Soluble TNF Receptors are Modulated by Vitamin D Status but not by Acute Perturbations in 25-Hydroxyvitamin D Following A Bolus of Supplemental Vitamin D
Received: 19-Aug-2017 / Accepted Date: 24-Aug-2017 / Published Date: 31-Aug-2017 DOI: 10.4172/2576-3881.1000118
Abstract
The first objective of this study was to identify if soluble tumor necrosis factor-receptor 1 (sTNFr1) and -receptor 2 (sTNFr2) are modulated by vitamin D status (insufficient vs. sufficient). The second objective was to reveal if soluble TNF receptors fluctuate with serum 25-hydroxyvitamin D (25(OH)D) concentrations following a bolus of supplemental vitamin D. Reportedly healthy male adults were randomly (double-blind) assigned to a placebo (n=15) or vitamin D (100,000 IU of cholecalciferol; n=14) supplement. Supplements were taken as a bolus immediately after and on the same day as providing the first blood sample (baseline (Bsl)). Fasting blood samples were also obtained at 1-, 3-, 7-, and 24-d after the bolus. Serum 25(OH)D, 1,25-dihyroxyvitamin D (1,25(OH)D), tumor necrosis factor (TNF)-α, sTNFr1, and sTNFr2 were measured in each blood sample. At Bsl, subjects were classified as vitamin D insufficient (serum 25(OH)D
Keywords: TNF-α; Soluble TNF receptors; Vitamin D3
6918Introduction
Tumor necrosis factor (TNF)-α is a quintessential pro-inflammatory cytokine produced from immune and non-immune cells in response to diverse stimuli. Low levels of TNF-α expression is important for tissue remodeling and host defense responses, but excessive production over activates inflammatory responses and leads to tissue damage. Intracellular signals regulating the biological events governed by TNF- α are mediated through a 55 kDa TNF receptor 1 (TNFr1) and a 75 kDa TNF receptor 2 (TNFr2); both of which, are type I transmembrane glycoproteins and members of the TNF receptor family.
TNFr1 and TNFr2 are susceptible to proteolytic cleavage and alternative mRNA splicing. As a result, their soluble forms (sTNFr1 and sTNFr2) are commonly found in the circulation [1] and offer a more stable measure of long-term exposure to TNF-α [2,3]. Circulating soluble TNF receptor concentrations increase with disease [4] and could antagonize TNF-α, and subsequently, ameliorate TNF-α induced activities [2,5-7]. Conversely, soluble TNF receptor could preserve TNF-α activity, prolong the systemic half-life, and serve as a reservoir for TNF-α [2,6,8,9]. These empirical results establish the physiological importance of soluble TNF receptors to govern the inflammatory-driven properties induced by TNF-α, which provocatively, are modulated by vitamin D.
Vitamin D regulates inflammatory cytokines and is routinely assessed by serum 25-hydroxyvitamin D (25(OH)D) concentrations. Despite discrepancies [10-12], an increase in circulating 25(OH)D generally associates with a decrease in circulating TNF-α and TNF-α production [13-19]. Likewise, in isolated immune cells from patients with various diseases, 25(OH)D and 1,25-dihydroxyvitamin D (1,25(OH)D), the most biological active vitamin D metabolite, dose dependently inhibited TNF-α production [20-28]. Vitamin D also neutralizes pro-inflammatory events derived from TNF-α [29-31], collectively demonstrating its ability to regulate TNF-α levels and its biological properties. Few studies, however, report the influence of 25(OH)D on soluble TNF receptors. From sparse data, results suggest that vitamin D (i.e., 1,25(OH)D) suppressed the biological properties of TNF-α by facilitating the ectodomain shedding of TNFr1, and as a by-product, increased sTNFr1 in the culture medium [32]. In elderly, increases in sTNFr1 and sTNFr2 associated with an increase in 25(OH)D and a decrease in 1,25(OH)D [33]. Unfortunately, results are inconsistent as data from elderly men and women also demonstrate a decrease in sTNFr1 and sTNFr2 with an increase in serum 25(OH)D above a vitamin D-deficient demarcation (≥ 21.3 ng/mL) [34]. The inconsistency in the sparse literature is compounded by the void in our knowledge regarding the ability of supplemental vitamin D to modulate soluble TNF receptors concomitantly with serum 25(OH)D.
Based on the aforementioned inconsistencies and gap in our knowledge, the purpose of this investigation was two-fold. The first objective was to identify if sTNFr1 and sTNFr2 are modulated by vitamin D status prior to vitamin D supplementation. As the second objective, we sought to identify if soluble TNF receptors fluctuate following a large bolus of supplemental vitamin D that induces an abrupt increase in serum 25(OH)D and 1,25(OH)D. We hypothesized that vitamin D sufficiency and an increase in serum 25(OH)D following a bolus of supplemental vitamin D associate with an increase sTNFr1 and sTNFr2 concentrations. Addressing this hypothesis is important because it will advance our knowledge regarding the capacity of vitamin D to regulate soluble cytokine receptors that are instrumental to pro-inflammatory biology and immune system signaling.
Materials and Methods
Subjects
Reportedly healthy and modestly active (i.e., 30 min of continuous physical activity at least 3 times per week) males between 18 and 50 years of age were recruited to participate in this study (n=29, age, 33 (8) y; height, 178 (7) cm; body mass, 84.6 (11.8) kg; body mass index (BMI), 26.6 (3.3) kg/m2). Potential subjects were excluded from participation if they had a known history of metabolic bone disease, any skeletal muscle pathologies, cardiac or peripheral cardiovascular system abnormalities, clotting disorders, coronary artery disease, peripheral vascular disease, stroke, cancer, high cholesterol or triglycerides, high blood pressure, hypercalcemia or parathyroid dysfunction, iron deficiency within the past year, or impaired liver or kidney function. Potential subjects were also excluded from study participation if they had a known family history or diagnosis of chronic granulomatous disease, taking digoxin or antiarrhymic medications, warfarin, anti-coagulants, cholesterol lowering medication, diagnosed with diabetes mellitus, taking a daily dietary supplement, treated for vitamin D deficiency during the past year, using corticosteroid medication, planning on increasing or decreasing the amount of time spent in the sun or tanning bed, traveling south of 37ºN in latitude during study participation, or morbidly obese (BMI >40 kg/m2). The Central Region Institutional Review Board at Intermountain Healthcare (Salt Lake City, UT USA) approved this study. Subjects were informed of and provided written and verbal consent to the study protocol and procedures.
Study design and protocol
This study consisted of a randomized, double-blind, placebo controlled design. Subjects were randomly assigned to a placebo (PL; n=15) or vitamin D (VD; cholecalciferol at 100,000 IU; n=14) supplement group. Supplements were taken as a bolus immediately after and on the same day as providing the first blood sample (i.e., Baseline; Bsl). Tablets were taken under the supervision of the investigators. USANA Health Sciences Inc. (Salt Lake City, UT USA) donated and provided a quality control analysis of the supplements. Placebo and vitamin D tablets were identical in appearance and randomization was permutated into blocks of four.
During participation, subjects were asked to keep their dietary habits consistent with their regular routine during the previous year and to refrain from the use of dietary supplements. Subjects were also instructed to refrain from physical activity and using aspirin, ibuprofen, naproxen sodium, acetaminophen, or other anti-inflammatory agents 72 hours prior to a blood draw. Fasting blood samples were obtained prior to (Bsl) and 1-, 3-, 7-, and 24-d after ingesting the bolus.
Blood sample handling
Fasting blood samples were obtained from the antecubital vein. Plasma was separated by centrifugation (VWR International, Clinical 50 Centrifuge, Radnor, PA USA) at 1100 g for 15 min within 20 min of sample collection. Following separation, plasma samples were sent to ARUP Laboratories (Salt Lake City, UT USA) for the determination of parathyroid hormone (PTH), intact with calcium (see below). After coagulation, serum was separated by centrifugation (VWR International, Clinical 50 Centrifuge) at 1100 g for 10 min, and then aliquoted into several small micro-centrifuge tubes. Aliquoted serum samples were stored at -80°C (Revco Freezer, GC Laboratory Equipment, Asheville, NC USA) until later analyses.
Analytical procedures
Serum vitamin D metabolites: Serum 25(OH)D concentrations were measured in duplicate (coefficient of variation = 5.29%), as previously described [35]. In brief, analytes were separated on an Agilent high performance-liquid chromatography system (series 6460, Model G6460A, Santa Clara, CA USA) and detected on Agilent tandem mass spectrometer (Series 6410, Model G6410B, Santa Clara, CA USA) using atmospheric pressure chemical ionization detection (350°C gas temperature, 400°C vaporizer). The 25(OH)D3, deuterated 25(OH)D3 internal standard, and 25(OH)D2 precursor ions were 383.3, 386.3, and 395.4, respectively. The 25(OH)D3, deuterated 25(OH)D3, and 25(OH)D2 product ions were 365.3, 368.3, and 208.9, respectively. Serum 25(OH)D2 and 25(OH)D3 concentrations were corrected for recovery of the 25(OH)D3 internal standard. Serum 25(OH)D2 (limit of detection=2.0 ng/mL) was not detected in any of the samples. Therefore, serum 25(OH)D total concentrations are referred to as serum 25(OH)D concentrations hereafter. National Institute of Standards and Technology standards were measured in parallel to study samples to confirm the accuracy of the analytical procedure. Subjects were classified as vitamin D deficient, insufficient, or sufficient if they had a serum 25(OH)D concentration ≤ 20, between 21-29, or ≥ 30 ng/mL, respectively [36]. Serum 1,25(OH)D concentrations were determined using a quantitative radioimmunoassay (ARUP Laboratories) and VDBP concentrations (EMD Millipore, Billerica, MA USA) were determined using Luminex technology (MAGPix; Austin, TX USA).
Serum TNF-α, sTNFr1, and sTNFr2 concentrations
The multiplex technology of Luminex was used to analyze serum TNF-α (EMD Millipore) and soluble TNF-receptor (EMD Millipore) concentrations with high-sensitivity in The Physiology Research Laboratory at The Orthopedic Specialty Hospital (Murray, UT USA).
Plasma parathyroid hormone, intact with calcium
Plasma PTH, intact with calcium concentrations were measured using a quantitative electrochemiluminescent immunoassay at ARUP Laboratories.
Statistical analyses
Data were checked for normality prior to statistical analyses with a Shapiro-Wilk Test. Statistical significance of subject characteristics and data between vitamin D status groups (Insufficient and Sufficient) and supplements (Placebo and Vitamin D) at Bsl were assessed with either a Mann-Whitney U test or a t -test depending on the Shapiro-Wilk Test p-value. Statistical significance of data (concentration and concentration changes) between supplement groups (PL and VD) were assessed with separate Friedman two-way analysis of variance tests and followed by multiple pairwise comparisons when appropriate. A Spearman Rank Correlation was performed to examine the association between variables. Significance was set at p
Results
Vitamin D status group comparisons prior to supplementation
The number of subjects classified as vitamin D deficient, insufficient, or sufficient were two (6.9%), 15 (51.7%), and 12 (41.4%), respectively. Due to the small sample size in the vitamin D deficient group, we subsequently combined the deficient and insufficient groups (n=17, Insufficient) for the vitamin D status comparisons prior to supplementation. Circulating concentrations of 1,25(OH)D, VDBP, PTH, and calcium were not significantly different between the vitamin D Insufficient (serum 25(OH)D Table 1).
| Insufficient | Sufficient | |
|---|---|---|
| n | 17 | 12 |
| Age (y) | 31 (5) | 38 (17) |
| Height (cm) | 179 (11) | 177 (8) |
| Body mass (kg) | 88.2 (17.9) | 84.2 (15.0) |
| BMI (kg/m2) | 25.8 (4.1) | 26.0 (2.1) |
| Serum 25(OH)D (ng/mL) | 23.5 (5.9) | 33.3 (8.5)* |
| Serum 1,25(OH)D (pg/mL) | 55.0 (18.0) | 61.0 (13.5) |
| Serum VDBP (pg/mL) | 28.6 (13.3) | 34.5 (19.1) |
| Plasma PTH (pg/mL) | 39.0 (13.3) | 39.5 (18.5) |
| Plasma calcium (mg/DL) | 9.20 (0.30) | 9.45 (0.40) |
| Serum TNF-α (pg/mL) | 7.03 (4.84) | 7.52 (4.50) |
| Data presented as median (interquartile range) Insufficient, serum 25(OH)D Sufficient, serum 25(OH)D ≥ 30 ng/mL *pvs. Insufficient |
||
Table 1: Vitamin D Insufficient and Sufficient subject characteristics at Bsl.
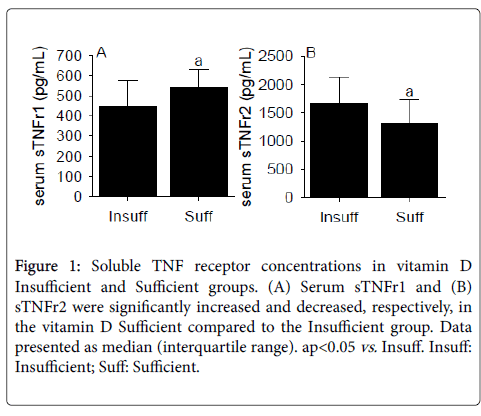
Serum TNF-α concentrations were not significantly different between the vitamin D Insufficient and Sufficient groups (Table 1). Conversely, serum sTNFr1 was significantly (p<0.05) increased and sTNFr2 was significantly (p<0.05) decreased in the vitamin D Sufficient compared to the Insufficient group (Figures 1A and 1B).
Figure 1: Soluble TNF receptor concentrations in vitamin D Insufficient and Sufficient groups. (A) Serum sTNFr1 and (B) sTNFr2 were significantly increased and decreased, respectively, in the vitamin D Sufficient compared to the Insufficient group. Data presented as median (interquartile range). apvs. Insuff. Insuff: Insufficient; Suff: Sufficient.
Subject characteristics and serum 25(OH)D concentrations
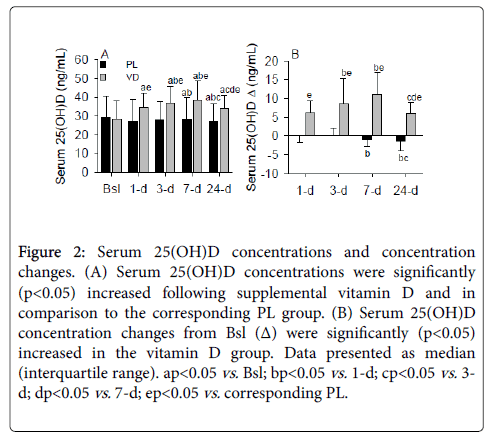
Subject characteristics, vitamin D status classification (deficient, insufficient, and sufficient; Table 2), plasma PTH, calcium (Table 3), and serum 25(OH)D concentrations (Figure 2A) were not significantly different between the PL and VD groups prior to supplementation. Following supplementation, serum 25(OH)D concentrations were significantly (p<0.05) increased in the VD group. Serum 25(OH)D concentrations displayed a transient peaked at 3- (~31%) and 7-d (~36%), and despite a gradual decrease thereafter, remained elevated at 24-d (~21%) compared to Bsl in the VD group. Importantly, all subjects with initial vitamin D insufficiency achieved vitamin D sufficiency at 3- or 7-d in the vitamin D group, and no subjects in the VD group achieved a serum 25(OH)D concentration deemed toxic (i.e., serum 25(OH)D) ≥ 100 ng/mL) [36] despite a substantial number of subjects possessing a sufficient concentration prior to supplementation. Serum 25(OH)D concentrations progressively decreased in the PL group at 7- (~3%) and 24-d (~6%).
| Placebo | Vitamin D | |
|---|---|---|
| n | 15 | 14 |
| Age (y) | 33 (8) | 29 (19) |
| Height (cm) | 179 (8) | 175 (10) |
| Body mass (kg) | 85.1 (11.5) | 85.0 (23.3) |
| BMI (kg/m2) | 25.6 (2.5) | 26.3 (7.7) |
| Vitamin D status Deficient (n) Insufficient (n) Sufficient (n) |
1 8 6 |
1 7 6 |
| Data presented as median (interquartile range) | ||
Table 2: Subject characteristics and vitamin D status prior to supplementation.
| Bsl | 1-d | 3-d | 7-d | 24-d | |
|---|---|---|---|---|---|
| Plasma PTH (pg/mL) | |||||
| PL | 39.0 (11.8) | 32.0 (10.0) | 34.0 (12.0) | 35.0 (12.5) | 34.0 (9.9) |
| VD | 38.0 (19.0) | 34.0 (17.0) | 26.5 (15.0) | 32.5 (17.0) | 30.0 (15.0) |
| Plasma calcium (mg/dL) | |||||
| PL | 9.20 (0.30) | 9.40 (0.45) | 9.30 (0.20) | 9.20 (0.28) | 9.30 (0.48) |
| VD | 9.45 (0.50) | 9.35 (0.60) | 9.45 (0.30) | 9.40 (0.50) | 9.60 (0.60) |
| Data presented as median (interquartile range) | |||||
Table 3: Plasma PTH and calcium prior to and following supplementation.
Figure 2: Serum 25(OH)D concentrations and concentration changes. (A) Serum 25(OH)D concentrations were significantly (p<0.05) increased following supplemental vitamin D and in comparison to the corresponding PL group. (B) Serum 25(OH)D concentration changes from Bsl (Δ) were significantly (p<0.05) increased in the vitamin D group. Data presented as median (interquartile range). apvs. Bsl; bpvs. 1-d; cpvs. 3- d; dpvs. 7-d; epvs. corresponding PL.
As expected, serum 25(OH)D concentration changes from Bsl (Δ) were significantly (p<0.05) increased in the VD group (Figure 2B). Specifically, serum 25(OH)D Δ were significantly (both p<0.05) increased at 3- and 7-d compared to 1- and 24-d. Also, serum 25(OH)D Δ were significantly (p<0.05) increased in the VD compared to the PL group. In the PL group, Δ were significantly decreased at 7- and 24-d (both p<0.05).
Serum 1,25(OH)D and VDBP concentrations
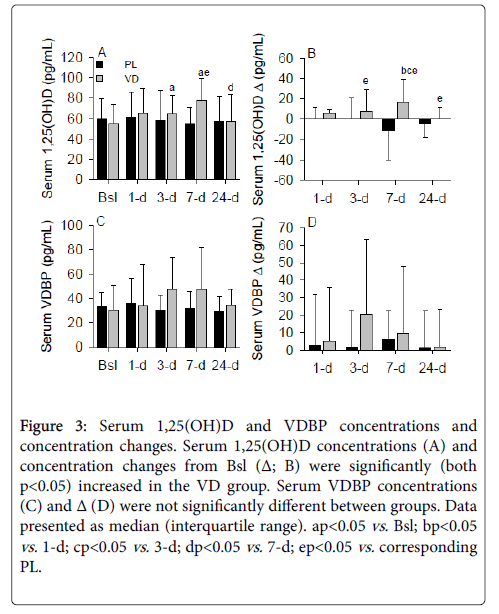
Following supplementation, serum 1,25(OH)D concentrations were significantly (p<0.05) increased at 3- and 7-d compared to Bsl in the VD group (Figure 3A). At 7-d, serum 1,25(OH)D concentrations were significantly (p<0.05) increased in the VD group compared to 24-d and to the corresponding 7-d concentration in the PL group. Serum 1,25(OH)D Δ were significantly (p<0.05) increased at 7-d in the VD group and at 3-, 7-, and 24-d in the VD compared to the PL group (Figure 3B). Serum 1,25(OH)D concentrations were not significantly different in the PL group.
Figure 3: Serum 1,25(OH)D and VDBP concentrations and concentration changes. Serum 1,25(OH)D concentrations (A) and concentration changes from Bsl (Δ; B) were significantly (both p<0.05) increased in the VD group. Serum VDBP concentrations (C) and Δ (D) were not significantly different between groups. Data presented as median (interquartile range). apvs. Bsl; bpvs. 1-d; cpvs. 3-d; dpvs. 7-d; epvs. corresponding PL.
The VDBP carries 85-90% of the total circulating 25(OH)D [37], and importantly, maintains and stabilizes serum 25(OH)D concentrations during variable vitamin D availability [38]. Although 25(OH)D and 1,25(OH)D are two of the major vitamin D metabolites with significant biological activity, VDBP also regulates the biologic effects of vitamin D as evident by its role in altering neutrophil recruitment [39]. Therefore, we examined the VDBP response to supplemental vitamin D. Despite significant alterations in serum 25(OH)D and 1,25(OH)D, serum VDBP concentrations were not significantly modulated by a large bolus of vitamin D (Figures 3C and 3D). However, a non-significant increase in the VDBP was apparent in the VD group at 3- and 7-d. This finding could assist with generating new hypotheses and determining appropriate sample sizes for future studies examining the role of supplemental vitamin D to modulate the VDBP.
Vitamin D metabolite correlations in the VD group
As demonstrated by our lab [40] and others [41], Bsl serum 25(OH)D inversely correlated (p<0.05) with the change in serum 25(OH)D following supplementation (Table 4). Extending those findings, we found that serum 25(OH)D and VDBP at Bsl positively and negatively correlated, respectively, with the Δ in VDBP at 7-d. The inverse association between Bsl and concentration changes in VDBP following supplemental vitamin D extends previous work [42] by demonstrating that the VDBP response to supplemental vitamin D is blunted with an increase in Bsl concentrations.
| Bsl | |||
|---|---|---|---|
| 25(OH)D | 1,25(OH)D | VDBP | |
| 25(OH)D 1-d Δ | -0.19 | 0.32 | -0.02 |
| 25(OH)D 3-d Δ | -0.26 | 0.06 | 0.28 |
| 25(OH)D 7-d Δ | -0.26 | 0.43 | -0.11 |
| 25(OH)D 24-d Δ | -0.61* | 0.21 | -0.13 |
| 1,25(OH)D 1-d Δ | 0.04 | -0.06 | 0.11 |
| 1,25(OH)D 3-d Δ | 0.15 | -0.24 | 0.18 |
| 1,25(OH)D 7-d Δ | 0.07 | -0.16 | 0.30 |
| 1,25(OH)D 24-d Δ | 0.06 | -0.10 | 0.20 |
| VDBP 1-d Δ | 0.41 | 0.05 | -0.12 |
| VDBP 3-d Δ | 0.38 | -0.24 | -0.49 |
| VDBP 7-d Δ | 0.56* | -0.22 | -0.68* |
| VDBP 24-d Δ | 0.11 | -0.18 | -0.45 |
| n=14; *p<0.05 | |||
Table 4: Vitamin D metabolite Spearman Rank correlation coefficients (ρ) in the VD group only.
Serum cytokine and soluble cytokine receptor concentrations
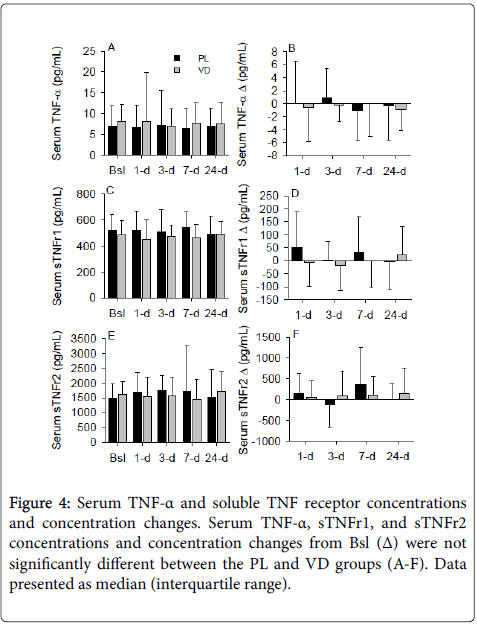
Despite numerous investigations illuminating the impact of vitamin D on serum TNF-α and the data from this study suggesting that vitamin D status alters soluble TNF receptors, we were unable to detect a difference in serum TNF-α, sTNFr1, or sTNFr2 concentrations between the PL and VD groups (Figures 4A-4F). In addition, vitamin D status prior to supplementation did not significantly modulate serum sTNFr1 and sTNFr2 concentrations following the bolus of supplemental vitamin D (data not shown).
Discussion
In this investigation, we provide the first evidence that vitamin D sufficiency increases sTNFr1 and decreases sTNFr2 without altering serum TNF-α in reportedly healthy, male adults. In contrast, however, soluble TNF receptor deviations are absent following a large bolus of supplemental vitamin D that induces an immediate but transient increase in serum 25(OH)D and 1,25(OH)D. These data suggest that soluble TNF receptors are prone to modulation by vitamin D status but less apt to fluctuations following a large bolus of vitamin D that mediates an abrupt and transient increase in serum 25(OH)D and 1,25(OH)D.
A novel finding of the present investigation was the sTNFr1 increase in reportedly healthy male adults with vitamin D sufficiency. This finding conflicts with previous reports demonstrating an increase in sTNFr1 with various diseases or low vitamin D [4,34,43]. Low vitamin D associates with an increase in inflammation [14], and inflammatory stimuli triggers the proteolysis of the TNF-α receptors by metalloproteinases that subsequently increase the soluble form in the circulation [2,44-46]. Also, in vitro and in vivo results indicate that inflammatory challenge compromises 25(OH)D levels, which consequentially, increases 1,25(OH)D [47-52]. In vascular smooth muscle cells, 1,25(OH)D exposure mediates the ectodomain shedding of TNFr1 and increases the amount of sTNFr1 found in cell culture medium [32]. Thus, inflammation or an increase in 1,25(OH)D (inflammatory- or non-inflammatory-driven) could contribute to the increase in sTNFr1. However, in the present investigation, neither serum 1,25(OH)D nor TNF-α were significantly different despite contrasting sTNFr1 concentrations between vitamin D status groups.
Another unique finding of this study and consistent with elderly data [34], is the decrease in sTNFr2 with vitamin D sufficiency. Proteolysis of the membrane bound receptor decreases the number of receptors on the cell surface and the sensitivity to TNF-α signal transduction [1,53]. Therefore, it is plausible that a decrease in the soluble form could relate to an increase in the membrane-bound TNFr2 and the capacity to augment TNF-α mediated events through the cell-associated receptor. Although it is unknown if the decrease in sTNFr2 associates with an increase in the cell-associated TNFr2 or alters TNF-α mediated events, it is noteworthy that a decrease in sTNFr2 improves survival following hospital admission in children with severe meningococcaemia [54] and reduces the risk of hip fracture in women [55]. Nevertheless, additional studies performed at the cellular and molecular levels are needed to reveal the mechanism(s) underlying the increase in sTNFr1 and decrease in sTNFr2 with vitamin D sufficiency.
Conflicting with the unique findings regarding the divergence in sTNF receptor concentrations with vitamin D status is the inability of supplemental vitamin D to mediate sTNFr1 and sTNFr2 fluctuations concurrently with serum 25(OH)D. One plausible explanation could reside with initial serum 25(OH)D concentrations. The average serum 25(OH)D concentration (28.2 (9.8) ng/mL) prior to supplementation was borderline sufficient. Further supporting the presence of gross vitamin D sufficiency, plasma calcium and parathyroid hormone concentrations were not significantly different between the vitamin D Insufficient and Sufficient groups prior to supplementation. The lack of subjects with low vitamin D could have important ramifications on the ability of supplemental vitamin D to alter soluble cytokine receptors as indirect evidence from our lab suggests that initially low serum 25(OH)D potentiates the ability of supplemental vitamin D to modify circulating cytokine (i.e., interferon-γ and interleukin-10) concentrations [56]. Additionally, this investigation consists of a 24-d protocol in reportedly healthy, male adults following a bolus of supplemental vitamin D. Based on the cytokine receptor differences between vitamin D status groups before supplementation, it is foreseeable that a daily or intermittent (i.e., weekly or monthly) long-term intervention of supplemental vitamin D intended to induce and maintain a sufficient serum 25(OH)D concentration in subjects with initial vitamin D deficiency (i.e.,
Consistent with previous data [40,41,57], there was a significant increase in serum 25(OH)D (from approximately 28.0 to 38.4 (peak at 7-d) ng/mL) following a large bolus of cholecalciferol that inversely correlated with Bsl concentrations. Extending on previous findings, this study provides new results suggesting subjects with high serum 25(OH)D at Bsl possess the greatest change in VDBP concentrations following a 100,000 IU bolus of cholecalciferol. However, despite the trend for increasing concentrations (Figure 2C), there was not a significant increase in serum VDBP following supplemental vitamin D, thereby leaving doubt in the causal relationship of 25(OH)D to mediate an increase in the VDBP. In agreement with this inference, results elsewhere suggest supplemental vitamin D increases serum 25(OH)D without perturbing VDBP concentrations in adults and elderly [42,58]. Those original findings are not universal, however, as data from hip fracture patients suggest an increase in serum VDBP following supplemental vitamin D [59]. Considering the robust inflammatory cascade and limb disuse conditions following the acute trauma of a hip fracture episode, it is plausible that the capacity of 25(OH)D to modulate serum VDBP is condition specific. This assumption, however, requires additional research for later resolve.
In addition to those mentioned above, there are other study limitations worthy of discussion. This study was delimited to reportedly healthy, male adults with unintentionally good vitamin D and corresponding circulating calcium levels. The ability of vitamin D to regulate soluble TNF receptors is calcium dependent [32], and therefore, alterations in circulating calcium with a fluctuating serum 25(OH)D concentration could be a necessity for vitamin D to modulate sTNFr1 and sTNFr2. Also, a single serum 25(OH)D concentration measure was used to identify vitamin D status before supplementation. Serum 25(OH)D concentration measures performed on separate occasions would be ideal to confirm a stable vitamin D status prior to supplementation. Next, it is unclear if the diverging soluble TNF receptor concentrations with vitamin D status moderate biological events mediated by acute or chronic disturbances in TNF-α. These limitations should be taken into consideration when designing future studies pertaining to vitamin D and soluble TNF receptors.
In summary, this study provides the first evidence that vitamin D sufficiency associates with an increase in sTNFr1 and a decrease in sTNFr2 without altering serum TNF-α in reportedly healthy, male adults. However, serum 25(OH)D and 1,25(OH)D concentrations increase while TNF-α and its soluble receptors were not significantly different following a large bolus of supplemental vitamin D. Based on these findings, we conclude that vitamin D sufficiency differentially regulates sTNFr1 and sTNFr2, while neither soluble receptor fluctuate with acute perturbations in serum 25(OH)D following supplemental vitamin D. Additional studies are clearly justified and desired to identify if soluble TNF receptor deviations moderated by vitamin D status or supplementation regulate the pleiotropic properties of TNF-α necessary for optimum host defenses in diverse physiological and pathophysiological conditions.
Acknowledgements
This study was funded in part by USANA Health Sciences, Inc. (Salt Lake City, UT USA) (TB). We would also like to thank Jenna Templeton, Howard Goldfine, Erik D. Schneider, and Mark Levy (USANA Health Sciences, Inc.) for measuring the serum 25(OH)D concentrations and Vanessa Henriksen and Bettinga Junghahn for critically reviewing this manuscript.
References
- Seckinger P, Isaaz S, Dayer JM (1989) Purification and biologic characterization of a specific tumor necrosis factor alpha inhibitor. J Biol Chem 264: 11966-11973.
- Van Zee KJ, Kohno T, Fischer E, Rock CS, Moldawer LL, et al. (1992) Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci USA 89: 4845-4849.
- Queiroz BZ, Pereira DS, Rosa NM, Lopes RA, Felicio DC, et al. (2015) Functional performance and plasma cytokine levels in elderly women with and without low back pain. J Back Musculoskelet Rehabil 28: 343-349.
- Chikanza IC, Roux-Lombard P, Dayer JM, Panayi GS (1993) Tumour necrosis factor soluble receptors behave as acute phase reactants following surgery in patients with rheumatoid arthritis, chronic osteomyelitis and osteoarthritis. Clin Exp Immunol 92: 19-22.
- Dekkers PE, Lauw FN, ten HT, te Velde AA, Lumley P, et al. (1999) The effect of a metalloproteinase inhibitor (GI5402) on tumor necrosis factor-alpha (TNF-alpha) and TNF-alpha receptors during human endotoxemia. Blood 94: 2252-2258.
- Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D (1992) Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med 175: 323-329.
- Shibata J, Goto H, Arisawa T, Niwa Y, Hayakawa T, et al. (1999) Regulation of tumour necrosis factor (TNF) induced apoptosis by soluble TNF receptors in Helicobacter pylori infection. Gut 45: 24-31.
- Aderka D, Sorkine P, Abu-Abid S, Lev D, Setton A, et al. (1998) Shedding kinetics of soluble tumor necrosis factor (TNF) receptors after systemic TNF leaking during isolated limb perfusion. Relevance to the pathophysiology of septic shock. J Clin Invest 101: 650-659.
- Mohler KM, Torrance DS, Smith CA, Goodwin RG, Stremler KE, et al. (1993) Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol 151: 1548-1561.
- Carrillo AE, Flynn MG, Pinkston C, Markofski MM, Jiang Y, et al. (2013) Impact of vitamin D supplementation during a resistance training intervention on body composition, muscle function, and glucose tolerance in overweight and obese adults. Clin Nutr 32: 375-381.
- Beilfuss J, Berg V, Sneve M, Jorde R, Kamycheva E (2012) Effects of a 1-year supplementation with cholecalciferol on interleukin-6, tumor necrosis factor-alpha and insulin resistance in overweight and obese subjects. Cytokine 60: 870-874.
- Schneider L, Colar da Silva AC, Werres Junior LC, Alegretti AP, Pereira dos Santos AS, et al. (2015) Vitamin D levels and cytokine profiles in patients with systemic lupus erythematosus. Lupus 24: 1191-1197.
- Khoo AL, Chai LY, Koenen HJ, Sweep FC, Joosten I, et al. (2011) Regulation of cytokine responses by seasonality of vitamin D status in healthy individuals. Clin Exp Immunol 164: 72-79.
- Barker T, Martins TB, Hill HR, Kjeldsberg CR, Dixon BM, et al. (2013) Circulating pro-inflammatory cytokines are elevated and peak power output correlates with 25-hydroxyvitamin D in vitamin D insufficient adults. Eur J Appl Physiol 113: 1523-1534.
- Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, et al. (2006) Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 83: 754-759.
- Milovanovic M, Pesic G, Nikolic V, Jevtovic-Stoimenov T, Vasic K, et al. (2012) Vitamin D deficiency is associated with increased IL-17 and TNFαlevels in patients with chronic heart failure. Arq Bras Cardiol 98: 259-265.
- Karim Y, Turner C, Dalton N, Roplekar R, Sankaralingam A, et al. (2013) The relationship between pro-resorptive inflammatory cytokines and the effect of high dose vitamin D supplementation on their circulating concentrations. Int Immunopharmacol 17: 693-697.
- Bellia A, Garcovich C, D'Adamo M, Lombardo M, Tesauro M, et al. (2013) Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern Emerg Med 8: 33-40.
- Peterson CA, Heffernan ME (2008) Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm (Lond) 5: 10.
- Zhang Y, Leung DYM, Richers BN, Liu Y, Remigio LK, et al. (2012) Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 188: 2127-2135.
- Khoo AL, Chai LY, Koenen HJ, Oosting M, Steinmeyer A, et al. (2011) Vitamin D(3) down-regulates proinflammatory cytokine response to Mycobacterium tuberculosis through pattern recognition receptors while inducing protective cathelicidin production. Cytokine 55: 294-300.
- Khoo AL, Chai LY, Koenen HJ, Kullberg BJ, Joosten I, et al. (2011) 1,25-dihydroxyvitamin D3 modulates cytokine production induced by Candida albicans: impact of seasonal variation of immune responses. J Infect Dis 203: 122-130.
- Czifra G, Toth B, Kovacs I, Biro T, Griger Z, et al. (2014) The in vitro treatment with vitamin D is ineffective on the expression of PKC isoenzymes, but decreases further the impaired production of IL-2 in the T lymphocytes of SLE patients. Rheumatol Int 34: 717-720.
- Lysandropoulos AP, Jaquiery E, Jilek S, Pantaleo G, Schluep M, et al. (2011) Vitamin D has a direct immunomodulatory effect on CD8+ T cells of patients with early multiple sclerosis and healthy control subjects. J Neuroimmunol 233: 240-244.
- Prabhu AS, Selvaraj P, Narayanan PR (2009) Effect of 1,25 dihydroxyvitamin D3 on intracellular IFN-gamma and TNF-alpha positive T cell subsets in pulmonary tuberculosis. Cytokine 45: 105-110.
- Di Rosa M, Malaguarnera G, De Gregorio C, Palumbo M, Nunnari G, et al. (2012) Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol 280: 36-43.
- Muller K, Haahr PM, Diamant M, Rieneck K, Kharazmi A, et al. (1992) 1,25-Dihydroxyvitamin D3 inhibits cytokine production by human blood monocytes at the post-transcriptional level. Cytokine 4: 506-512.
- Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, et al. (2009) 1Alpha,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine 45: 190-197.
- Chen Y, Kong J, Sun T, Li G, Szeto FL, et al. (2011) 1,25-Dihydroxyvitamin D suppresses inflammation-induced expression of plasminogen activator inhibitor-1 by blocking nuclear factor-kappaB activation. Arch Biochem Biophys 507: 241-247.
- Martinesi M, Bruni S, Stio M, Treves C (2006) 1,25-Dihydroxyvitamin D3 inhibits tumor necrosis factor-alpha-induced adhesion molecule expression in endothelial cells. Cell Biol Int 30: 365-375
- Mackay F, Rothe J, Bluethmann H, Loetscher H, Lesslauer W (1994) Differential responses of fibroblasts from wild-type and TNF-R55-deficient mice to mouse and human TNF-alpha activation. J Immunol 153: 5274-5284.
- Yang WS, Kim HW, Lee JM, Han NJ, Lee MJ, et al. (2015) 1,25-dihydroxyvitamin D3 causes ADAM10-dependent ectodomain shedding of tumor necrosis factor receptor 1 in vascular smooth muscle cells. Mol Pharmacol 87: 533-542.
- Srikanth P, Chun RF, Hewison M, Adams JS, Bouillon R, et al. (2016) Associations of total and free 25OHD and 1,25(OH)2D with serum markers of inflammation in older men. Osteoporos Int 27: 2291-2300.
- De VF, Lauretani F, Bauer J, Bautmans I, Shardell M, et al. (2014) Relationship between vitamin D and inflammatory markers in older individuals. Age (Dordr ) 36: 9694.
- Barker T, Martins TB, Hill HR, Kjeldsberg CR, Henriksen VT, et al. (2012) Different doses of supplemental vitamin D maintain interleukin-5 without altering skeletal muscle strength: a randomized, double-blind, placebo-controlled study in vitamin D sufficient adults. Nutr Metab (Lond) 9: 16.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, et al. (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96: 1911-1930.
- Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, et al. (1986) Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab 63: 954-959.
- Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, et al. (1999) Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest 103: 239-251.
- Trujillo G, Habiel DM, Ge L, Ramadass M, Cooke NE, et al. (2013) Neutrophil recruitment to the lung in both C5a- and CXCL1-induced alveolitis is impaired in vitamin D-binding protein-deficient mice. J Immunol 191: 848-856.
- Barker T, Schneider ED, Dixon BM, Henriksen VT, Weaver LK (2013) Supplemental vitamin D enhances the recovery in peak isometric force shortly after intense exercise. Nutr Metab 10: 69.
- Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, et al. (1998) Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr 68: 854-858.
- Ponda MP, McGee D, Breslow JL (2014) Vitamin D-binding protein levels do not influence the effect of vitamin D repletion on serum PTH and calcium: data from a randomized, controlled trial. J Clin Endocrinol Metab 99: 2494-2499.
- Kern WV, Engel A, Schieffer S, Prummer O, Kern P (1993) Circulating tumor necrosis factor alpha (TNF), soluble TNF receptors, and interleukin-6 in human subacute bacterial endocarditis. Infect Immun 61: 5413-5416.
- Crowe PD, Walter BN, Mohler KM, Otten-Evans C, Black RA, et al. (1995) A metalloprotease inhibitor blocks shedding of the 80-kD TNF receptor and TNF processing in T lymphocytes. J Exp Med 181: 1205-1210.
- Schall TJ, Lewis M, Koller KJ, Lee A, Rice GC, et al. (1990) Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell 61: 361-370.
- Mullberg J, Durie FH, Otten-Evans C, Alderson MR, Rose-John S, et al. (1995) A metalloprotease inhibitor blocks shedding of the IL-6 receptor and the p60 TNF receptor. J Immunol 155: 5198-5205.
- Barker T, Trawick RH (2012) Changes in the circulating 1,25(OH)D-to-25(OH)D ratio correlate with IFN-gamma alterations. Cytokine 26: 1-3.
- Reichel H, Koeffler HP, Barbers R, Norman AW (1987) Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab 65: 1201-1209.
- Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, et al. (2006) Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res 21: 37-47.
- Stoffels K, Overbergh L, Bouillon R, Mathieu C (2007) Immune regulation of 1alpha-hydroxylase in murine peritoneal macrophages: unravelling the IFNgamma pathway. J Steroid Biochem Mol Biol 103: 567-571.
- Edfeldt K, Liu PT, Chun R, Fabri M, Schenk M, et al. (2010) T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci USA 107: 22593-22598.
- Koeffler HP, Reichel H, Bishop JE, Norman AW (1985) Gamma-Interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem Biophys Res Commun 127: 596-603.
- Williams LM, Gibbons DL, Gearing A, Maini RN, Feldmann M, et al. (1996) Paradoxical effects of a synthetic metalloproteinase inhibitor that blocks both p55 and p75 TNF receptor shedding and TNF alpha processing in RA synovial membrane cell cultures. J Clin Invest 97: 2833-2841.
- Girardin E, Roux-Lombard P, Grau GE, Suter P, Gallati H, et al. (1992) Imbalance between tumour necrosis factor-alpha and soluble TNF receptor concentrations in severe meningococcaemia. Immunology 76: 20-23.
- Ing SW, Orchard TS, Lu B, LaMonte MJ, Barbour KE, et al. (2015) TNF Receptors Predict Hip Fracture Risk in the WHI Study and Fatty Acid Intake Does Not Modify This Association. J Clin Endocrinol Metab 100: 3380-3387.
- Barker T, Rogers VE, Levy M, Templeton J, Goldfine H, et al. (2015) Supplemental vitamin D increases serum cytokines in those with initially low 25-hydroxyvitamin D: A randomized, double blind, placebo-controlled study. Cytokine 71: 132-138.
- Ilahi M, Armas LA, Heaney RP (2008) Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr 87: 688-691.
- Sollid ST, Hutchinson MY, Berg V, Fuskevag OM, Figenschau Y, et al. (2016) Effects of vitamin D binding protein phenotypes and vitamin D supplementation on serum total 25(OH)D and directly measured free 25(OH)D. Eur J Endocrinol 174: 445-452.
- Glendenning P, Chew GT, Inderjeeth CA, Taranto M, Fraser WD (2013) Calculated free and bioavailable vitamin D metabolite concentrations in vitamin D-deficient hip fracture patients after supplementation with cholecalciferol and ergocalciferol. Bone 56: 271-275.
Citation: Barker T, Brown KB, Rogers VE (2017) Soluble TNF Receptors are Modulated by Vitamin D Status but not by Acute Perturbations in 25-Hydroxyvitamin D Following A Bolus of Supplemental Vitamin D. J Cytokine Biol 2: 118. DOI: 10.4172/2576-3881.1000118
Copyright: © 2017 Barker T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4315
- [From(publication date): 0-2017 - Jul 20, 2025]
- Breakdown by view type
- HTML page views: 3421
- PDF downloads: 894