Soy Isoflavones (from Glycine max) in Menopause Health and Diseases
Received: 02-Dec-2017 / Accepted Date: 10-Dec-2017 / Published Date: 17-Dec-2017 DOI: 10.4172/2168-9652.1000225
Abstract
As per the third consensus meeting of Indian Menopause Society reports, the number of postmenopausal women in India is approximately 43 million and it is projected to be 103 million by 2026. While the average age for menopause in India is 47.5 years, there is an alarming increase of premature menopause among the Indian women has been reported recently. It has been observed that, nearly four per cent women of age group of 29-34 years attain menopause and its incidence increases to eight per cent in women of age group between 35 and 39 years. The reason for this early menopause is still unclear, but it is a major public health problem because of its influence on the development of components of metabolic syndrome. Despite HRT improves postmenopausal complications, the prolonged use of HRT was not recommended now due to its influence on the development of certain types of cancers; endometrial cancer and breast cancer in addition to its negative impact on cardiovascular diseases. Hence, understanding the cellular and molecular mechanisms which amenable for the pathogenesis of postmenopausal complications may open new clinical therapeutic targets for the management of these complications. Soy isoflavones, a natural phytoestrogen, which shares the structural and functional homogeneity with estrogen. Several studies have reported that soy isoflavones have beneficial effects against ovariectomy (surgical menopause) as well as diet induced metabolic complications in animal models; however, its efficacy in terms of improving metabolic complications associated with postmenopausal state is largely unclear.
Keywords: Post-menopause; Obesity; Metabolic syndrome; Soy isoflavones
Introduction
Menopause is a critical milestone in the life of women. Menopause is defined as “loss of estrogen production due to ovarian dysfunction”. Premenopausal women are more protected from developing metabolic syndrome and its associated metabolic complications; type 2 diabetes and cardiovascular diseases (CVD) when compared to men of similar age [1,2]. This protection is lost after menopause due to changes in the sex steroidal hormone profile. The public health impacts of postmenopausal complications are substantial since women spend virtually a one-third of their lives in the postmenopausal state worldwide [3]. In the direction of reports of the third consensus meeting of the Indian Menopause society, the number of postmenopausal women in India is approximately 43 million and it may reach 103 million by 2026 [4]. These observations highlight the importance of empathizing the molecular and cellular mechanisms that underlie the pathology of metabolic complications concomitant with postmenopausal population.
Postmenopausal cardiovascular complications
Evidence from recent studies reported that the occurrence/risk of obesity and metabolic syndrome (MetS) are three times higher in postmenopausal women when compared to the premenopausal women [5]. The ground for the increases in the weight gain and obesity after menopause is not clear, but it is considered as an important growing public health problem worldwide [6,7]. Recently, Clegg reported that estrogen deficiency after menopause promotes weight gain and fat accumulation in the waist region which ultimately leads obesity in postmenopausal population [8]. The lack of estrogen due to ovarian dysfunction after menopause accelerates endocrine and metabolic derangements predisposing to obesity, cardiovascular diseases and metabolic syndrome [9,10]. Consequently, the influence of estrogen deficiency in the patho-biology of obesity associated metabolic diseases in postmenopausal population is emerging as a plausible therapeutic challenge in the clinical gerontology of 21st century. In addition, there are other environmental factors such as intake of fat rich diet after menopause unique in the present generation which can further worsen the scenario. Perhaps it could be the combination of the aforementioned factors (i.e., estrogen deficiency and intake of fat rich diet) which leads to the pathogenesis; but the actual trigger for the postmenopausal obesity require further studies. The better understanding of the effect of how estrogen deficiency linked with the energy imbalance leads to lipid metabolic derangements promises to open a new novel clinical therapeutic strategy for an increasing large segment of the female population.
Postmenopausal inflammation
Past few decades most of the research focused on molecular link among oxidative stress and inflammation in response to aging and numerous metabolic and pathological consequences have been recognized, which have etiological origin of inflammation and the term “inflammaging” has been coined [11-13]. Thus, the modulation of oxidative stress and inflammation in the setting of ageing is a matter of prominent interest for gerontological research in modern years. Menopause, an age-related loss of ovarian function adds further complexity to the aging milieu. Many women experience weight gain and fat accumulation in the waist region during their menopausal period [14]. It is already reported that postmenopausal women are more prone to develop adiposity/obesity and its associated pathological sequelae when compared to the men of peer age [15]. Adipose tissue is not only a lipid storage organ, it is also considered as one of the positive instigators in the expansion of components of metabolic syndrome [16,17]. Increased visceral adiposity and obesity accompanied with age related exhaustion of ovarian function are implicated in the pathology of CVD [18]. The discrepancy in the production of inflammatory cytokines has been identified as a one of the major triggers, which involved in the development of CVD associated with postmenopausal obesity [19-22]. Furthermore, the heightened expression of inflammatory cytokine TNFα has been observed in response to estrogen decline after menopause, which positively correlates with increased CVD risk [23]. Moreover, evidence from clinically relevant studies also showed the elevated levels of proinflammatory cytokines in postmenopausal populace [24,25]. Controversially, Yasui T et al. reported that in early stage of menopause (particularly less than five years after menopause), the proinflammatory cytokines levels in the serum were increased and return to the normal levels in the late stage, with values parallel to pre menopause phase [26]. Recently Ludgero et al. stated that ovariectomy in C57BL/6J mice did not escalate the levels of TNFα and IL6 in serum and ovarian adipose tissue. However, when ovariectomy was followed by high fat diet, these inflammatory proteins levels were elevated [27]. Thus, it is unclear whether estrogen deficiency could attribute to the development of inflammatory response in postmenopausal women and the skepticism has developed lately concerning the role of estrogen deficiency in causing inflammatory response. Therefore, the etiology of inflammation in postmenopausal women is still a complex issue and further studies are required to explore the same.
Postmenopausal oxidative stress
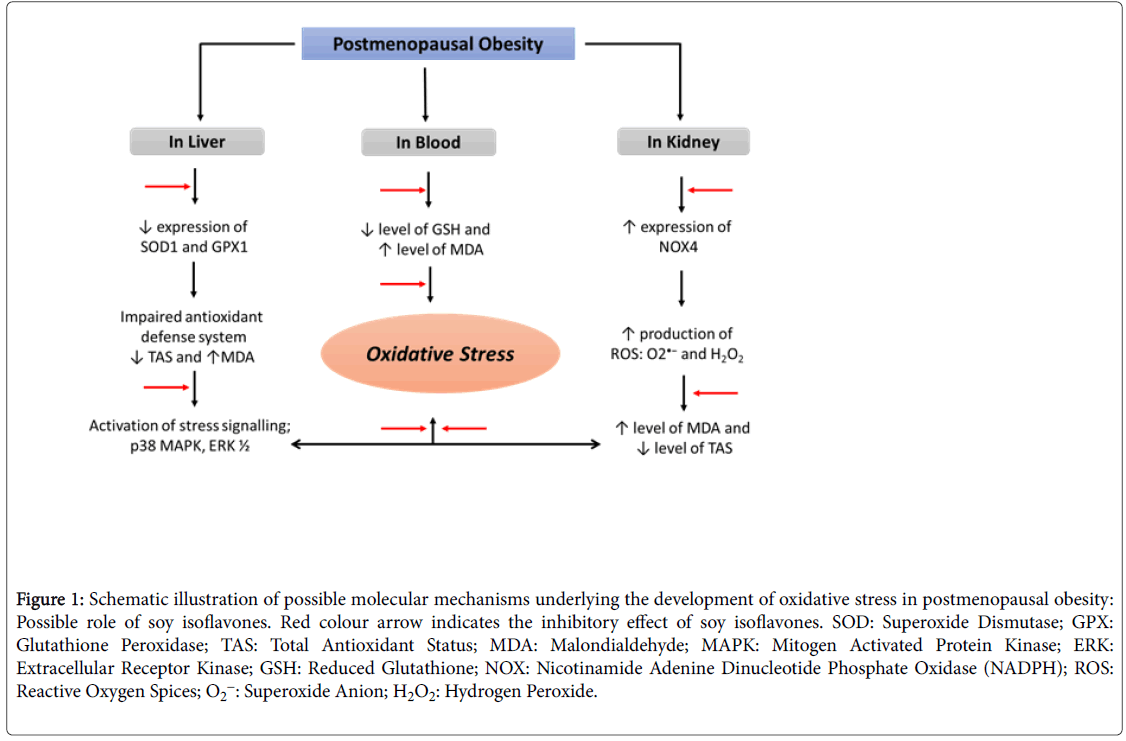
Oxidative stress, a dysregulation in the redox homeostasis believed to play a crucial role in ageing sequelae and caused by over production of highly reactive oxygen species (ROS), which overwhelm the body anti-oxidant defense system [28,29]. There is an adequate balance between the oxidants and anti-oxidants levels in healthy premenopausal women and the level of oxidative stress in these women is insufficient in terms of disturbing the biology of ovaries until the onset of menopause. Menopause creates a redox imbalance and subsequent oxidative stress due to the decline of the natural antioxidant hormone estrogen [30]. Despite the fact that estrogen deficiency leads to oxidative stress, the question often arises whether oxidative stress causes menopause or is it the menopause, which is responsible for the oxidative stress, is still unclear. Recently, Sanchez et al. stated that the menopause alone can be one of the potential triggering or risk factors for oxidative stress [31]. However, the mechanisms amenable for the pathogenesis of menopause induced oxidative stress remains unclear and the problem needs to be addressed (Figure 1).
Figure 1: Schematic illustration of possible molecular mechanisms underlying the development of oxidative stress in postmenopausal obesity: Possible role of soy isoflavones. Red colour arrow indicates the inhibitory effect of soy isoflavones. SOD: Superoxide Dismutase; GPX: Glutathione Peroxidase; TAS: Total Antioxidant Status; MDA: Malondialdehyde; MAPK: Mitogen Activated Protein Kinase; ERK: Extracellular Receptor Kinase; GSH: Reduced Glutathione; NOX: Nicotinamide Adenine Dinucleotide Phosphate Oxidase (NADPH); ROS: Reactive Oxygen Spices; O2-: Superoxide Anion; H2O2: Hydrogen Peroxide.
Postmenopausal insulin resistance
Insulin resistance (IR), a state of impaired ability of peripheral tissues to respond to normal circulating insulin, is the predominant defect in type 2 diabetes (type 2 DM) [32]. Menopause is one of the higher risks for developing IR and subsequently type 2 DM [33]. However, the question remains about why postmenopausal women have an increased risk of diabetes when compared with peer-aged men. The incidence of diabetes is less in women than in men aged less than 60 years. While women after 60 years of their age, the risk of developing diabetes increased more than men of similar age [34]. These findings clearly indicate that the fluxes in the production of sex hormones after menopause might be accompanied with the risk of diabetes [35]. Though, the association between menopause and type 2 diabetes has been established [36-40], conclusion about whether the menopause by themselves influence the pathology of type 2 diabetes independently of aging remains controversial. In 2005, the crosssectional study conducted by Donato et al. among the Italian women, reported a positive correlation between the menopausal state and diabetes independently of age [41]. However, a recent study conducted by Lee et al., among the Japanese population fails to show the positive association between the menopause state and diabetes when amendment is made for chronological age [42]. In the face of the aforementioned findings, it is controversial whether menopause by itself led to diabetes or not.
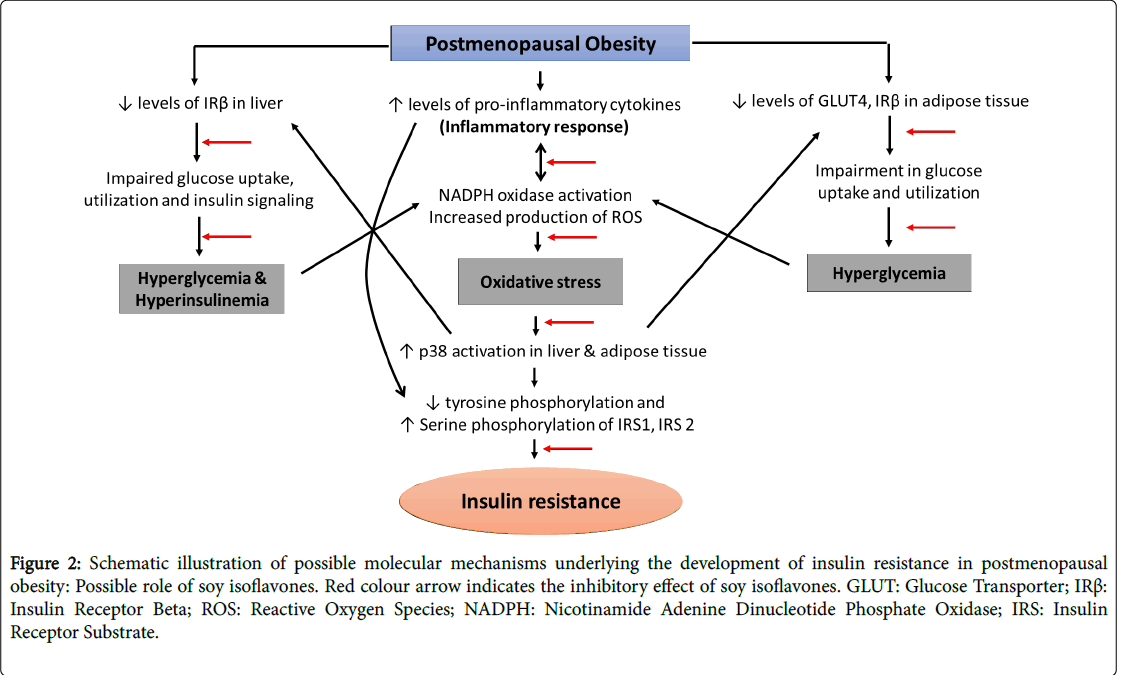
Evidence, extending from in vitro experiments and animal to human studies has showed compelling evidence for the pivotal role of oxidative stress, inflammation and adiposity in the pathogenesis of insulin resistance concomitant with postmenopausal women [43-46]. Conversely, insulin resistance has been shown to cause imbalance in the redox status, pro versus anti-inflammatory cytokines and deranged lipid homeostasis with the resultant oxidative stress, inflammation and adiposity/obesity respectively in experimental animals [47-52]. Taken together, these observations suggest the concept that oxidative stress, inflammation, obesity and insulin resistance participate in a vicious cycle, by which each of the four factors can recruit and amplify each other. This cycle plays a central role in the development of other metabolic complications associated with the postmenopausal population. Though, several molecular mechanisms amenable for the development of postmenopausal complications has been established, the etiological origin and management of metabolic complications concomitant with these complications remain unclear and the problem needs to be explored (Figure 2).
Figure 2: Schematic illustration of possible molecular mechanisms underlying the development of insulin resistance in postmenopausal obesity: Possible role of soy isoflavones. Red colour arrow indicates the inhibitory effect of soy isoflavones. GLUT: Glucose Transporter; IRß: Insulin Receptor Beta; ROS: Reactive Oxygen Species; NADPH: Nicotinamide Adenine Dinucleotide Phosphate Oxidase; IRS: Insulin Receptor Substrate.
Hormone replacement therapy
Hormone replacement therapy (HRT) also known as menopausal replacement therapy has been extensively investigated as a promising treatment for the debilitating signs and sequelae allied with the menopausal populace [53]. HRT can be effective at alleviating the moderate to severe symptoms of the menopausal transition [53]. Lindheim et al. and Margolis et al. reported that HRT can alleviate glucose intolerance and insulin resistance in postmenopausal women and improves whole body glucose homeostasis [33,54]. The Nurses Health Study reported 40% attenuation in CVD risk in women who used HRT after menopause [55]. In addition, estrogen suppressed cardiovascular resistance and enhanced coronary blood flow and attenuated myocardial ischemia [56,57]. Recently, it has been stated that there is a convinced window during the postmenopausal period in which HRT is helpful. Outside this time frame, prolonged HRT can have detrimental effects. Evidence from large-scale clinical trials, particularly the Women’s Health Initiative (WHI), reported that women who receive HRT during their menopausal period exhibited an increased incidence of both CVD and stroke [58]. However, there is no justification for this discrepancy, i.e., whether estrogen is useful or harmful for women in term of cardiovascular function. Furthermore, prior studies also reported that, prolonged use of HRT might raise the risk of developing certain type of cancer; endothelial cancer and breast cancer in addition to its negative impact on cardiovascular events [58,59]. In light of the above unexpected findings that HRT could actually aggravate cardiovascular dysfunction and causes cancer, there is accumulative evidence that the alternative therapies appear to be a critical factor in determining the overall outcome of HRT. The search is for a natural or synthetic origin which will possess less undesirable effects and can alternate/replace or reduce the need of present strategy HRT.
Beneficial role of soy isoflavones
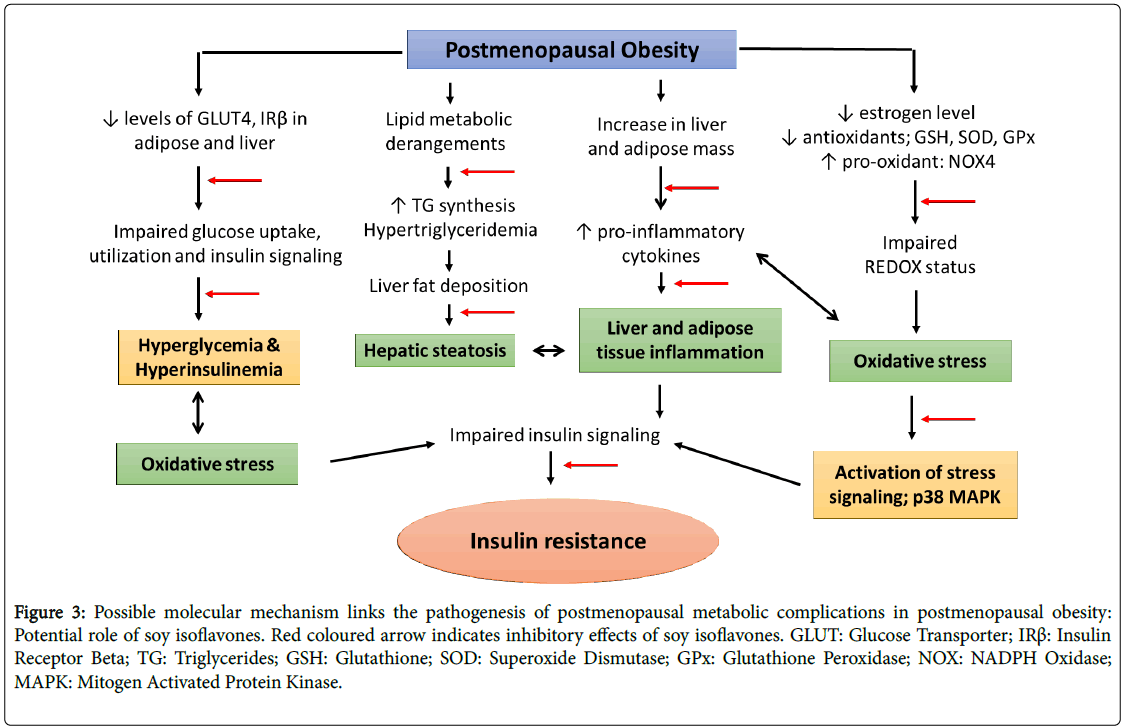
Soy isoflavones, a group of naturally occurring phytoestrogens shares the structural and functional homogeneity with estrogen. Consumption of phytoestrogens rich diet may have protective effects on postmenopausal symptoms [60]. In addition, the consumption of natural phytoestrogens as an alternative intervention is allied with lower occurrence of postmenopausal symptoms; breast cancer and endometrial cancer in Asian women [61,62]. Numerous studies including epidemiological studies to human and animal experiments have also showed beneficial effects of soy isoflavones against the inflammatory associated cardiovascular diseases [63-67]. The beneficial effects of soy isoflavones have been studied in animal model; however its efficacy in terms of ameliorating the metabolic complications concomitant with postmenopausal model of obesity is largely unclear (Figure 3).
Figure 3: Possible molecular mechanism links the pathogenesis of postmenopausal metabolic complications in postmenopausal obesity: Potential role of soy isoflavones. Red coloured arrow indicates inhibitory effects of soy isoflavones. GLUT: Glucose Transporter; IRß: Insulin Receptor Beta; TG: Triglycerides; GSH: Glutathione; SOD: Superoxide Dismutase; GPx: Glutathione Peroxidase; NOX: NADPH Oxidase; MAPK: Mitogen Activated Protein Kinase.
Conclusion
Both experimental menopause and experimental obesity in isolation and in combination caused oxidative stress, inflammation, insulin resistance and lipid derangements and hepatic steatosis. The severity of these complications was further augmented when ovariectomy was followed by high fat diet, suggesting a synergistic role of postmenopausal state and the intake of fat rich diet in the development of metabolic complications. Treatment with soy isoflavones markedly alleviated these metabolic complications suggesting the use of this natural phytoestrogen as a strategy for relieving the metabolic complications associated with the postmenopausal women. The present study further strengthening the concept that, the soy isoflavones exhibits potent anti-oxidant, anti-inflammatory, antidiabetic and anti-lipidemic properties.
Based on the above findings, we may conclude that women after postmenopausal state may restrict intake of fat rich diet especially those who have a genetic predisposition for the development of components of metabolic syndrome. Consumption of soy based food after menopause may be beneficial for the management of metabolic complications.
References
- Grygiel-Górniak B, Marcinkowska J, Szczepanik A, Przysławski J (2014) The nutritional and oxidative stress implications of post-menopausal age. Pol Arch Med Wewn 124: 298-305.
- Shapiro Y, Mashavi M, Luckish E, Shargorodsky M (2014) Diabetes and menopause aggravate age-dependent deterioration in arterial stiffness. Menopause 21: 1234-8.
- Rogers NH, Perfield JW, Strissel KJ, Obin MS, Greenberg AS (2009) Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150: 2161-2168.
- Unni J (2010) Third consensus meeting of Indian Menopause Society (2008) - A summary. J Life Health 1: 43-47.
- Eshtiaghi R, Esteghamati A, Nakhjavani M (2010) Menopause is an independent predictor of metabolic syndrome in Iranian women. Maturitas 65: 262-266.
- Awa WL, Fach E, Krakow D (2012) Type 2 diabetes from pediatric to geriatric age; analysis of gender and obesity among 120 183 patients from the German/Austrian DVP database. Eur J Endocrinol 167: 245-254.
- Weitlisbach V, Marques-Vidal P, Kuulasmaa K, Karvanen J, Paccaud F (2013) The relation of body mass index and abdominal obesity wiht dyslipidemia in 27 general populations of the WHO MONICA project. Nutr Metab Cardiovasc Dis NMCD 2: 432-442.
- Clegg D J (2012) Mini review; the year in review of estrogen regulation of metabolism. Mol Endocrinol 26: 1957-1960.
- Carr MC (2003) The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88: 2404-2411.
- Mauvais-Jarvis F (2011) Estrogen and androgen receptors; regulator of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab 22: 24-33.
- Bal A, Unlu E, Bahar G, Aydog E, Eksioglu E, et al. (2007) Comparison of serum IL-1 beta, sIL-2R, IL-6 and TNF-alpha levels with disease activity parameters in ankylosing spondylitis. Clin Rheumatol 26: 211-215.
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, et al. (2007) Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128: 92-105.
- Toth MJ, Ades PA, Tischler MD, Tracy RP, LeWinter MM (2006) Immune activation is associated with reduced skeletal muscle mass and physical function in chronic heart failure. Int J Cardiol 109: 179-87.
- Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, et al. (2012) Understanding weight gain at menopause. Climacteric J Int Menopause Soc 15: 419-429.
- Sharma S, Bakshi R, Tandon VR, Mahajan A (2008) Post-menopausal obesity. JK Sci 10: 105-106.
- Chedraui P (2009) Metabolic changes and the menopause in Ecuador. Maturitas 63: S19.
- McCarthy JJ (2006) Gene by sex interaction in the etiology of coronary heart disease and the preceding metabolic syndrome. Nat Metab Cardiovasc Dis 17: 153-161.
- Teede HJ, Lombard C, Deeks AA (2010) Obesity, metabolic complications and the menopause: An opportunity for prevention. Climacteric 13: 203-209.
- Loos BG (2005) Systemic markers of inflammation in periodontitis. J Peridontol 76: 2106-2115.
- McFarlane SI, Muniyappa R, Shin JJ, Bahtiyar G, Sowers JR (2004) Osteoporosis and cardiovascular disease: Brittle bones and boned arteries, is there a link? Endocrine 23: 1-10.
- Ross R (1999) Atherosclerosis-an inflammatory disease. N Engl J Med 340: 115-126.
- Willerson JT, Ridker PM (2004) Inflammation as a cardiovascular risk factor. Circulation 109: II2-10.
- Baldini V, Mastropasqua M, Francucci CM, D’Erasmo E (2005) Cardiovascular disease and osteoporosis. J Endocrinol Investig 28: 69-72.
- Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP (2005) Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation 12: 255-269.
- Weitzmann MN, Pacifici R (2006) Estrogen deficiency and bone loss: An inflammatory tale. J Clin Invest 116: 1186-1194.
- Yasui T, Maegawa M, Tomita J, Miyatani Y, Yamada M, et al. (2007) Changes in serum cytokine concentrations during the menopausal transition. Maturitas 56: 396-403.
- Ludgero-Correia A, Aguila MB, Mandarim-de-Lacerda CA, Faria TS (2012) Effects of high-fat diet on plasma lipids, adiposity and inflammatory markers in ovariectomized C57BL/6 mice. Nutrition 28: 316-323.
- Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S (2012) The role of oxidative stress of female reproduction. A review. Reprod Biol Endocrinol RBE 10:1-32.
- Ruder EH, Hartman TJ, Bumberg J, Goldberg MB (2008) Oxidative stress and antioxidants: Exposure and impact on female infertility. Hum Reprod Update 14: 345-357.
- Behrman HR, Kodaman PH, Preston SL, Gao S (2001) Oxidative stress and the ovary. J Soc Gynecol Investig 8: S40–42.
- Sánchez-RodrÃguez MA, ZacarÃas-Flores M, Arronte-Rosales A, Correa-Muñoz E, Mendoza-Núñez VM (2012) Menopause as risk factor for oxidative stress. Menopause N Y N 19: 361-367.
- Zimmet P, Alberti KG, Shaw J (2001) Global and societal implications of the diabetes epidemic. Nature 414: 782-787.
- Lindheim SR, Buchanan TA, Duffy DM, Vijod MA, Kojima T, et al. (1994) Comparison of estimates of insulin sensitivity in pre- and post-menopausal women using the insulin tolerance test and the frequently sampled intravenous glucose tolerance test. J Soc Gynecol Investig 1: 150-154.
- Yoriko H, Kasumi A, Yasumi S, Hitoshi S, Yasuji A, et al. (2013) Effect of post-menopausal status and age at menopause on type 2 diabetes and pre-diabetes in Japanese individuals: Toranomon hospital health management centre study 17 (TOPICS 17). Diabetes Care 36: 4007-4014.
- Szmuilowicz ED, Stuenkel CV, Seely EW (2009) Influence of menopause on diabetes and diabetes risk. Nat Rev Endocrinol 5: 553-558.
- Feng Y, Hong X, Wilker E, Zhang W, Zang T, et al. (2008) Effects of age at menarche, reproductive years and menopause on metabolic risk factors for cardiovascular diseases. Atherosclerosis 196: 590-597.
- He L, Tang X, Li N, Wang JW, Zhang ZX, et al. (2012) Menopause with cardiovascular disease and its risk factors among rural Chinese women in Beijing: A population based study. Maturitas 72: 132-138.
- Kim C, Edelstein SL, Crandall JP, Dabelea D, Kitabchi AE, et al. (2011) Diabetes Prevention Program Research Group. Menopause and risk of diabetes in the Diabetes Prevention Program. Menopause 18: 857-868.
- Park HA, Park JK, Park SA, Lee JS (2010) Age, menopause and cardiovascular risk factors among Korean middle-aged women: The 2005 Korea National Health and Nutrition Examination Survey. J Womens Health (Larchmt) 19: 869-876.
- Zivkovic TB, Vuksanovic M, Jelic MA, Stojanovic J, Buric B, et al. (2011) Obesity and metabolic syndrome during the menopause transition in Serbian women. Climacteric 14: 643-648.
- Di Donato P, Giulini NA, Bacchi MA, Cicchetti G, Comitini G, et al. (2005) Gruppo di Studio Progetto Menopausa Italia. Risk factors for type 2 diabetes in women attending menopause clinics in Italy: A cross-sectional study. Climacteric 8:287-293.
- Lee JS, Hayashi K, Mishra G, Yasui T, Kubota T, et al. (2013) Independent association between age at natural menopause and hypercholesterolemia, hypertension and diabetes mellitus: Japan nurses’ health study. J Atheroscler Thromb 20: 161-169.
- Abildgaard J, Henstridge DC, Pedersen AT, Langley KG, Scheele C, et al. (2014) In vitro palmitate treatment of myotubes from post-menopausal women leads to ceramide accumulation, inflammation and affected insulin signalling. PLoS One 9:e101555.
- Macciò A, Madeddu C (2011) Obesity, inflammation and post-menopausal breast cancer: Therapeutic implications. ScientificWorldJournal 11: 2020-2036.
- Phillips GB, Jing T, Heymsfield SB (2008) Does insulin resistance, visceral adiposity or a sex hormone alteration underlie the metabolic syndrome? Studies in Women. Metabolism 57: 838-844.
- Sorkin JD, Vasaitis TS, Streeten E, Ryan AS, Goldberg AP (2014) Evidence for threshold effects of 25-hydroxyvitamin D on glucose tolerance and insulin resistance in black and white obese post-menopausal women12. J Nutr 144:734-742.
- Jung UJ, Choi MS (2014) Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and non-alcoholic fatty liver disease. Int J Mol Sci 15: 6184-6223.
- Roberts CK, Hevener AL, Barnard RJ (2013) Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr Physiol 3:1-58.
- Samuel VT, Petersen KF, Shulman GI (2010) Lipid-induced insulin resistance: Unravelling the mechanism. Lancet 375: 2267-2277.
- Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L (2013) Obesity-associated oxidative stress: Strategies finalized to improve redox state. Int J Mol Sci 14: 10497-10538.
- Styskal J, Van Remmen H, Richardson A, Salmon AB (2012) Oxidative stress and diabetes: What can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic Biol Med 52: 46-58.
- Tangvarasittichai S (2015) Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes 6: 456-80.
- Mueck AO, Seeger H (2004) Estrogens acting as cardiovascular agents: Direct vascular actions. Curr Med Chem Cardiovasc Haematol Agents 2: 35-42.
- Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, et al. (2004) Effect of oestrogen plus progestin on the incidence of diabetes in post-menopausal women: Results from the women’s health initiative hormone trial. Diabetologia 47: 1175-1187.
- Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Willett WC, et al. (1996) Post-menopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med 335: 453-461.
- Raddino R, Manca C, Poli E, Bolognesi R, Visioli O (1986) Effects of 17 beta-estradiol on the isolated rabbit heart. Arch Int Pharmacodyn Ther 281: 57-65.
- Reis SE, Gloth ST, Blumenthal RS, Resar JR, Zacur HA, et al. (1994) Ethinyl estradiol acutely attenuates abnormal coronary vasomotor responses to acetylcholine in post-menopausal women. Circulation 89: 52-60.
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, et al. (2002) Risks and benefits of estrogen plus progestin in healthy post-menopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. J Am Med Assoc 288: 321-333.
- McKenzie J, Fisher BM, Jaap AJ, Stanley A, Paterson K, et al. (2006) Effects of HRT on liver enzyme levels in women with type 2 diabetes: A randomized placebo-controlled trial. Clin Endocrinol (Oxf) 65: 40-44.
- Lethaby A, Marjoribanks J, Kronenberg F, Roberts H, Eden J, et al. (2013) Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev 12: CD001395.
- Adlercreutz H (2002) Phyto-oestrogens and cancer. Lancet Oncol 3: 364-373.
- Ingram D, Sanders K, Kolybaba M, Lopez D (1997) Case-control study of phyto-oestrogens and breast cancer. Lancet 350: 990-994.
- Kagan A, Harris BR, Winkelstein W Jr, Johnson KG, Kato H, et al. (1974) Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: Demo-graphic, physical, dietary and biochemical characteristics. J Chronic Dis 27: 345-364.
- Cassidy A, Hooper L (2006) Phytoestrogens and cardiovascular disease. J Br Menopause Soc 12: 49-56.
- Hall WL, Vafeiadou K, Hallund J, Bugel S, Koebnick C, et al. (2005) Soy-isoflavone-enriched foods and inflammatory biomarkers of cardiovascular disease risk in post-menopausal women: Interactions with genotype and equal production. Am J Clin Nutr 82: 1260-1268.
- Curran EM, Judy BM, Newton LG, Lubahn DB, Rottinghaus GE, et al. (2004) Dietary soy phytoestrogens and ER alpha signalling modulate interferon gamma production in response to bacterial infection. Clin Exp Immunol 135: 219-225.
- Verdrengh M, Jonsson IM, Holmdahl R, Tarkowski (2003) Genistein as an anti-inflammatory agent. Inflamm Res 52: 341-346.
Citation: Sankar P, Bobby Z, Mirza AA (2017) Soy Isoflavones (from Glycine max) in Menopause Health and Diseases. Biochem Physiol 6: 225. DOI: 10.4172/2168-9652.1000225
Copyright: 2017 Sankar P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 9178
- [From(publication date): 0-2017 - Dec 22, 2025]
- Breakdown by view type
- HTML page views: 8071
- PDF downloads: 1107