Review Article Open Access
Sources for Inflammation and Accelerated Aging in Well Controlled HIV Patients on Antiretroviral Therapy
| Gnoni ML1*, Friedstrom S2, Blatt S2, Fernandez H2 and Ramirez JA3 | |
| 1Department of Medicine, Louisville, Kentucky; GoodSamaritan Hospital (Trihealth system) Department of Internal Medicine, Cincinnati, USA | |
| 2Department of Medicine, Division of Infectious Diseases, GoodSamaritan Hospital, Cincinnati, USA | |
| 3Division of Infectious Diseases, University of Louisville, Kentucky, USA | |
| Corresponding Author : | Gnoni ML Department of Medicine, Louisville Kentucky; GoodSamaritan Hospital (Trihealth system) Department of Internal Medicine, Cincinnati, USA Tel: 5025483525 E-mail: Martin_Gnoni@trihealth.com |
| Received: September 1, 2015 Accepted: September 28, 2015 Published: September 30, 2015 | |
| Citation: Gnoni ML, Friedstrom S, Blatt S, Fernandez H, Ramirez JA (2015) Sources for Inflammation and Accelerated Aging in Well Controlled HIV Patients on Antiretroviral Therapy. J Infect Dis Ther 3:239. doi:10.4172/2332-0877.1000239 | |
| Copyright: © 2015 Gnoni et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
After the introduction of highly active antiretroviral therapy (HAART) in the middle 1990, the mortality and morbidity of HIV has decreased dramatically. However, even well controlled patients on HAART are now suffering from “accelerated aging” with increased incidence of cardiovascular, respiratory, neurologic, metabolic, renal, and liver disease. Persistent low-level replication, coinfections, deposition of collagen in lymphoid tissue, microbial translocation in the GI and respiratory mucosa, overproduction of cellular debris, and immune-senescence may all contribute to its pathogenicity. Further exploration of these possible mechanisms will help us to define optimal trials to decrease the accelerated aging in HIV patients.
| Keywords | |
| HIV Infection; Aging; Antiretroviral Therapy; Immunoscenescense; Inflammation; Reservoirs | |
| Introduction | |
| The concept of “accelerated aging” | |
| The HIV pandemic is quite different from the original described in the 80’s with a cluster of cases of PJP. It has reached every country around the planet but has hit particularly hard in undeveloped countries such as sub-Saharan Africa and Southeast Asia. New data has shown a total of 36.9 million persons living with HIV worldwide at the end of 2014 (adults 34.3, women 17.4, children<15 years 220.000) with a total of 2 million recently infected and 1.2 million deaths due to AIDS during the same period. Nearly three quarters of the global burden of disease exists in sub-Saharan Africa (25.8 million) with an alarming prevalence exceeding 20 percent in some countries such as Botswana, Swaziland, and Lesotho. After sub-Saharan Africa, Asia and the Pacific have the second highest number of cases (5.0 million) [http://www.unaids.org]. At the end of 2013, 1.2 million people in the United States were living with HIV infection [http://www.cdc.gov]. | |
| The access to therapy is also asymmetric with a significant decrease in mortality after the introduction of Highly Active Antiretroviral Therapy [HAART] in the middle 1990’s in developed areas such as US, Canada, European countries, and Australia. However, most of the countries with a higher prevalence still have very limited access to therapy and therefore a high incidence of AIDS-defining illness as a cause of death. | |
| Antiretroviral therapy [ART] has changed the natural history of the disease and most HIV patients with access to therapy reach lifelong viral suppression with almost no incidence of opportunistic infections. Despite the control of HIV replication in plasma and the decrease in overall mortality, there is evidence that even treated HIV infected patients have a high prevalence of non-AIDS defining illnesses, including cardiovascular, , neurologic, metabolic, renal, and liver disease. Different types of solid and hematologic malignancies can be seen as well [1-5]. As a result, even well controlled HIV-infected patients have a shorter lifespan than matched HIV uninfected individuals. | |
| The genesis of these chronic diseases appears to be related to a generalized state of chronic inflammation and immune activation. Theoretically, chronic systemic inflammation and immune activation (a “catabolic” state) could cause damage to multiple organ systems, which is reflected in an increase in mortality and morbidity seen in this population. These observations have lead to the concept of “HIV and aging” defined by accelerated aging of multiple organs [2,4,5]. This is not a new concept in the physiology of aging since “Inflammaging” describes how chronic inflammation contributes to progressive and disseminated organ damage and aging [6]. The nature of the biochemical and cellular insults that give rise to the process of inflammaging in non-HIV infected individuals remains largely unknown, but the understanding of the accelerated aging process seen on well controlled HIV patients on ART might contribute to its clarification. | |
| Chronic inflammation is not only linked to the concept of aging on this population but to the concept of progressive immune dysfunction as well. Interestingly natural hosts of the simian immunodeficiency virus [SIV], which fail to develop immunodeficiency and AIDSdefining conditions, have low levels of immune activation and chronic inflammation [7]. This is important since SIV and HIV cause progressive immune dysfunction and chronic inflammation in nonnatural hosts. Of note, ART in HIV patients improves the level of the biomarkers of chronic inflammation and immune dysfunction but never bring them back to their baseline levels, reflecting the expected response in non-natural hosts [8]. | |
Supporting this theory, it has been seen that immune cells in wellcontrolled HIV patients show characteristic phenotypic changes compatible with “immune-senescence” or “immune exhaustion” which makes them work not very effectively [2,4,5]. This is also extremely important since “cellular senescence” is a hallmark of the aging process. Examples of this phenomenon are the decrease proliferative capacity and IL2 production, altered receptor signaling, and altered cell surface markers including the loss of CD28+ expression seen in this population [9]. The rate of elimination of “senescent cells” can be diminished in the context of immune depression as well, which makes chronic HIV infection the perfect candidate to promote an accelerated aging of organs [10]. Making this scenario even more complex, it has been recently seen that senescent cells suffer profound alterations of their “secretome” if they are included in a rich pro-inflammatory environment (with a high content of cytokines and matrix
|
|
| Chronic immune dysfunction, immune activation, immunesenescence, and systemic inflammation likely predict and contributes to the increased morbidity and mortality associated with non-AIDS defining illnesses (“Aging”) in the context of a well-controlled HIV [1,2,12-22] but the intrinsic mechanisms of its production remains to be clarify. | |
| The objective of this review is to explore the possible sources of chronic inflammation and immune activation in well-controlled HIV patients on ART, with the goal of defining new and novel targets for therapy in addition to antiretrovirals. An extensive literature review was done using PubMed, Ovid, and Clinical Key. | |
| Biomarkers of chronic inflammation | |
| Until recently the mechanisms involved in the pathogenesis of chronic inflammation and immune dysfunction in the virologically suppressed HIV patients were not very well understood. Currently it is well known now that the treatment-mediated immune reconstitution is often incomplete and the levels of inflammatory markers persist elevated [7]. | |
| Interestingly, some of the biomarkers of chronic inflammation and immune dysfunction have been linked specifically to an increase in the cardiovascular risk and overall mortality in HIV patients. Elevated CRP for example has been associated with an increase risk of acute myocardial infarction [22]. Similarly, IL-6 and D-dimer were strongly related to all-cause mortality in this population [21].Supporting the observations that the coagulation cascade could be an important contributor to the increase on the cardiovascular risk on these population, it was recently showed that CRP and fibrinogen are strong and independent risk factors of mortality in HIV patients [23]. Since most of these observations were seen after adjusting for viral load and CD4-T cells, these events most likely were independently driven by the state of chronic inflammation and immune activation regardless of the degree of immunosuppression. Of note, chronic inflammation and the coagulation cascade system have common biochemical paths and both might contribute to the disease process. Some of these biomarkers of inflammation have emerged recently as possible strong predictors of morbidity and mortality in the setting of treated HIV infection [24]. | |
| These are times when the medical community has to address the two main issues that are pending in order to reach a possible functional cure and decrease even further the mortality and morbidity of our suppressed HIV patients: to decrease not only the state of chronic inflammation-immune activation-dysfunction but the reservoir size as well. | |
| Factors that might contribute to the state of chronic inflammation and immune activation-dysfunction in wellcontrolled HIV patients on ART | |
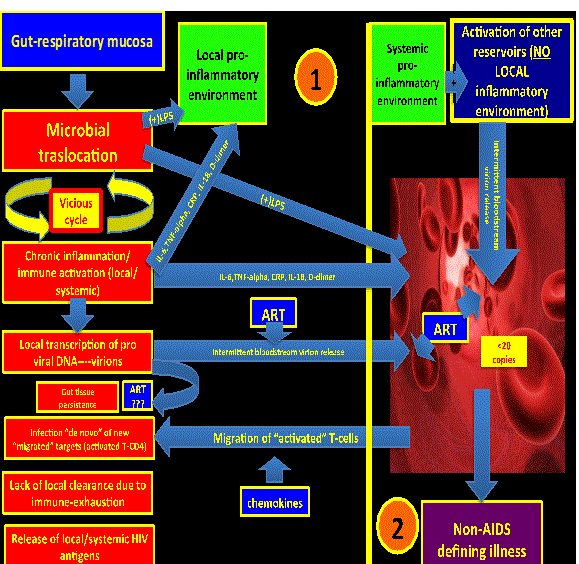
| Persistent ongoing low level HIV replication (persistence): Even when HIV replication in plasma has been diminished to undetectable levels, there is still active low-level replication on difficult-to-reach areas (microglia, lymph nodes, gut mucosa, respiratory mucosa, etc.) or regions with a persistent local pro-inflammatory environment (gut mucosa and respiratory mucosa). Antiretroviral therapy is ineffective in reaching the integrated pro-viral DNA established in the reservoirs since it represents a target not reachable for the current FDA approved drugs (Figure 1). | |
| It is clear that local pro-inflammatory environments promote HIV persistence trough at least two main mechanisms: a) Local immuneactivation and immune-senescence that promote an ineffective immune-clearance of the HIV itself; and b) repetitive rounds of “de novo” infections since there is a high number of activated T-cells trafficking to areas of inflammation (preferred “targets” for newly released virions). | |
| It is believed that ART has a minimal (if any) activity on those environments. In fact, it is possible to detect HIV RNA in rectal tissue of virologically-supressed HIV patients on ART [7,8]. Even though some of the intensification trials (mainly with Raltegravir) have shown some promising results in decreasing the reservoir size, seems to be that persistent low level HIV replication alone is not enough to explain such a degree of high persistent systemic inflammation, being more likely just a contributor [25-27]. It is also not known whether the relationship between residual viral replication and persistent immune activation is casual or other mechanisms are involved [8] which would suggest a multifactorial and complex process. | |
| Deposition of collagen in lymphoid tissue leading to fibrosis with partial-suboptimal immune recovery | |
| The alteration of the architecture of the lymphoid tissue is driven by persistent inflammation which leads to collagen deposition [8]. This would lead to incomplete T-cell homeostasis and persistent immunodeficiency with lack of effective control not only of the residual low-level HIV replication but viral co-infections (CMV) as well. This structurally abnormal tissue would favor a close contact between the dysfunctional and activated immune cells, promoting cellto- cell HIV transmission as a mechanism of local persistence [24]. The local pro-inflammatory environment and the high number of activated (although dysfunctional) CD4+T cells help to promote this vicious cycle of pro-inflammation, lack of immune clearance and repetitive rounds of local “de novo” infections. Lymph node fibrosis persists despite effective plasma viral suppression and is mainly produced by the anti-inflammatory pathway, mainly trough secretion of transforming growth factor beta-1 [TGF-B1] [28]. Probably different compartments have different balances between inflammatory/antiinflammatory pathways in this complex infection. | |
| Lymphoid fibrosis can be consider then as both cause and consequence of this process which, in turn, leads not only to the lack of control of the HIV replication itself, but other viral infections (CMV, EBV, HCV, HBV, etc.) and the microbial translocation in mucosal surfaces as well. | |
| HIV-associated gut mucosal immune dysfunction | |
| Regardless of the mode of transmission, HIV replicates especially in the gut-associated lymphoid tissue (GALT) where it causes profound functional and anatomical changes. Recent evidence has shown that immune activation and chronic inflammation is due, at least in part, to the translocation of microbial products from the lumen of the intestine to the systemic circulation (“microbial translocation”) [7,8,19,20,29-32] driving a local and systemic pro-inflammatory state. It has been extensively shown that SIV (in non-natural hosts) and HIV infection leads to breaches in the tight junctions between epithelial cells in the gut mucosa allowing microbial products, and chemokines to travel through it [20,29,32,33]. Not only the anatomy but also the functionality of the mucosa is affected. | |
| The GALT represents an area of persistent, dynamic, and inexhaustible antigenic stimulation of the local innate immune system. Bacterial products from the “gut-microbiome” like lipopolysaccharides (LPS) can stimulate the innate immune system through the pattern recognition receptors such as toll-like receptors (TLRs) generating local and systemic inflammation [20,33]. | |
| The fact that the innate immune system is not as affective as it should be (“immune-senescence”) along with the breaches in the epithelial tight unions and decrease in the numbers of important regulatory cells (Th-17, CD103+ dendritic cells) makes this constant immune stimulation vicious cycle very difficult to interrupt. | |
| …” The inflamed and highly permeable gut mucosa is the perfect generator of pro-inflammatory cytokines, chemokines, and activated immune cells that promotes not only local persistence but systemic inflammation as well…” | |
| It has been proven that an increase in the sCD4 (a soluble marker of monocyte activation after binding to LPS) predicts early mortality in HIV patients [24] representing this finding the first link between microbial translocation and mortality on HIV individuals. | |
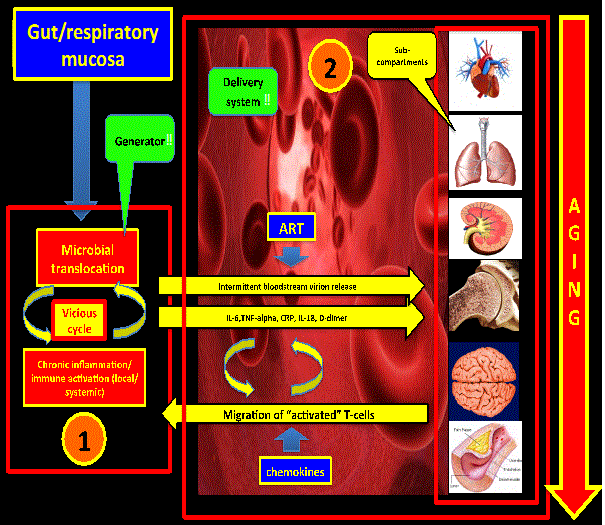
| Considering the above, the gut mucosa could represent a “generator” (“hot-spot #1”) of pro-inflammatory cytokines and microbial products (LPS) that would cause distal tissue damage. This hot-spot might feed with inflammatory signals other distant reservoirs with subsequent pro-viral DNA activation. Of note, simian immunodeficiency virus in natural hosts does not produce immune activation, chronic inflammation and distal tissue damage due to the fact that there is not microbial translocation in the gut mucosa [20] (Figure 2). | |
| ….” May be the way that HIV and SIV found in order to “cope with non-natural hosts” is to create these “gaps” (“hot spots”) in areas of continuous antigenic stimulation (GI and respiratory tract) in order to persist….” | |
| A study showed not only direct evidence of microbial translocation with local damage in areas of mucosal breakdown in the gut but also within areas of distal lymph nodes and the liver, showing that hot spots (like the GI mucosa) can also affect distal tissues [33]. There are multiple reports in the literature showing that there is an association between activated monocytes trough LPS exposure and atheromatous plaque progression on HIV patients, addressing the importance of microbial translocation not only in the genesis of chronic inflammation but for the increase in the overall cardiovascular risk and mortality as well. Importantly, the functionality of the gut mucosa is not fully restored despite suppressive ART. | |
| HIV-associated respiratory mucosal immune dysfunction | |
| Probably some of the concepts described above for the gut mucosa also apply to the respiratory mucosa. Even though there has been a significant decrease over the years of the cases of PJP and Kaposi sarcoma of the lung after the introduction of HAART on the 90’s, wellcontrolled HIV patients are moving toward the acquisition of chronic pulmonary diseases (COPD, Pulmonary hypertension, Interstitial Lung Diseases, etc.) [34-61]. | |
| As we described in our recent review [62] there might be a micro - inflammatory environment in the respiratory mucosa as well (“hotspot# 2”) where a dysfunctional and dynamic respiratory flora (respiratory microbiome) interacts with activated and dysfunctional immune-cells (from the innate and adaptive system) in order to create and promote local inflammation. This local pro-inflammatory environment could promote not only local (COPD, pulmonary hypertension) and distal tissue damage (Aging) but to be a local reservoir for the HIV itself as well. This dynamic process is called “lung remodeling” and agents like Tetracyclines are part of an ongoing NIH funded study in order to decerase the progression to COPD on wellcontrolled HIV patients [https://clinicaltrials.gov]. The role of immune dysfunction in the local lung environment and its contribution to the local and systemic chronic inflammatory state and local HIV persistence remains to be better defined. | |
| Co-pathogens | |
| Co-infection with pathogens able to produce a chronic carrier state like hepatitis C virus, cytomegalovirus, herpes human virus, and chronic endemic parasitic infections (Toxoplasmosis, Toxocariasis, Chagas) are common among HIV infected patients. It has been proposed that the chronic antigen exposure can activate immune cells rendering targets for new rounds of “de novo” infections [24]. This theory has driven some clinical trials targeting these chronic viral infections as a way to decrease the chronic inflammation and immune activation in HIV suppressed patients on ART [63] with heterogeneous results between studies. | |
| Other sources of inflammation | |
| On well-controlled HIV patients on HAART the source for the chronic “Inflammation and aging” could be, at least in part, damaged macromolecules and cells (self-debris). | |
| The HIV itself, immune-activated cells, free radicals from oxidative stress, chronic co-pathogens burden, gut/lung microbial translocation, HAART toxicity (mitochondrial toxicity with NRTIs), illicit drug use, alcohol abuse, malnutrition, and systemic inflammation all may generate a continuous antigenic stimulation for a dysfunctional and senescent immune system. These damaged molecules or debris could be misrecognized as “damage”-associated molecular patterns (DAMPs) by the innate immune system [6] starting the inflammatory process. The Nlrp3 inflammasome is a well-known source of inflammation and aging. It is a multiprotein complex that can activate pro-caspase-1 in response to cellular danger, which final result is the secretion of proinflammatory cytokines like IL-1B and IL-18 [64]. Since the Nlrp3 inflammasome is particularly sensitive to reactive oxygen species [ROS] derived from mitochondrial damage, the hypothesis that HAART (specially NRTIs) is a possible contributor to inflammation on these population, results attractive. | |
| Immune senescence | |
| It is well known that chronic HIV infection promote the “aging” not only of different organs but of the immune system as well. Continuous and persistent antigenic exposure (being “overwhelmed”) cannot be handled effectively by our immune system being the final outcome the “aging” of the immune system itself (Immune senescence). | |
| Early immune senescence may be seen as the genesis of the aging as well since senescent cells promote the secretion of pro-inflammatory cytokines (termed “senescent-associated secretory phenotype or SASP”). Of note, elimination of senescent cells from prematurely aged mice prevents aging of some [65]. As long as people age the adaptive immunity declines whereas the innate immunity predominates. It may be possible that in well-controlled HIV patients these changes are even more critical, with the changes in the innate immunity being driven by the continuous and dynamic antigen exposure on the gut and respiratory mucosa (“hot-spots”). | |
| Conclusion | |
| There is a debate in the literature regarding which of these seven factors is the initial insult that drives the persistent state of immuneactivation and chronic inflammation that promotes the “accelerated aging” in this population. An attractive hypothesis is one that consider these local pro-inflammatory environments (gut and respiratory mucosa) as the main generators favored by the fact that they are on direct contact with a continuous, persistent, and dynamic antigenic load (“lung and gut microbiome”). There is sufficient evidence in the literature to confirm that the gut mucosa meets not only the criteria to be one of the main generators of inflammation but a defined reservoir as well. As we discussed in our recent review [66] regarding the possibility of the respiratory mucosa being the second most important generator of inflammation, a lot of questions remain to be answered first in order to confirm that hypothesis. Even though HIV is a very complex and multi-compartmental disease, these two compartments may represent the main two generators of chemical signals that promote distal organ damage and viral replication on distal reservoirs like the CNS. | |
| The link between these two compartments and the accelerated aging process might be the microbial translocation on these two anatomical and functionally defective mucosal surfaces. Following this hypothetical model there might be then three main compartments: GI mucosa, respiratory mucosa, and the systemic compartment (which includes other well-known reservoirs like peripheral monocytes and CNS). New and novel targets needs to be defined in order to reach a functional cure, decrease the aging process, and prolong survival. Novel therapies (interleukins [66], biological agents, etc) combined with well-known old drugs [67] needs to be tested on well-designed prospective randomized controlled trials along with antiretroviral therapy with the ultimate goal of slowing down the ongoing accelerated aging process. | |
| Further exploration of pathogenicity models needs to be explored not only with serum markers of inflammation but with tissue samples (biopsies of the GI mucosa, bronchoalveolar lavage) as well. | |
| Acknowledgment | |
| Steibrunner, Jenni for helping us in the writing process. | |
References
- Alcaide ML, Parmigiani A, Pallikkuth S, Roach M, Freguja R, et al. (2013) Immune activation in HIV-infected aging women on antiretrovirals--implications for age-associated comorbidities: a cross-sectional pilot study. PLoS One 8: e63804.
- Chou JP, Ramirez CM, Wu JE, Effros RB (2013) Accelerated aging in HIV/AIDS: novel biomarkers of senescent human CD8+ T cells. PLoS One 8: e64702.
- Deeks SG, Tracy R, Douek DC (2013) Systemic effects of inflammation on health during chronic HIV infection. Immunity 39: 633-645.
- Meir-Shafrir K, Pollack S (2012) Accelerated Aging in HIV Patients. Rambam Maimonides Med J 3: e0025.
- Pathai S, Bajillan H, Landay AL, High KP (2013) Is HIV a Model of Accelerated or Accentuated Aging? The journals of gerontology Series A, Biological sciences and medical sciences.
- Franceschi C, Campisi J (2014) Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. The journals of gerontology Series A, Biological sciences and medical sciences 69 1:S4-S9.
- Hunt PW (2012) HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep 9: 139-147.
- Klatt NR, Chomont N, Douek DC, Deeks SG (2013) Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev 254: 326-342.
- Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, et al (2008) Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 47:542-553.
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153: 1194-1217.
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS (2010) The essence of senescence. Genes Dev 24: 2463-2479.
- Amin SG (2001) Control charts 101: a guide to health care applications. Qual Manag Health Care 9: 1-27.
- Benneyan JC, Lloyd RC, Plsek PE (2003) Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care 12: 458-464.
- Alfahad TR, Nath A (2013) Update on HIV-associated neurocognitive disorders. Curr Neurol Neurosci Rep 13: 387.
- Boulware DR, Hullsiek KH, Puronen CE, Rupert A, Baker JV, et al. (2011) Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 203: 1637-1646.
- Chambers LA, Wilson MG, Rueda S, Gogolishvili D, Shi MQ et al. (2013) Evidence Informing the Intersection of HIV, Aging and Health: A Scoping Review. AIDS and behavior.
- Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, et al. (2013) Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis 208: 1737-1746.
- Hsue PY, Deeks SG, Hunt PW (2012) Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis 205 Suppl 3: S375-382.
- Jaipersad AS, Lip GY, Silverman S, Shantsila E (2014) The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol 63: 1-11.
- Klatt NR, Funderburg NT, Brenchley JM (2013) Microbial translocation, immune activation, and HIV disease. Trends Microbiol 21: 6-13.
- Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, et al. (2008) Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 5: e203.
- Triant VA, Meigs JB, Grinspoon SK (2009) Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr 51: 268-273.
- Phyllis C, Tien MAIC, Zolopa AR, Benson C, Tracy R, et al. (2010) Inflammation and Mortality in HIV-Infected Adults: Analysis of the FRAM Study Cohort. Journal of acquired immune deficiency 55:316-322.
- Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW (2013) Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol 119: 51-83.
- Dinoso JB, Kim SY, Wiegand AM, Palmer SE, Gange SJ, et al (2009) Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proceedings of the National Academy of Sciences of the United States of America 106: 9403-9408.
- Gandhi RT, Zheng L, Bosch RJ, Chan ES, Margolis DM, et al. (2010) The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med 7.
- Hatano H, Hayes TL, Dahl V, Sinclair E, Lee TH, et al. (2011) A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis 203: 960-968.
- Hatano H (2013) Immune activation and HIV persistence: considerations for novel therapeutic interventions. Curr Opin HIV AIDS 8: 211-216.
- Reus S, Portilla J, Sánchez-Payá J, Giner L, Francés R, et al. (2013) Low-level HIV viremia is associated with microbial translocation and inflammation. J Acquir Immune Defic Syndr 62: 129-134.
- Schnabl B (2013) Linking intestinal homeostasis and liver disease. Curr Opin Gastroenterol 29: 264-270.
- Seneviratne AN, Sivagurunathan B, Monaco C (2012) Toll-like receptors and macrophage activation in atherosclerosis. Clin Chim Acta 413: 3-14.
- Vassallo M, Mercié P, Cottalorda J, Ticchioni M, Dellamonica P (2012) The role of lipopolysaccharide as a marker of immune activation in HIV-1 infected patients: a systematic literature review. Virol J 9: 174.
- Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, et al. (2010) Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog 6: e1001052.
- Campo M, Oursler KK, Huang L, Goetz MB, Rimland D, et al. (2014) Association of chronic cough and pulmonary function with 6-minute walk test performance in HIV infection. J Acquir Immune Defic Syndr 65: 557-563.
- Capocci S, Lipman M (2013) Respiratory infections in HIV-infected adults: epidemiology, clinical features, diagnosis and treatment. Current opinion in pulmonary medicine 19: 238-243.
- Collard HR, Morris A, Daley CL, Humbert M, Meyer KC (2012) Clinical year in review IV: HIV, mycobacterial disease, pulmonary hypertension, and interstitial lung disease. Proc Am Thorac Soc 9: 204-209.
- Cribbs SK, Park Y, Guidot DM, Martin GS, Brown LA, et al. (2014) Metabolomics of bronchoalveolar lavage differentiate healthy HIV-1-infected subjects from controls. AIDS Res Hum Retroviruses 30: 579-585.
- Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, et al. (2011) HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med 183: 388-395.
- Crothers K, McGinnis K, Kleerup E, Wongtrakool C, Hoo GS, et al. (2013) HIV infection is associated with reduced pulmonary diffusing capacity. J Acquir Immune Defic Syndr 64: 271-278.
- Dai HL, Zhang M, Xiao ZC, Guang XF, Yin XL (2014) Pulmonary arterial hypertension in HIV infection: a concise review. Heart Lung Circ 23: 299-302.
- Drummond MB, Kirk GD, Astemborski J, Marshall MM, Mehta SH, et al. (2012) Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax 67: 309-314.
- Drummond MB, Kirk GD, McCormack MC, Marshall MM, Ricketts EP, et al. (2010) HIV and COPD: impact of risk behaviors and diseases on quality of life. Qual Life Res 19: 1295-1302.
- Flannery B, Heffernan RT, Harrison LH, Ray SM, Reingold AL, et al. (2006) Changes in invasive Pneumococcal disease among HIV-infected adults living in the era of childhood pneumococcal immunization. Ann Intern Med 144: 1-9.
- George MP, Kannass M, Huang L, Sciurba FC, Morris A (2009) Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS One 4: e6328.
- Gingo MR, Balasubramani GK, Kingsley L, Rinaldo CR Jr, Alden CB, et al. (2013) The impact of HAART on the respiratory complications of HIV infection: longitudinal trends in the MACS and WIHS cohorts. PLoS One 8: e58812.
- Gingo MR, George MP, Kessinger CJ, Lucht L, Rissler B, et al. (2010) Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med 182: 790-796.
- Gingo MR, Morris A (2013) Pathogenesis of HIV and the lung. Curr HIV/AIDS Rep 10: 42-50.
- Gordon SB, Jagoe RT, Jarman ER, North JC, Pridmore A, et al. (2013) The alveolar microenvironment of patients infected with human immunodeficiency virus does not modify alveolar macrophage interactions with Streptococcus pneumoniae. Clin Vaccine Immunol 20: 882-891.
- Hirani A, Cavallazzi R, Vasu T, Pachinburavan M, Kraft WK, et al. (2011) Prevalence of obstructive lung disease in HIV population: a cross sectional study. Respir Med 105: 1655-1661.
- Hou W, Fu J, Ge Y, Du J, Hua S (2013) Incidence and risk of lung cancer in HIV-infected patients. J Cancer Res Clin Oncol 139: 1781-1794.
- Kunisaki KM (2014) Will expanded ART use reduce the burden of HIV-associated chronic lung disease? Curr Opin HIV AIDS 9: 27-33.
- Kynyk JA, Parsons JP, Para MF, Koletar SL, Diaz PT, et al. (2012) HIV and asthma, is there an association? Respir Med 106: 493-499.
- Lambert AA, Merlo CA, Kirk GD (2013) Human immunodeficiency virus-associated lung malignancies. Clin Chest Med 34: 255-272.
- Madeddu G, Fois AG, Calia GM, Babudieri S, Soddu V, et al. (2013) Chronic obstructive pulmonary disease: an emerging comorbidity in HIV-infected patients in the HAART era? Infection 41: 347-353.
- Morris A, Crothers K, Beck JM, Huang L; American Thoracic Society Committee on HIV Pulmonary Disease (2011) An official ATS workshop report: Emerging issues and current controversies in HIV-associated pulmonary diseases. Proc Am Thorac Soc 8: 17-26.
- Opravil M, Sereni D (2008) Natural history of HIV-associated pulmonary arterial hypertension: trends in the HAART era. AIDS 22 Suppl 3: S35-40.
- Petrache I, Diab K, Knox KS, Twigg HL 3rd, Stephens RS, et al. (2008) HIV associated pulmonary emphysema: a review of the literature and inquiry into its mechanism. Thorax 63: 463-469.
- Raju R, Peters BS, Breen RA (2012) Lung infections in the HIV-infected adult. Curr Opin Pulm Med 18: 253-258.
- Shcherba M, Shuter J, Haigentz M Jr (2013) Current questions in HIV-associated lung cancer. Curr Opin Oncol 25: 511-517.
- Sigel K, Wisnivesky J, Gordon K, Dubrow R, Justice A, et al. (2012) HIV as an independent risk factor for incident lung cancer. AIDS 26: 1017-1025.
- Winstone TA, Man SF, Hull M, Montaner JS, Sin DD (2013) Epidemic of lung cancer in patients with HIV infection. Chest 143: 305-314.
- Wein LM, Atkinson MP (2009) Assessing infection control measures for pandemic influenza. Risk Anal 29: 949-962.
- Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, et al. (2011) Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis 203: 1474-1483.
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, et al. (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104-107.
- Coppé JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5: 99-118.
- Gnoni M, Ramirez J (2014) Pulmonary Complications of HIV: Pulmonary Immunity (Chapter 3). European respiratory Society monographs; edited by Charles Feldman, Eva Polverino, and Julio Ramirez.
- Chevalier MF, Jülg B, Pyo A, Flanders M, Ranasinghe S, et al. (2011) HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J Virol 85: 733-741.
- Gnoni M, Otero D, Friedstrom S, Blatt S, Ramirez J (2015) Possible role of tetracyclines on decreasing the accelerated aging process of well-controlled HIV patients on antiretroviral therapy. HIV & AIDS Review, available online August 5 2015.
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 14225
- [From(publication date):
October-2015 - Aug 19, 2025] - Breakdown by view type
- HTML page views : 9563
- PDF downloads : 4662
