40 | - | 9155 | 1.42 | 5.75 | 5752 | 1.76 | 3.09 |
| Mobile phase ratio (0.1 M NaH2PO4: methanol: acetonitrile) - 60:155:25 v/v/v | 8588 | 1.39 | - | 9318 | 1.40 | 5.77 | 5827 | 1.74 | 3.03 |
| Mobile phase pH–4.6 | 8501 | 1.40 | - | 9177 | 1.44 | 5.79 | 5849 | 1.76 | 3.20 |
| Mobile phase pH–5.0 | 8516 | 1.41 | - | 9122 | 1.43 | 5.77 | 5799 | 1.75 | 3.19 |
| Detection wavelength–243 nm | 8496 | 1.41 | - | 8961 | 1.42 | 5.83 | 5715 | 1.77 | 3.24 |
| Detection wavelength–247 nm | 8412 | 1.39 | - | 9084 | 1.43 | 5.78 | 5789 | 1.73 | 3.24 |
Table 6: Robustness of the method.
Conclusion
For the first time, a stability indicating HPLC with photodiode array detector method has been developed and validated for the simultaneous assay of drospirenone, ethinyl estradiol and levomefolate in bulk and tablet dosage form. All validation parameters satisfied the acceptance criteria of the ICH guideline. The developed method is good enough to separate the peaks of drospirenone, ethinyl estradiol and levomefolate from the degradation products produced during stress degradation. Therefore, it was concluded the developed and validated stability indicating method can be employed for the routine estimation of drospirenone, ethinyl estradiol and levomefolate in quality control laboratories and for stability studies.
Acknowledgements
The author would like to thank Bharathiar University (Coimbatore, Tamil Nadu, India), Sacred Heart College (Autonomous) (Tirupattur, Tamil Nadu, India) and Rainbow Pharma Training Lab (Hyderabad, Telangana, India).
References
- Rapkin AJ, Winer SA (2007) Drospirenone: a novel progestin. Expert Opin Pharmacother 8: 989-999.
- Breech LL, Braverman PK (2009) Safety, efficacy, actions, and patient acceptability of drospirenone/ethinyl estradiol contraceptive pills in the treatment of premenstrual dysphoric disorder. Int J Womens Health 1: 85.
- Lopez LM, Kaptein AA, Helmerhorst FM (2009) Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev 1: 2.
- Arowojolu AO, Gallo MF, Lopez LM, Grimes DA (2012) Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev 6: CD004425.
- Krattenmacher R (2000) Drospirenone: pharmacology and pharmacokinetics of a unique progestogen. Contraception 62: 29-38.
- Sitruk-Ware R (2005) Pharmacology of different progestogens: the special case of drospirenone. Climacteric 8: 4-12.
- Elks J (2014) The dictionary of drugs: Chemical data: Chemical data, structures and bibliographies. Springer, p: 522.
- Sood R, Faubion SS, Kuhle CL, Thielen JM, Shuster LT (2014) Prescribing menopausal hormone therapy: an evidence-based approach. International Journal of Women's Health 6: 47.
- Stanczyk FZ, Archer DF, Bhavnani BR (2013) Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception 87: 706-727.
- Ciesiółka S, Budna J, Jopek K, Bryja A, Kranc W, et al. (2016) Influence of Estradiol-17beta on Progesterone and Estrogen Receptor mRNA Expression in Porcine Follicular Granulosa Cells during Short-Term, In Vitro Real-Time Cell Proliferation. BioMed Research International, Article ID: 8431018.
- Montgomery B, Nelson PS, Vessella R, Kalhorn T, Hess D, et al. (2010) Estradiol suppresses tissue androgens and prostate cancer growth in castration resistant prostate cancer. BMC Cancer 10: 244.
- Pietrzik K, Bailey L, Shane B (2010) Folic acid and L-5-methyltetrahydrofolate. Clinical Pharmacokinetics 49: 535-548.
- Miraglia N, Agostinetto M, Bianchi D, Valoti E (2016) Enhanced oral bioavailability of a novel folate salt: comparison with folic acid and a calcium folate salt in a pharmacokinetic study in rats. Minerva Ginecologica 68: 99-105.
- Czeizel AE, Dudás I, Vereczkey A, Bánhidy F (2013) Folate deficiency and folic acid supplementation: the prevention of neural-tube defects and congenital heart defects. Nutrients 5: 4760-4775.
- Rapkin RB, Creinin MD (2011) The combined oral contraceptive pill containing drospirenone and ethinyl estradiol plus levomefolate calcium. Expert Opinion on Pharmacotherapy 12: 2403-2410.
- Fruzzetti F (2012) Beyaz®: an oral contraceptive fortified with folate. Women’s Health 8: 13-19.
- Nelson AL (2012) Comprehensive evaluation of Safyral® 2012. Women’s Health 8: 619-633.
- PubMed Health. US National Library of Medicine. Available at: https://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0010056/
- Diefenbach K, Trummer D, Ebert F, Lissy M, Koch M, et al. (2013) EE-drospirenone-levomefolate calcium versus EE-drospirenone+folic acid: folate status during 24 weeks of treatment and over 20 weeks following treatment cessation. International Journal of Women's Health 5: 149.
- International Conference on Harmonization (ICH) of technical requirements for the registration of pharmaceutical for human use stability testing of new drugs substance and products Q1A (R2) (2003).
- US Food and Drug Administration (2015) Guidance for Industry. Analytical Procedures and Methods Validation for Drugs and Biologics. US Food and Drug Administration. Silver Spring, MD, USA.
- International Conference on Harmonization (ICH) Guidelines (2005) Validation of analytical procedures technical requirements for registration of pharmaceuticals for human use: Text and Methodology Q2 (R1), International Conference on Harmonization, Geneva, Switzerland.
Citation: Chandran S, Xavier Rajarathinam SR, Kalaiselvan A (2018) Simultaneous Quantification of Drospirenone, Ethinyl Estradiol and Levomefolate by Stability Indicating RP-HPLC Method. J Anal Bioanal Tech 9:408. DOI: 10.4172/2155-9872.1000408
Copyright: © 2018 Chandran S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 7186
- [From(publication date): 0-2018 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 6131
- PDF downloads: 1055