Study on Copper Ion Deposited Polyester Fabric
Received: 01-Nov-2022 / Manuscript No. JMSN-22-78590 / Editor assigned: 04-Nov-2022 / PreQC No. JMSN-22-78590 / Reviewed: 18-Nov-2022 / QC No. JMSN-22-78590 / Revised: 25-Nov-2022 / Manuscript No. JMSN-22-78590 / Published Date: 30-Nov-2022 DOI: 10.4172/jmsn.100059
Abstract
Deposition of thin metal particles on textiles can be more advantages than the conventional method of incorporating metals into textiles. A single bath method of synthesizing the copper nanoparticles and deposition of the same on polyester fabric is carried out using electroplating technique. The copper nanoparticles were generated in the treatment bath by increasing the time from 30 to 150 minutes. The effect of varying the time for the generation of copper ions, on the deposition percentage was studied. Further, copper nanoparticle deposited fabric is studied for its property like morphological structure and electrical conductivity. With an increase in treatment time, decrease in the electrical resistance of the fabric was observed from 300KΩ/cm for untreated polyester to 118KΩ/cm for copper ion deposited fabric with a treatment time of 150 minutes.
Keywords
Copper nanoparticle, Electroplating, Electrical conductivity
Introduction
The growth in digital technology is demanding for highly flexible electronics. Integration of new technologies and materials into the field of textiles is growing at a faster rate. Integration of conductivity in textiles (Conductive Textiles) finds numerous applications in the field of military, healthcare, wearable electronics etc. The terms flexible electronics or wearable electronics are used interchangeably [1]. These flexible electronics are developed with the integration of electronics into textiles to produce conductive textiles. Textiles are in general insulative/semi conductive in nature [2]. To make them conductive; metals, conductive polymers were either incorporated in their structure or coated on their surface. Textiles with metals like copper, silver and gold etc., are conventionally produced by either wrapping metallic fibres (Au, Ag) on the textile fibre during spinning or by wrapping them onto fabric during their manufacturing process like weaving, knitting or non-woven. As an alternative nano particles can also be deposited or coated onto textiles using different methods like sputter coating, electroplating, coating metal powder with binders, electro-less plating, vacuum deposition etc.,1-8. Hence, in this work copper ions generated through electroplating techniques were deposited on polyester fabrics in single bath and its properties were studied [3].
Experimental
Materials
Commercially available polyester fabrics with plain weave structure (EPI X PPI = 30X 30), GSM of 150 was used. The chemicals: Sodium chloride, Acetic acid, Labolene and Citric acid were purchased from SRL chemicals. All the chemicals used were of reagent grade.
Methods
Pretreatment
The polyester fabrics were initially washed with 2g/l of Labolene (surfactant), rinsed in deionized water and then dried. The pretreatment process removes dirt from the fabrics. This fabric is taken as the control sample.
Preparation of Metal Ions and Deposition of Metal Ions on Fabric
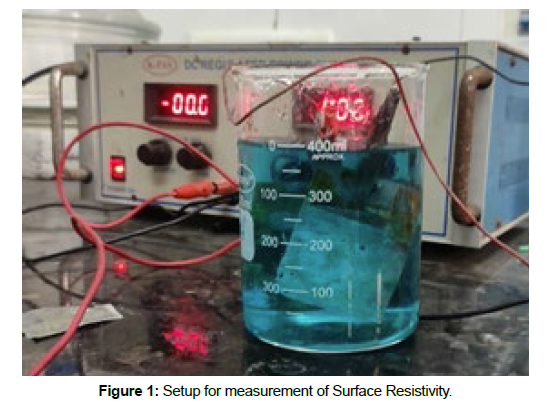
Bath for the preparation of metal ions was prepared using 4g/l of the acetic acid and 10gpl of sodium chloride. For the generation of metal ions, two copper plates were immersed into the solution prepared and then the plates were connected to the electrolytic power source. A constant of 1.5±0.3A was maintained during the complete process. The polyester fabrics were immersed in the bath once the metal ions are generated in the bath. The time the fabric is immersed in the bath was varied and studied [4]. Once the metal ions start depositing on the fabric, 5gpl of citric acid was added to the bath to enhance the deposition of the metal ions. The treatment time of 30, 60, 90, 120, 150 minutes were studied as given in Table 1. The metal deposited fabrics were dried at 80°C for 5 min and their properties were studied. The setup for metal deposition is shown in Figure 1.
| Sl. No. | Sample Code | Time of Treatment (Minutes) |
|---|---|---|
| 1 | C – Control Fabric | 0 |
| 2 | C1 | 30 |
| 3 | C2 | 60 |
| 4 | C3 | 90 |
| 5 | C4 | 120 |
| 6 | C5 | 150 |
Table 1: Sample code for different treatment time.
Testing
Surface Morphology
The metal deposited fabrics were observed in the polarizing microscope (Nikon eclipse LB 100N POL) to study their morphological structure
Electrical Properties
Surface resistivity gives the electrical resistance of materials across the top layer of a conductive material. The surface resistance of copper deposited fabric was measured according to the American Association of Textile Chemists and Colorists test method 76-1995. Two copper electrodes having dimensions 20x30 mm2 separated by 20 mm were placed on the fabric sample and a load of one kg mass was applied on top of the electrodes. The fabric surface electrical resistance was measured by using a high precision multimeter [5]. The surface resistivity of fabric samples was measured at 25⁰C±2⁰C temperature and relative humidity of 65% ± 2%. The setup used for measuring the surface resistivity of the fabric is given in the figure 2.
Increase in Weight of the Sample
Weight gain of copper deposited polyester fabrics was examined for different treatment times at 30, 60, 90,120,150 minutes.
Results and Discussion
Electrical Conductivity of copper deposited fabrics
The electrical resistivity of copper deposited fabrics were measured using two probe multimeter. The control sample (C) showed very higher electrical resistivity. The resistivity was measured in 5 different areas in each sample and the average results are given in Table 2. The electrical resistivity decreased as the time of exposure increased from sample C1 to C4 drastically. On further increasing the time of exposure for the sample C5 to 150 minutes, noticeable change in the electrical resistivity is not observed.
| Sample Code | Electrical Resitivity (KΩ/cm) |
|---|---|
| C1 | 300 |
| C2 | 270 |
| C3 | 180.4 |
| C4 | 120.5 |
| C5 | 118 |
Table 2: Surface Resistivity of the Copper deposited fabric.
Increase in Weight of the Samples
The weight of the copper ion deposited fabrics was investigated. The effect of treatment time was measured for all the sample (30, 60, 90, 12,150 minutes). It is clear from the results given in Table 3 that with increasing treatment time, there was no significant increase in weight of the fabric.
| Treatment Time (Minutes) | Weight in Grams |
|---|---|
| Control Fabric | 2.5 |
| 30 | 2.52 |
| 60 | 2.52 |
| 90 | 2.53 |
| 120 | 2.53 |
| 150 | 2.54 |
Table 3: Weight Gain percentage of Copper Deposited Fabric.
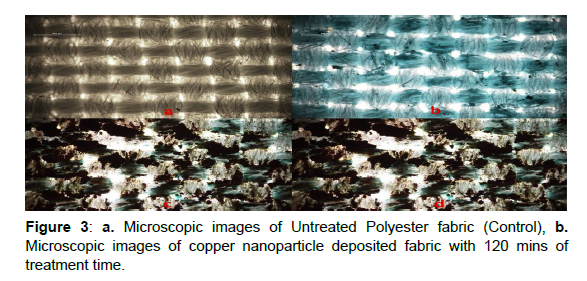
Surface Morphology
The microscopic image of the copper coated fabrics is shown in Figure 3. The untreated polyester fabric is shown in Figure 3a and the sample C5 is shown in Figure 3b. The samples (Figure 3b.) deposited with copper particles clearly shows the deposition of nano copper particles on the surface of the fabric with a blue tint. Further the fabric surface is not covered by the copper particle like in case of copper coated fabrics.
Conclusion
The copper nanoparticles deposited polyester fabric shows a minimum electrical resistivity of 118 KΩ/cm. Further the study of weight gain percentage and surface morphology also reveals that the nanoparticle deposition does not result in a significant increase of the fabric. This method proves to be a promising method for development of conductive textiles.
References
- Hao Liu, Lili Zhu, JieXue, Lei Hao, Jin Li, et al. (2016) A Novel Two-Step Method for Fabricating Silver Plating Cotton Fabrics, Hindawi Publishing Corporation. J Nanomater 2016: 1–11.
- JiangS Q, E Newton, CWM Yuen, CW Kan (2004) ‘Chemical Silver Plating and Its Application to TextileFabric Design’. J Appl Polym Sci 96(3): 919–926.
- JiangS Q, E Newton, CWM Yuen, CW Kan (2006) ‘Chemical Silver Plating on Polyester/Cotton Blended Fabric’. J Appl Polym Sci 100(6): 4383-4387.
- Kamal K, Kara D, Sathiyamoorthy (2007) Influence of process parameters for coating of nickel–phosphorous on carbon fibers. J Mater Process Technol 209(6): 3022-3029.
- RuiXu, Wei Wang, Jiayi Sun, Yu Wang, Chenrong Wang, et al. (2019) ‘A flexible, conductive and simple pressure sensor prepared by electroless silver plated polyester fabric’. Colloids Surf A Physicochem Eng Asp 578: 123554.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Farzana H, Neelakandan (2022) Study on Copper Ion Deposited Polyester Fabric. J Mater Sci Nanomater 6: 059. DOI: 10.4172/jmsn.100059
Copyright: © 2022 Farzana H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2427
- [From(publication date): 0-2022 - Nov 29, 2025]
- Breakdown by view type
- HTML page views: 1963
- PDF downloads: 464