Stimulation of Anterior Thalamic Nucleus Protects Hippocampus Neural Injury in Kainic Acid-Induced Epileptic Rats
Received: 10-Sep-2017 / Accepted Date: 18-Sep-2017 / Published Date: 25-Sep-2017 DOI: 10.4172/2161-0460.1000377
Abstract
Anterior nucleus of thalamus (ANT) stimulation has been proved to be effective in the treatment of refractory epilepsy, but the underlying mechanisms remain to be elucidated. We examined the role of ANT stimulation on hippocampal neuron death after seizures induced by kainic acid (KA). Our data showed that ANT stimulation could significantly rescue neurons from death induced by seizures, by reducing the release of cytochrome c (cyto c) and also via apoptosis-inducing factor (AIF) induced by seizures through inhibiting the activated caspase-9 and caspase-3. Our data suggest that ANT stimulation may protect against neuronal loss and reduce neuronal injury in the ipsilateral CA3 region of the hippocampus in the KA-induced epileptic rats, and the underlying mechanism may be mediated by inhibiting mitochondrial caspase-dependent (cyto c release and the subsequent cleavage of caspase-) and caspase-independent (nuclear translocation of AIF) apoptosis pathways.
Keywords: Epilepsy; Anterior nucleus of thalamus; Stimulation; Apoptosis; Neuroprotection; Cytochrome c; Apoptosis-inducing factor
Introduction
Epilepsy is a progressive neurodegenerative disorder characterized by an enduring predisposition to generate seizures [1]. Temporal lobe epilepsy (TLE) is the most common type of pharmaco-resistant epilepsy in adults, and one third patients is poorly controlled or their seizures are refractory to drug treatment [2]. The resection of mesial temporal structures can lead to the absence of seizures in more than 70% of patients [3]. However, such surgery is not suitable for up to 30% TLE patients [4]. High frequency stimulation (HFS) has become an alternative treatment for intractable epilepsy, as HFS can be tailored to address the specific requirements of the patients. Although several small-scale studies on patients with anterior nucleus of thalamus (ANT) stimulation showed a similar significant reduction of approximately 50% in the number of seizures [5], the underlying mechanism of how ANT stimulation decreases seizure frequency is still unknown. Apoptotic cell death has emerged as an important factor in brain injury produced by prolonged epileptic seizures or status epilepticus [6]. Our previous study has demonstrated that hippocampal high frequency stimulation has protective on hippocampal neurons by inhibition of apotosis in epilepsy macaques [7]. Furthermore, we have proposed hypotheses that ANT-DBS could have neuroprotective effects in epilepsy patients by regulating the metabolism of amino acids [8]. Yet, it is still unclear whether ANT stimulation has protection on hippocampal neurons. Kainic acid (KA), a potent neuroexcitatory amino acid has been widely used to establish TLE models, which closely imitates the pathophysiological alterations in patients with TLE [9,10]. Intra-hippocampal KA injection in rats has been widely used a model to study the mechanisms of seizure-induced neuronal cell death in the hippocampus [11,12]. Therefore, in the present study, we examined the protective effects and potential mechanisms of ANT stimulation on hippocampal neurons in kainic acid epilepsy rats.
Materials and Methods
Animals
Male Sprague Dawley rats weighing 280 g-320 g were provided by Experimental Animal Center of Military Medical Sciences, China. Animals were individually housed in a cage (constant temperature, 22-25°C; humidity, 60-70%; illumination, 12/12 h cycles) with free access to food and water. All experiments were performed during the light phase of the cycle. The care and handling of animals were conducted in compliance with the Chinese Animal Welfare Act and was approved by the responsible governmental agency at Capital Medical University. Sixty-four rats were randomized into four groups: (1) control group (n=16), underwent administration of saline; (2) KA model group (n=16), underwent administration of KA; (3) sham stimulation group (n=16), received KA injections with implantation of electrodes into the ipsilateral ANT but without stimulation; (4) stimulation group (n=16), received KA injections with implantation of electrodes into the ipsilateral ANT and stimulation.
Seizure model
All rats were anesthetized with chloral hydrate (350 mg/kg, i.p.) and mounted in a stereotaxic frame (David Kopf Instruments, USA). Right injections of KA into the CA3 region of the hippocampus were injected at the following coordinate: A/P=5.6 mm, R=4.5 mm, V=-5.5mm [13]. KA, at a dose of 0.5 μg/0.2 μL (in phosphate buffer) for a 250 g rat (and adjusted according to body weight), was injected slowly during 10 min with a 0.5 μl Hamilton micro syringe in KA group, sham stimulation group and stimulation group [14]. Rats of the control group were stereotaxically injected with equivalent saline instead. The rats were continuously monitored for behavioral seizures for 24 h after injection.
Implantation of electrode
Three weeks later, the concentric bipolar stimulation electrodes (outer diameter 200 mm, inner diameter 50 mm, CBBPF50, FHC, USA) were glued to the guide cannula with a specially made device and the electrode tips were 2 mm below the end of the guide cannula. One cannula was implanted for right ANT (AP=-2.0 mm, R=1.8 mm, V=4.7 mm) within sham stimulation group and stimulation group [13]. The cannulas were cemented in place using dental cement to affix them to three stainless steel screws fastened to the skull. Penicillin (6 × 107 U) was administered intraperitoneally three times a day for the first three days after surgery. After a recovery of seven days, continuous unilateral ANT stimulation was then performed using a stimulator system (Master-8 and ISO-flex, Israel). Parameters were: 500 μA, 60 μs and 130 Hz. However, the sham stimulation group was manipulated in the same way but without electrical stimulation.
Fixation and processing of tissue
After three months, the rats were harvested and ipsilateral hippocampus resection was then performed. A portion of each resected sample was immediately placed in liquid nitrogen for subsequent western blot analysis; another portion was fixed in 10% buffered formalin and 2.5% glutaraldehyde for paraffin embedding, Nissl staining, apoptosis detection and electron microscope.
Nissl staining and apoptosis detection
Tissues were post-fixed for 24 h and then placed for another 24 h in 30% sucrose in 0.1 M phosphate buffer. Coronal sections (12 μm) underwent Nissl staining with Toluidine Blue and terminal deoxynucleotidyl transferase-mediated Dutp nick-end labeling (Tunel) staining with a peroxidase in situ apoptosis detection kit (Roche Molecular Biochemincals, Indianapolis, IN). Tunel staining was performed was used as recommended by the manufacturer. Cell counts were performed on n=6 brain sections for the entire hippocampal CA3 subfields. And the numbers of surviving hippocampal CA3 region neurons or the Tunel-positve neurons at 100 μm in diameter were automatically counted in blind manner.
Preparation of cystosolic fraction and mitochondrial fractions
The cytosolic fraction was generated according to preivous reported methods [15]. Briefly, tissue was homogenized in buffer containing 100 mM sucrose, 1 mM EGTA, 20 mM MOPS, pH 7.4 with 5% Ficoll, 0.01% digitonin, 1 μg/ml pepstatin, 2 μg/ml leupeptin and 100 μg/ml PMSF and incubated for 15 min on ice. Samples were then centrifuged at 2500x g for 10 min to precipitate the nuclei and cellular debris. The supernatant was centrifuged at 15,000x g for 15 min to pellet mitochondria. Mitochondria were collected at the 1-1.5 M interphase by lateral suction through the tube, washed in four volumes of MS buffer with centrifugation at 15,000x g between washes, and resuspended in a final volume of 200 ml MS buffer. The supernatant was subsequently centrifuged at 100, 000x g for 60 min to obtain the cytosol (supernatant) [15].
Western blotting
Protein was extracted from samples in 100 μl lysis buffer and concentration was determined using the bicinchoninic acid protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA). Samples of 4-10 μg protein were resolved by 10% SDS-PAGE, and transferred to a polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA, USA).
These were blocked with 5% nonfat dry milk in Tris-buffered saline and 0.1% Tween 20, and probed with antibodies against actin (1:5000, Abcam), apoptosis-inducing factor (AIF, 1:2000, Sigma), cytochrome c (cyto c) (1:1000, Sigma), caspase-9 (1:1000, Sigma) cleaved caspase-3 (1:1000, Sigma). The band was detected using a chemiluminescent peroxidase substrate followed by exposure to X-ray film. The density of bands on the membrane were scanned and analyzed using an image software (Alpha Innotech, San Leandro, CA, USA).
Transmission electron micorscopy
For electron microscopy, the samples were fixed with 1% w/v osmium tetroxide, dehydrated in a graded ethanol and embedded in Epon. Ultrathin sections (70 nm) were cut with a microtome (Bromma, Sweden), counterstained with uranyl acetate and lead citrate and viewed using an electron microscope (Hitachi H7650, Tokyo, Japan).
Statistical Analysis
Statistical analysis was performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as mean ± SD. Data were analyzed using one-way analysis of variance (ANOVA) with a post hoc test. A P value <0.05 was considered statistically significant.
Results
Effects of ANT stimulation on hippocampal neuronal cells
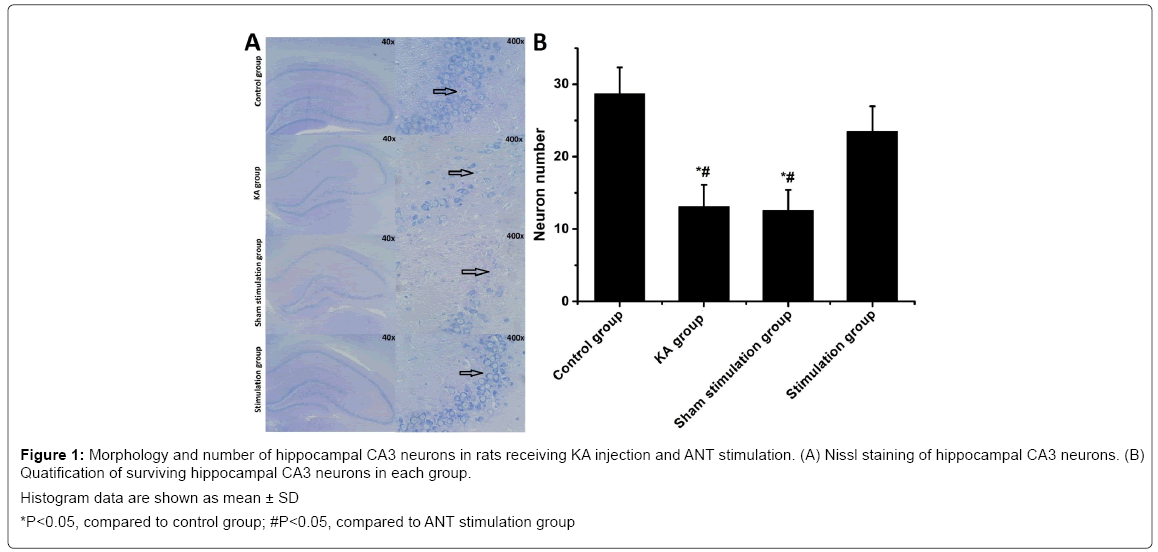
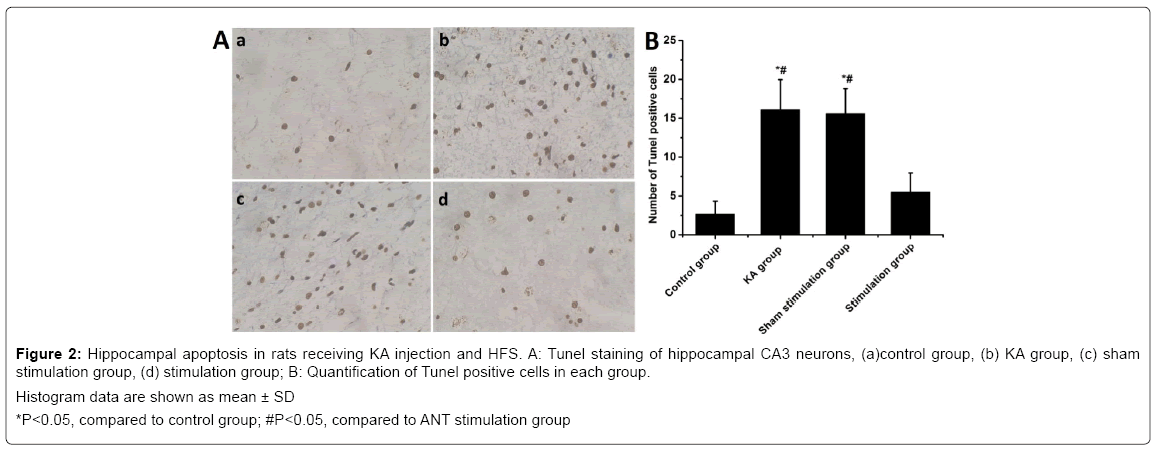
Nissl staining revealed that seizures led to significant neuronal loss in the hippocampal CA3 regions of the KA group. The number of surviving neurons was decreased significantly compared with the control (P<0.05), but ANT stimulation in stimulation group significantly attenuated the neuronal loss induced by seizure compared with KA group (Figure 1). However, no significant difference in neuronal loss were found between KA group and sham stimulation group (P>0.05). A large number of Tunel positive neurons were found in ipsilateral CA3 of the KA group compared with that of the control group. In contrast, stimulation group with ANT stimulation show remarkedly decreased Tunel positive cells compared with KA group (Figure 2). However, there was no significant difference in the number of CA3 Tunel positive cells between sham stimulation group and KA group. These results demonstrated that KA-induced seizures caused prominent neuronal loss in the hippocampus and ANT stimulation could rescue neurons from death induced by seizures. Electron microscopically, loss of nucleolus and granularly coagulated chromosome aggregated under the membrane with high electron density were observed in the KA group and the sham stimulation group (Figure S1). However, no damaged neural cells were found in the controls and in the stimulation group.
Figure 1: Morphology and number of hippocampal CA3 neurons in rats receiving KA injection and ANT stimulation. (A) Nissl staining of hippocampal CA3 neurons. (B) Quatification of surviving hippocampal CA3 neurons in each group.
Histogram data are shown as mean ± SD
*P<0.05, compared to control group; #P<0.05, compared to ANT stimulation group
Figure 2: Hippocampal apoptosis in rats receiving KA injection and HFS. A: Tunel staining of hippocampal CA3 neurons, (a)control group, (b) KA group, (c) sham stimulation group, (d) stimulation group; B: Quantification of Tunel positive cells in each group.
Histogram data are shown as mean ± SD
*P<0.05, compared to control group; #P<0.05, compared to ANT stimulation group
ANT stimulation reduces mitochondrial release of cyto c, AIF following seizures
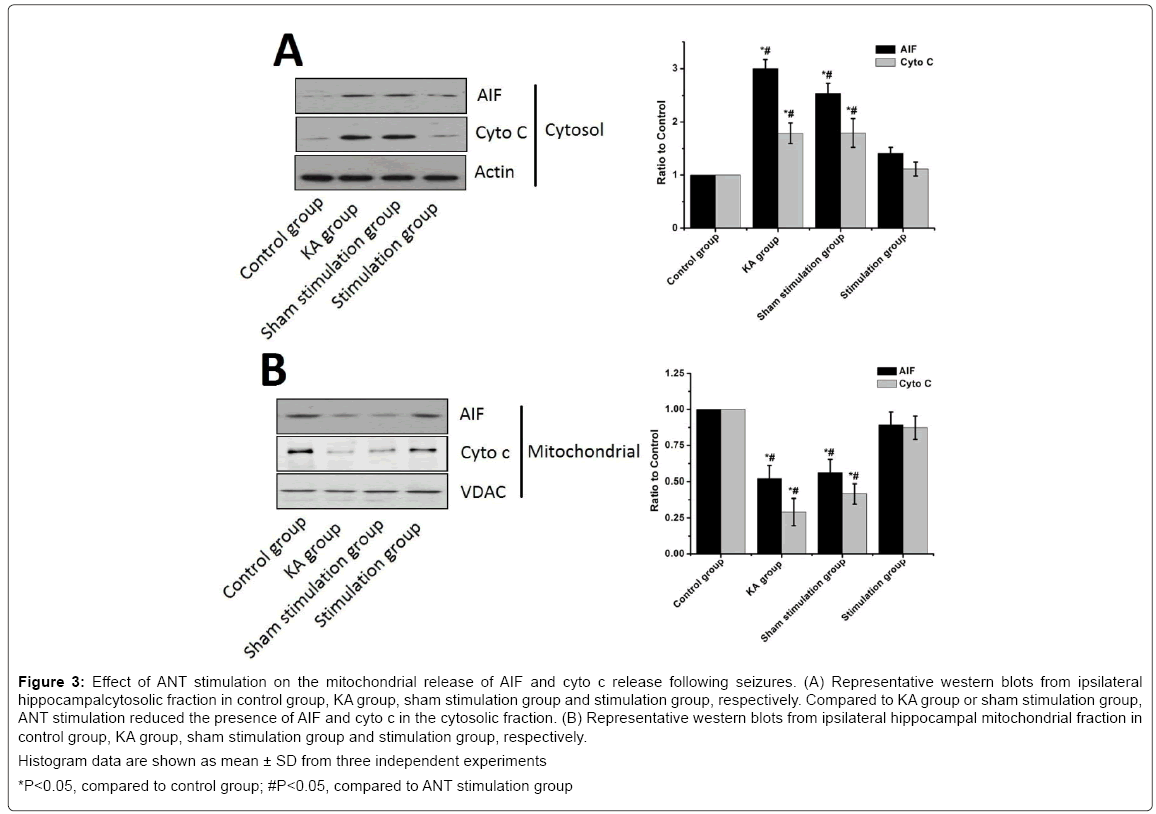
To investigate the effect of ANT stimulation on the mitochondrial release of cyto c and AIF induced by seizures, we detected the appearance of cyto c and AIF in the cytosolic fraction from the hippocampus samples. Western blot analysis revealed that the levels of cyto c and AIF largely increased following KA injection in the KA group compared with that of the control group whereas ANT stimulation reduced the levels of cyto c and AIF in the stimulation group compared with the KA group (P<0.05) (Figure 3). There were no significant difference in cyto c and AIF between the KA group and the sham stimulation group (P>0.05). Moreover, a significant decrease of cyto c and AIR in the mitochondrial fraction was noted in the KA group compared the control group and stimulation group, respectively.
Figure 3: Effect of ANT stimulation on the mitochondrial release of AIF and cyto c release following seizures. (A) Representative western blots from ipsilateral hippocampalcytosolic fraction in control group, KA group, sham stimulation group and stimulation group, respectively. Compared to KA group or sham stimulation group, ANT stimulation reduced the presence of AIF and cyto c in the cytosolic fraction. (B) Representative western blots from ipsilateral hippocampal mitochondrial fraction in control group, KA group, sham stimulation group and stimulation group, respectively.
Histogram data are shown as mean ± SD from three independent experiments
*P<0.05, compared to control group; #P<0.05, compared to ANT stimulation group
ANT stimulation inhibits caspas-9 and caspase-3 activation triggered by seizures
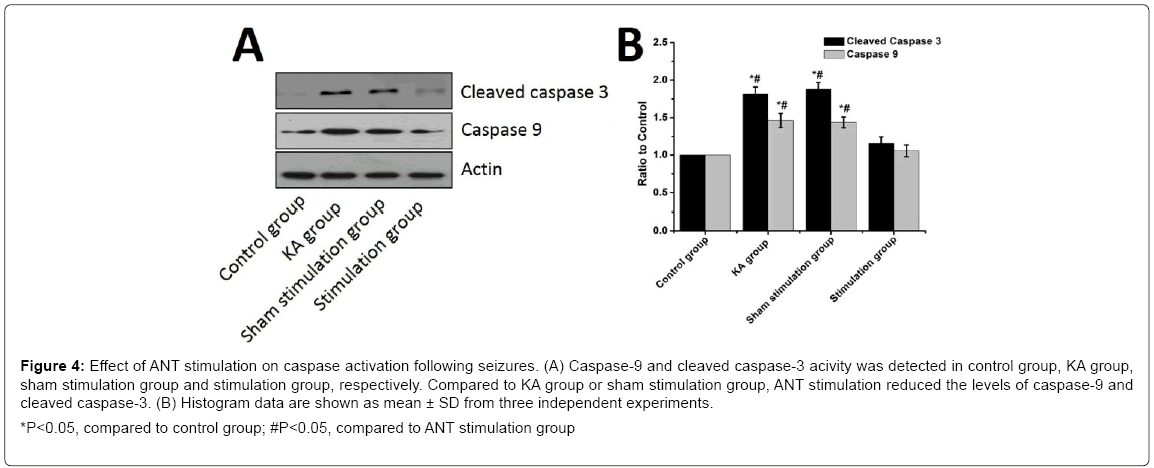
To further investigate the underlying neuroprotective mechanisms of ANT stimulation on brain injury induced by seizures, we utilized Western Blotting to measure the levels of caspase-9 and active cleavage product of caspase-3. The levels of caspase 9 and the active cleavage product of caspase-3 were significantly increased in the KA group compared to the control (P<0.05) while ANT stimulation reduced the levels of caspase-9 and cleaved caspase-3 compared to the KA group (P<0.05) (Figure 4). Nevertheless, there was no significant difference in caspase-3 and caspas-9 activity between KA group and sham stimulation group (P>0.05). These results illustrate a possible neuroprotective mechanism against seizures for ANT stimulation through the inhibition of capase-9 and caspase-3.
Figure 4: Effect of ANT stimulation on caspase activation following seizures. (A) Caspase-9 and cleaved caspase-3 acivity was detected in control group, KA group, sham stimulation group and stimulation group, respectively. Compared to KA group or sham stimulation group, ANT stimulation reduced the levels of caspase-9 and cleaved caspase-3. (B) Histogram data are shown as mean ± SD from three independent experiments.
*P<0.05, compared to control group; #P<0.05, compared to ANT stimulation group
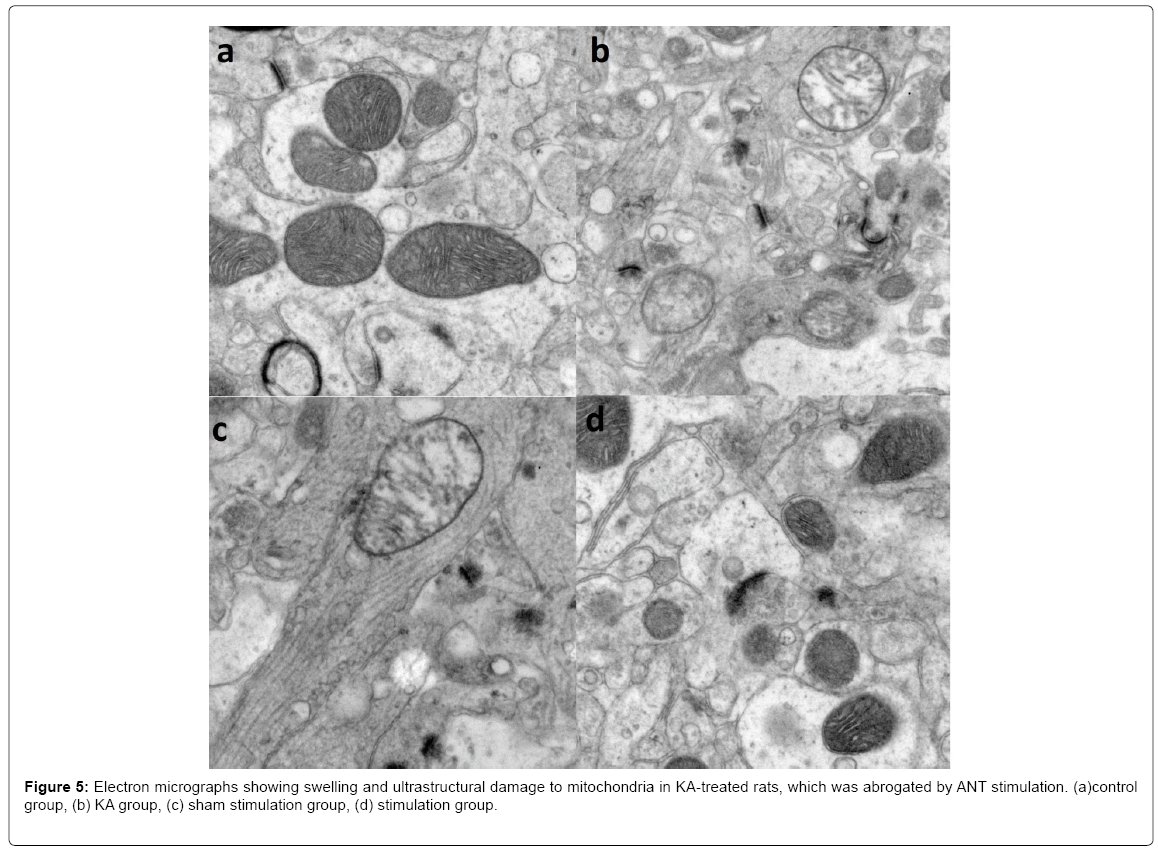
Electron microscopic observation
An examination by electron microscopy revealed that KA treatment caused mitochondrial swelling as well as malformation and disintegration of cristae in the KA group and the sham stimulation group (Figure 5). However, mitochondria in the control group were round and had numerous transverse cristae with parallel alignment. And mitochondrial structures in the stimulation group were similar to that of the control group, providing direct evidence that ANT stimulation mitigate KA-induced neuronal apoptosis by limiting mitochondrial injury.
Discussion
In the present study, neuronal death in the hippocampus was clearly observed following KA-induced seizures in rats, which was consistent with previous report [6]. Our results illustrated that ANT stimulation could significantly decrease the numbers of TUNEL positive cells and increase the number of surviving cells, indicating that ANT stimulation could rescue neurons from death induced by seizures and has a protective effect on hippocampal neuronals in KA-induced seizures of rats. In addition, ANT simulation reduced the release of cyto c and AIF induced by seizures while inhibiting the activated caspase-9 and caspase-3. Our results suggested that the neuroprotection of ANT stimulation against hippocampal cell death after KA treatment is mediated through the inhibition of caspase-dependent (cyto c release and the subsequent cleavage of caspase-) and caspase-independent (nuclear translocation of AIF) apoptosis pathways, which may be an important mechanism for ANT stimulation in epilepsy treatment.
Apoptosis is a complex process in which individual cells undergo self-destruction without inducing an inflammatory response. Several groups have reported that seizures induced by KA in adult rats caused ipsilateral death of CA3 neurons in the hippocampus [16,17]. There is increasing evidence that neuronal cell death induced by seizures activates mitochondria pathway [18]. Mitochondria play an important role in cell death by realys of signals for both caspase-dependent and caspase-independent death pathways [19]. The mitochondrion subserve important cellular functions to control of neuronal cell death, which is critical site for the initiation and/or reinforcement of cell death pathways [20]. It is well known that the release of cyto c by mitochondria is directly linked to induction of apoptosis [21]. Cyto c could interact with Apaf-1 and Datp, forming the apoptosome and causing the activation of caspase-9, which activates caspase-3 leading to chromation condensation, DNA fragmentation and apoptotic cell death in the caspse-dependent apoptosis pathways [15,22]. Previous reports demonstrated that the levels of cyto c, caspase-9 and cleaved caspase-3 were elevated and inhibition of the mitochondria release of cyto c could attenuate apoptosis after seizures [23,24]. In the current study, we also observed the similar results of release cyto c from mitochondria and activation of caspase-9 and caspase-3 following seizures and in contrast, decrease of motochondria release of cyto c and inhibitionof caspase-9 and caspase-3 activation by ANT stimulation. These results suggested that the ANT stimulation could exert neuroprotective effects against neuronal death by inhibiting mitochondrial caspase-dependent apoptosis pathways activation following KA-induced seizures.
Recent reports showed that AIF is involved in seizure induced apoptotic cell death in the mitochondrial caspase-independent apoptosis pathways [19]. AIF is a key factor in caspase-independent death pathway when it translocates from mitochondria to the nucleus, where it results in peripheral chormatin condensation and highmolecular weight DNA fragmentation that culminates in cell death [25,26]. Heo et al. reported that intra-hippocampal KA injection results in AIF translocation from mitochondria to nucleus in mouse hippocampal neurons as well as DNA cleavage [27]. Our study observed the similar results that the release of AIF from mitochondria following seizures, indicating that AIF could actually contribute to cell death induced by seizures. In addition, it has been reported that neurons with reduced AIF are protected against excitotoxicity specifically through the activation of kainic acid receptors and Hq mice with a mutation reducing AIF expression by 80% [28] have significantly less hippocampal damage than wild-type littermates in an in vivo seizure model induced by kainic treatment [19]. Meanwhile, we also found that ANT stimulation decrease mitochondria release of AIF illustrating that ANT stimulation could exert neuroprotective effects against neuronal death by inhibiting mitochondrial caspase-independent apoptosis pathways activation following KA-induced seizures.
However, the mechanism responsible of ANT stimulation to prevent mitochondiria release of cyto c and AIF remains obscure. Recent work has suggested that mitochondria are highly dynamic organelles and the changes in mitochondrial morphology could affect the susceptibility of cell to apoptosis in epilepsy [29]. The ultrastructural damage of mitochondria in the hippocampus may be a key to the pathogenesis of status epilepticus [30]. The mitochondrial dysfunction could cause the release of cyto c and AIF. The electron microscopic examination of mitochondrial ultrastructure in hippocampal neurons in this study demonstrated that swelling and disruption of mitochondrion in KA and sham stimulation group with ANT stimulation maintain most mitochondrial structural integrity in stimulation group. One possible mechanism of ANT stimulation to prevent mitochondria release of cyto c and AIF is that ANT stimulation mitigates KA-induced neuronal apoptosis by limiting mitochondrial injury.
Conclusion
We provided evidence that ANT stimulation attenuates neuronal death in hippocampus induced by seizures and exerts neuroprotective effect on hippocampal neurons. The underlying mechanisms may be mediated by inhibiting mitochondrial caspase-dependent (cyto c release and the subsequent cleavage of caspase-) and caspase-independent (nuclear translocation of AIF) apoptosis pathways.
Acknowledgement
This study was supported by the National Natural Science Foundation of China (GRTant NO81471375).
References
- Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, et al. (2005) Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 46: 470-472.
- Armstrong C M (2011) Entorhinal-hippocampal interactions: Interneuronal regulation and changes in epilepsy. Dissertations & Theses - Gradworks.
- Sun Z, Yuan D, Sun Y, Zhang J, Zuo H, et al. (2014) Clinical curative effect analysis and predictors of prognosis in patients with temporal lobe epilepsy after anterior temporal lobectomy: Results after five years. Chin Med J (England) 127: 2588-2593.
- Kwan P, Brodie MJ (2000) Early identification of refractory epilepsy. N Engl J Med 342: 314-319.
- Salanova V, Witt T, Worth R, Henry TR, Gross RE, et al. (2015) Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 84: 1017-1025.
- Henshall DC, Engel T (2013) Contribution of apoptosis-associated signaling pathways to epileptogenesis: Lessons from bcl-2 family knock-outs. Front Cell Neurosci 7: 110.
- Chen N, Gao Y, Yan N, Liu C, Zhang JG, et al. (2014) High-frequency stimulation of the hippocampus protects against seizure activity and hippocampal neuronal apoptosis induced by kainic acid administration in macaques. Neuroscience 256: 370-378.
- Chen N, Yan N, Liu C, Ge Y, Zhang JG, et al. (2013) Neuroprotective effects of electrical stimulation of the anterior nucleus of the thalamus for hippocampus neurons in intractable epilepsy. Med Hypotheses 80: 517-519.
- Sloviter RS, Zappone CA, Harvey BD, Frotscher M (2006) Kainic acid-induced recurrent mossy fiber innervation of dentate gyrus inhibitory interneurons: Possible anatomical substrate of granule cell hyper-inhibition in chronically epileptic rats. J Comp Neurol 494: 944-960.
- Bragin A, Azizyan A, Almajano J, Wilson CL, Engel J Jr (2005) Analysis of chronic seizure onsets after intrahippocampal kainic acid injection in freely moving rats. Epilepsia 46: 1592-1598.
- Ben-Ari Y, Cossart R (2000) Kainate, a double agent that generates seizures: Two decades of progress. Trends Neurosci 23: 580-587.
- Henshall DC, Bonislawski DP, Skradski SL, Araki T, Lan JQ, et al. (2001) Formation of the Apaf-1/cytochrome c complex precedes activation of caspase-9 during seizure-induced neuronal death. Cell Death Differ 8: 1169-1181.
- Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. (6th Edtn), Amsterdam, Boston.
- Liu HG, Yang AC, Meng DW, Chen N, Zhang JG (2012) Stimulation of the anterior nucleus of the thalamus induces changes in amino acids in the hippocampi of epileptic rats. Brain Res 1477: 37-44.
- Li T, Lu C, Xia Z, Xiao B, Luo Y (2006) Inhibition of caspase-8 attenuates neuronal death induced by limbic seizures in a cytochrome c-dependent and Smac/DIABLO-independent way. Brain Res 1098: 204-211.
- Li T, Fan Y, Luo Y, Xiao B, Lu C (2006) In vivo delivery of a XIAP (BIR3-RING) fusion protein containing the protein transduction domain protects against neuronal death induced by seizures. Exp Neurol 197: 301-308.
- Li T, Lytle N, Lan JQ, Sandau US, Boison D (2012) Local disruption of glial adenosine homeostasis in mice associates with focal electrographic seizures: A first step in epileptogenesis? Glia 60: 83-95.
- Henshall DC, Meldrum BS (2012) Cell death and survival mechanisms after single and repeated brief seizures. Epelipsia 51: 35.
- Cheung EC, Melanson-Drapeau L, Cregan SP, Vanderluit JL, Ferguson KL, et al. (2005) Apoptosis-inducing factor is a key factor in neuronal cell death propagated by BAX-dependent and BAX-independent mechanisms. J Neurosci 25: 1324-1334.
- Kroemer G, Reed JC (2000) Mitochondrial control of cell death. Nat Med 6: 513-519.
- Cai J, Yang J, Jones DP (1998) Mitochondrial control of apoptosis: The role of cytochrome c. Biochim Biophys Acta 1366: 139-149.
- Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, et al. (2001) A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 411: 112-116.
- Henshall DC, Bonislawski DP, Skradski SL, Araki T, Lan JQ, et al. (2001) Formation of the Apaf-1/cytochrome c complex precedes activation of caspase-9 during seizure-induced neuronal death. Cell Death Differ 8: 1169-1181.
- Li T, Lu C, Xia Z, Xiao B, Luo Y (2006) Inhibition of caspase-8 attenuates neuronal death induced by limbic seizures in a cytochrome c-dependent and Smac/DIABLO-independent way. Brain Res 1098: 204-211.
- SusinSA, Lorenzo HK, Zamzami N, MarzoI, Snow BE, et al. (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397: 441-446.
- Candé C, Cohen I, Daugas E, Ravagnan L, Larochette N, et al. (2002) Apoptosis-inducing factor (AIF): A novel caspase-independent death effector released from mitochondria. Biochimie 84: 215-222.
- Shin HY, Cho YJ, Cho KJ, Kim HW, Kim HJ, et al. (2006) Minocycline inhibits caspase-dependent and-independent cell death pathways and is neuroprotective against hippocampal damage after treatment with kainic acid in mice. Neurosci Lett 398: 195-200.
- KleinJA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, et al. (2002) The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature 419: 367-374.
- Bossy-Wetzel E, Barsoum MJ, Godzik A, Schwarzenbacher R, Lipton SA (2003) Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol 15: 706-716.
- Chuang YC, Chang AY, Lin JW, Hsu SP, Chan SH, et al. (2004) Mitochondrial dysfunction and ultrastructural damage in the hippocampus during kainic acid-induced status epilepticus in the rat. Epilepsia 45: 1202-1209.
Citation: Yan N, Chen N, Gao W, Wang W (2017) Stimulation of Anterior Thalamic Nucleus Protects Hippocampus Neural Injury in Kainic Acid-Induced Epileptic Rats. J Alzheimers Dis Parkinsonism 7: 377. DOI: 10.4172/2161-0460.1000377
Copyright: © 2017 Yan N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7387
- [From(publication date): 0-2017 - Nov 22, 2025]
- Breakdown by view type
- HTML page views: 6340
- PDF downloads: 1047