Successful Weight Loss Before Bariatric Surgery as an Indicator for Perioperative Complications, Adherence, And Postoperative Weight Reduction? A Retrospective Analysis
Received: 26-Dec-2021 / Manuscript No. JOWT-21-50623 / Editor assigned: 28-Dec-2021 / PreQC No. JOWT-21-50623(PQ) / Reviewed: 08-Jan-2022 / QC No. JOWT-21-50623 / Revised: 13-Jan-2022 / Manuscript No. JOWT-21-50623(R) / Accepted Date: 15-Jan-2022 / Published Date: 20-Jan-2022 DOI: 10.4172/2165-7904.1000476
Abstract
Objective: Participating in a weight loss program before bariatric surgery is an established preparation tool in many specialized bariatric clinics. A certain amount of weight loss before surgery can even be a mandatory requirement for some clinics or insurance companies. The objective of this study is to investigate whether successful preoperative weight loss through adherence to a low-calorie diet three weeks prior to bariatric surgery could predict a difference in the duration of the procedure, intraoperative blood loss, need for revision, excess weight loss after 12 months and the participation in postoperative follow-up visits.
Methods: All cases that underwent sleeve gastrostomy or Roux-en-Y gastric bypass in a bariatric department in Germany between January 2018 and October 2019 were retrospectively evaluated for our study. Data were obtained on gender, age, BMI, weight before and after preoperative weight loss, weight after 1, 3, 6, and 12 months, operative times, haemoglobin and haematocrit one day before and two days after surgery, the need for revision 4 weeks after the procedure, and the number of follow-up visits. Groups were formed according to the achieved excess weight loss before surgery and analysed inter alia using Kruskal Wallis H Test
Results: The study included 201 patients. Both, patients that underwent sleeve gastrostomy (n=66) or Roux-en-Y gastric bypass (n=135), participated significantly more often in follow-up visits (P = 0.004 and P = 0.012), if they lost a higher amount of excess weight prior to surgery compared to patients that lost less. Patients that underwent Rouxen- Y gastric bypass also showed higher adherence to follow-up visits than patients that underwent sleeve gastrostomy. Blood loss, operative times, need for revision and weight loss after one year did not differ between groups that lost a high, medium, or low amount of excess weight preoperatively.
Conclusions: Preoperative weight loss showed to be a good predictor of follow-up adherence after bariatric surgery. As adherence and participation in regular follow-up visits appear to be crucial for the long-term success of bariatric interventions, the possible prediction of patient adherence is set to increasingly gain importance.
Keywords
Bariatric surgery; Obesity; Preoperative weight loss; Adherence; Follow-up
Introduction
Bariatric surgery has become one of the most effective long-term treatments for obesity, as a change in diet and exercise is ineffective for a vast majority of patients [1-3]. Nonetheless, bariatric procedures remain technically challenging, resource-intensive, and may be subject to risk [4]. Patients need to follow strict medical guidelines and must be willing to modify their lifestyle postoperatively [5]. To maximize efficacy the appropriate preoperative selection of patients is critical.
Preoperative factors that predict successful outcomes therefore must be taken into consideration when working with patients that could profit from bariatric surgery. A possible predictor might be the excess weight loss (EWL) a patient accomplishes in a (mandatory) preoperative weight loss regimen (PWL).
Participating in a weight loss regimen before bariatric surgery can be seen as an established preparation tool in many bariatric clinics [6]. A certain amount of weight loss before surgery can even be a mandatory requirement for some clinics or insurance companies [6]. The purpose of this approach is based on arguments like a decrease in liver size and reduced intrahepatic fat [7-9] or higher risks of infection or bleeding [10] and poor mechanical conditions for laparoscopy in patients with obesity [11]. However, data on the effectiveness and exact execution of preoperative weight loss regimens remain ambiguous [12-16].
The primary objective of this study was to investigate whether successful preoperative weight loss through adherence to a low-calorie diet three weeks prior to bariatric surgery could predict a difference in the duration of the procedure, intraoperative blood loss, need for revision, EWL after 12 months, and the participation in postoperative follow-up visits.
Materials And Methods
After approval of the local ethics committee, all cases that underwent sleeve gastrectomy (SG) or Roux-en-Y gastric bypass (RYGB) in a bariatric clinic in Germany between January 2018 and October 2019 were retrospectively evaluated. Patients who underwent primary treatment, had a BMI ≥ 40 or ≥ 35 kg/m2 with at least one severe obesity-related comorbidity, were above the age of 18, participated in a three-week-long preoperative weight loss regimen and on whom data on height, weight before and after the preoperative diet and haemoglobin and haematocrit one day before surgery and two days after surgery were available, were included into our analysis.
Patients who had any complex abdominal surgery in the past, or became pregnant in the observation period, or had a gastric band or balloon, were excluded from our study.
Before surgery, all patients had to be assessed and cleared by their primary care physician, a psychologist, a registered Dietitian, and an endocrinologist, or a different specialist for internal medicine. Patients had to demonstrate attempts to lose weight through physical activity and change of diet.
The decision on the performed procedure was mainly made by the bariatric surgeon. After careful anamnesis and physical examination, criteria like regular medication, nicotine abuse, or abdominal girth were taken into account. If patients had a strong preference towards one procedure their wishes were considered if those did not increase the risk of a poor outcome. In all cases, the current medical guidelines for bariatric and metabolic guidelines in Germany were the basis for the surgical decision.
A complete blood count, an electrocardiogram, a pulmonary function testing, and an oesophageal gastroscopy were also compulsorily performed before surgery. All patients were instructed and supervised by a multidisciplinary team up to 3-6 months prior to surgery consisting of bariatric surgeons, registered Dietitian, and bariatric nurses. Patients were instructed and supervised on healthy nutrition and lifestyle habits (e.g. drinking enough water, daily physical activity, sleeping habits) and the need for supplementary intake of vitamins postoperatively. Three weeks prior to surgery patients were weighed and received specific diet counselling. During the following three weeks patients were required to use fluid meal replacements of high protein content including Opt fast (216 kcal, 20g of protein, 6g of fat, 19g of carbohydrates) or another product brand that had a similar nutrient composition, up to four times a day and consume less than 1.200 kcal per day. If the patients wanted to consume more than the fluid meal replacements, they were allowed to consume only one serving size per day of steamed vegetables. One day prior and two days after the operation a complete blood count was determined, including a differential blood count, liver, kidney, and fat values as well as a vitamin status.
All procedures were performed by the same experienced bariatric surgeons following departmental standards. The surgical position was in 45° Trendelenburg, using a 10 mm and 30°-view endoscope. SG was sized using a 40F or gastric tube and 60mm mm staple, only finished after an intraoperative leak test with methylene blue. Surgical drains were routinely used for SG-patients. The antecolic-antegastric approach was used for RYGB, with a 50 cm bilio pancreatic limb length and a 150 cm Roux limb length. The gastro jejunal anastomosis was stapled end-to-side using a 30 mm staple; the jejunojejunal anastomosis was stapled side-to-side using a 45 mm staple. After surgery, patients were summoned for follow-up visits to the bariatric centre to see one of the physicians and dieticians. Follow-up visits were provided after 1, 3, 6, and 12 months and after that once every year.
Data collection was performed after receiving a positive vote from the ethics committee, using the internal hospital database and Excel spread sheets. All data were anonym zed and statistically evaluated using SPSS Version 26. A P-value of
Data were obtained on gender, age, BMI, weight before and after the preoperative weight loss regimen, weight after 1, 3, 6, and 12 months, operative times, haemoglobin and haematocrit one day before and two days after surgery, the need for revision 4 weeks after the procedure, and the number of follow-up visits.
The total calculated volume of blood loss, in millilitres, at 48h was calculated indirectly using the following equations [17, 18]:
1) PBV = (k1 x h3) + (k2 x w) + k3
PBV = patient’s blood volume (L)
h = height (m)
w = weight (kg)
k1 = 0.3669 (men) and 0.3561 (women)
k2 = 0.03219 (men) and 0.03308 (women)
k3 = 0.6041 (men) and 0.1833 (women)
2) RBC = PBV x (Hctpreop – Hct48h postop)
RBC = red blood cell volume loss (mL)
Hctpreop = preoperative haematocrit
Hct48h postop = postoperative haematocrit after 48 3) Total volume loss = RBC
Hctavg
Hctavg = arithmetic average of Hctpreop and Hct48h postop
Excess weight loss was calculated using the following formula [19]:
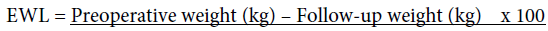
Preoperative weight (kg) – Ideal weight (kg)
Ideal weight was defined as the weight that a patient would have, with a BMI of 25 kg /m2.
Groups were built by arranging all patients according to their achieved EWL after the preoperative weight loss regimen from low to high. Then they were divided into three parts, each containing a third of the population. This was done separately for the SG and RYGB-group.
Kruskal-Wallis H Test was used to compare patients with a high (hEWL), medium (mEWL), and low (lEWL) EWL after the preoperative diet. In cases where the Kruskal-Wallis H Test showed a significant difference between the three groups a post hoc test using Dunn's test with Bonferroni correction was used. Mann-Whitney U Test was utilized to compare RYGB to SG patients.
Results
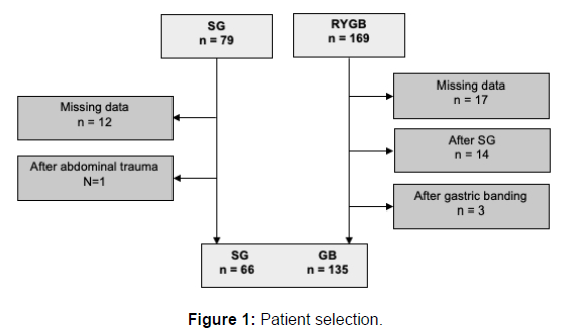
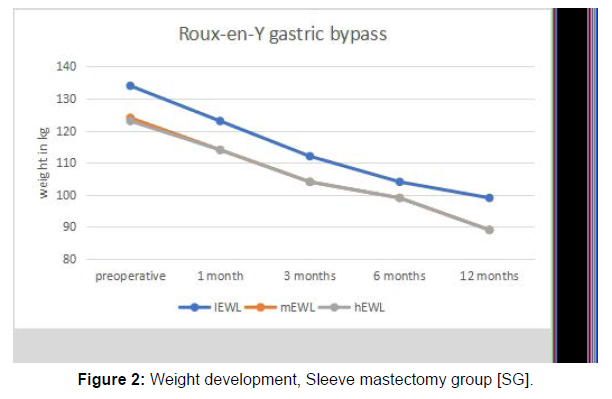
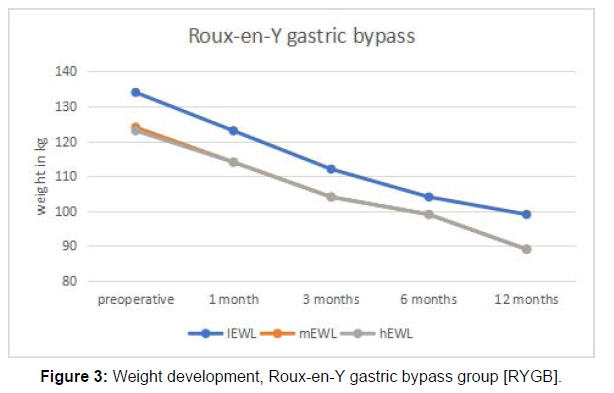
After investigating all 248 patients for inclusion and exclusion criteria (Figure 1) 201 patients were left, of whom 135 underwent RYGB and 66 underwent SG. The mean age was 41 years with a minimum of 18 years and a maximum of 69 years. The proportion of females to males was 78, 1 % to 21, 9%. Mean BMI before surgery was 48,5 kg/ m2, mean EWL after the preoperative weight loss regimen was 9,62 %, and mean EWL one year after surgery was 55,79% (Table 1). Initial body weight was defined as the weight measured three weeks prior to surgery, before undergoing the weight loss regimen. Preoperative weight was specified as the weight measured after the preoperative weight loss regimen, one day before surgery. Weight development 1 month, 3 months, 6 months, and 12 months after either SG or RYGB can be seen in Figures 2 and Figure 3.
| Sleeve gastrostomy (n = 66) | Gastric bypass (n = 135) | |
|---|---|---|
| Age (years), median (IQR): | ||
| hEWL | 43.3 (35.8 – 51.3) | 45.4 (36.5 – 54.5) |
| mEWL | 49.4 (30.8 – 49.5) | 37.5 (29.5 – 44) |
| lEWL | 37.2 (29 – 41.8) | 43.3 (34.5 – 51.5) |
| Female gender (%) | ||
| hEWL | 82 | 69 |
| mEWL | 68 | 91 |
| lEWL | 68 | 82 |
| Initial body weight (kg), median (IQR): | ||
| hEWL | 141.4 (120.5 – 159.2) | 133.2 (116.5 – 145) |
| mEWL | 147.7 (129 – 155.3) | 129.7 (120 – 139) |
| lEWL | 153 (138.7 – 171.8) | 136.6 (122 – 153) |
| Initial BMI (kg/m2), median (IQR): | ||
| hEWL | 50 (44.5 – 55) | 46.5 (42.1 – 50.2) |
| mEWL | 51 (50.8 – 55.2) | 47.3 (43.7 – 50.6) |
| lEWL | 50.8 (45.4 – 55) | 48.5 (43.7 – 51.8) |
| EWL after 12 months (%), median (IQR): | ||
| hEWL | 67.3 (48 – 83.6) | 56.8 (47.4 – 68.7) |
| mEWL | 58.6 (43.8 – 72.3) | 58.3 (46.9 – 65. 7) |
| lEWL | 56.4 (42.4 – 69.1) | 53.7 (40.8 – 66.9) |
| h/m/lEWL = high/medium/low excess weight loss in %; IQR = interquartile range of 4 mandatory follow-up visits, h/m/lEWL = high/medium/low excess weight loss in % | ||
Table 1: Demographics.
EWL after the preoperative weight loss regimen The mean EWL after the preoperative weight loss regimen in the SG group was 10, 91%, with a minimum of -1,58% and a maximum of 50,07 %.
In the RYGB group, the mean EWL after the preoperative weight loss regimen was 9,8% with a minimum of -9,54% and a maximum of 26,47%.
After arranging all patients according to their achieved EWL after their three weeks of preoperative weight loss, from low to high, equal groups were formed. The SG-group was parted into three equal groups, each containing 22 patients. While the RYGB-group was divided into three groups, each containing 45 patients. Threshold values for all groups can be seen in Table 2.
| EWL preoperative | operative times (min) | blood loss (mL) | need for revision | follow-up visits* | ||
|---|---|---|---|---|---|---|
| sleeve gastrostomy | hEWL | 13.32 - 50,07 | 81.77 | 858 | 0 | 3.91 |
| mEWL | 5.94 - 12.85 | 82.2 | 1187 | 2 | 3.45 | |
| lEWL | -7.49 | 77.91 | 970 | 0 | 3.36 | |
| mean | 10.91 | 80.6 | 1004 | 0.03 | 3.58 | |
| gastric bypass | hEWL | 11.98 - 26.47 | 117.13 | 985 | 1 | 3.91 |
| mEWL | 7.71 - 11.97 | 118.31 | 1044 | 1 | 3.84 | |
| lEWL | -17.23 | 132.84 | 1185 | 0 | 3.6 | |
| mean | 9.8 | 122 | 1076 | 0.01 | 3.79 | |
Table 2: EWL thresholds.
Operative times
In the SG group, operative times varied between 41 minutes and 130 minutes with a mean operative time of 80, 64 minutes. The mean operative time for the hEWL-group was 81, 77 minutes, for the mEWLgroup 82, 23 minutes, and the lEWL 77, 91 minutes.
Kruskal-Wallis H Test was conducted to examine the differences in operative times according to the EWL through the preoperative weight loss regimen. No significant differences (Chi-square = 1.646, p = .536, df = 2) were found among the three groups.
In the RYGB group, the mean operative time was 122 minutes, with a minimum of 63 minutes and a maximum of 210 minutes. The mean operative time for the hEWL-group was 117,13 minutes, for the mEWL-group 118,31 minutes, and the lEWL 132,84 minutes.
Kruskal-Wallis H Test was conducted to examine the differences in operative times according to the EWL through the preoperative weight loss regimen. No significant differences (Chi-square = 4.623, p = .099, df = 3) were found among the three groups.
Perioperative complications (blood loss and need for revision)
The mean perioperative blood loss in all procedures including SG and RYGB groups was around 1052 ml with a minimum of 102 ml and a maximum of 4635 ml.
In the SG group perioperative blood loss ranged from 103 to 4635 ml (mean 1004 ml). No significant difference (Chi square = 3.672, p = .159, df = 2) of perioperative blood loss was found among the three groups with hEWL (mean: 858 ml), mEWL (mean: 1187 ml) and lEWL (mean: 970 ml).
The need for revision in the first four weeks after SG occurred two times in the mEWL group. In both cases, a suture insufficiency had to be rectified, of whom one led to peritonitis with a retro splenic abscess. Kruskal-Wallis H Test showed significant differences in the number of surgical revisions (Chi-square = 6.190, p = .045, df = 2) among the three groups.
A post-hoc test using Dunn's test with Bonferroni correction showed significant differences between the hEWL group and the mEWL group (p= .031, r= .32, z/t= -2.155, SE= 2.088) as well as between the mEWL and the lEWL group (p= .031, r= .32, z/t= 2.155, SE= 2.088).
In the RYGB group perioperative blood loss ranged from 102 to 4028 ml (mean 1076 ml). No significant difference (Chi square = 4.334, p = .115, df = 2) of perioperative blood loss was found among the three groups with hEWL (mean: 985 ml), mEWL (mean: 1044 ml) and lEWL (mean: 1185 ml).
In two cases a surgical revision was needed, once because of a haemoglobin relevant bleeding and once because of a suture insufficiency that led to peritonitis. Kruskal-Wallis H Test showed no significant difference in the number of surgical revisions (Chi-square = 1.008, p = .604, df = 2) among the three groups.
Follow-up visits
Patients of both groups (SG and RYGB) participated in 3,72 followup visits on average. Adherence in the RYGB group was significantly higher than in the SG group (U= 3629, z=-2.873, p=.004)
In the SG group the mean participation in follow-up visits was 3,58, with hEWL (mean: 3,91), mEWL (mean: 3,45) and lEWL (mean: 3,36). Kruskal-Wallis H Test showed a significant difference in the number of attended follow-up visits among the three groups (Chi square = 10.900, p = .004, df = 2). A post-hoc test using Dunn's test with Bonferroni correction showed significant differences between the hEWL group and the mEWL group (p= .011, r= .38, z/t= 2.527, SE= 4.892) as well as between the mEWL and the lEWL group (p= .002, r= .47, z/t= 3.103, SE= 4.892).
In the RYGB group the mean participation in follow-up visits was 3, 79, with hEWL (mean: 3,91), mEWL (mean: 3,84) and lEWL (mean: 3,6). Kruskal-Wallis H Test showed a significant difference in the number of attended follow-up visits among the three groups (Chi square = 8.783, p = .012, df = 2). A post-hoc test using Dunn's test with Bonferroni correction showed significant differences between the hEWL group and the lEWL group (p= .005, r= .3, z/t= 2.806, SE= 5.475).
Discussion
There is a scientific consensus, that bariatric surgery serves as one of the most effective long-term treatments for obesity, while also reducing medical comorbidities [1, 2, 20]. Preoperative weight loss regimens have become a standard preparation tool in most bariatric centers all over the world but are lacking consistent evidence supporting the practice [12-15]. One reason might be the heterogenic study designs covering the topic [21]. Weight loss programs differ in length, supervision, nutritional guidelines, and their objectives.
Some clinics or insurance companies prescribe EWL of at least 5%. This number is supported by several studies [22-24]. However, Giordano et al. [23] found that while an EWL of >5% prior to RYGB is associated with lower morbidity, only an EWL greater than 10% may improve weight loss results after 12 months. Hutcheon et al. [25] found that only patients with a EWL of >8% experienced a significantly greater weight loss after 12 months, with shorter operative duration and hospital length of stay. Our study showed, that EWL above 13,32% (SG) and above 11,98% (RYGB) resulted in significantly higher rates of follow-up visits compared to groups of EWL lower than 5,91% (SG) and lower than 7,69% (RYGB). Uniform threshold values thereby remain uncertain.
In their meta-analysis, Ochner et al. [26] found, that preoperative weight loss using meal replacements might be the most effective way of producing weight loss prior to surgery. Their study also showed that preoperative weight loss was usually not detrimental for patients and improved outcomes at least in one field (postoperative weight loss, rate of complications, reduction in comorbidities). Our study supports those findings, the preoperative weight loss was achieved using meal replacements and showed to be 9, 62%, which is an average result according to the literature [26].
Our study did not show a significant difference in weight loss after 12 months for patients that lost high, medium, or low amounts of weight preoperative. Previous studies have shown that in weight loss interventions, early weight loss is associated with greater weight loss after intervention and years later [27-29]. However, most of these studies state that early weight loss should be defined as the achieved weight loss in the first 1-2 months, as adherence in the first 1-3 weeks is typically excellent [27, 30]. Therefore, it might not be appropriate to anticipate the same results for our study design.
Concerning the question, if a three-week-long regimen was long enough to already have an impact, Van Wissen et al. [9] stated in their systematic review, that even studies with a short duration of two weeks proved a reduction in liver size by 5,1%. However, their study showed that in general, the duration of the preoperative weight loss regimen did correlate with the achieved reduction in liver size. For comparison, programs of 12 weeks found a reduction of 18,7% and programs of 4 weeks a reduction of 12% in liver size. Nonetheless, supporting the thesis, that a three-week-long regimen should still have a significant benefit. Yolsuriyangwon et al. [31] investigated the amount of weight loss as their primary objective and found that even one week and two weeks of a liquid very-low-calorie diet prior to bariatric surgery provided a modest amount of weight loss.
When preparing and selecting patients for bariatric surgery convenient preoperative indicators for good outcomes are unspecified but needed. Successful preoperative weight loss might be a good tool because of its easy measurability and comparability.
Our data found that preoperative weight loss was associated with higher adherence to scheduled visits postoperatively. This could support the thesis that successful preoperative weight loss reflects the motivation that a patient would have postoperatively to comply with the wide medical requirements. Conversely, this could mean that patients, who lost an above-average amount of weight preoperatively, achieved these results due to higher adherence. Searching for literature regarding this thesis, mainly contradictory results can be found. Bergh et al. [32] for example examined preoperative predictors of adherence to dietary and physical activity recommendations and weight loss and could not find ones that were associated with weight loss. A recent study by Cadena-Obando et al. [33] also stated that predictive factors for successful outcomes after bariatric surgery remain controversial but could be population-specific and therefore are worth evaluating.
At least in the light of current literature, a high proportion of postoperative complications seem to be due to a lack of adherence or incorrect implementation of medical requirements and could be easily detected and addressed in a postoperative follow-up program. Swanson et al. [34] appoint an insufficient fluid intake as the main reason for a hospital readmission in the first 30 postoperative days and abdominal pain as the most common leading symptom.
A well-known long-term complication, especially in patients after RYGB, is the formation of gallstones (40% after 3 years). Weight reduction of more than 1.5 kg per week seems to be associated with their occurrence [35]. Regular follow-ups with a sonographic assessment of the gallbladder can detect stones before they become symptomatic and can thereby prevent emergency surgery in cases of gall bladder inflammation. A randomized study even showed that the use of ursodiol could reduce the incidence of gallstones from 32 to 2% [36].
Frequently occurring vitamin and mineral deficiencies are detected through regular blood tests and given sufficient compliance, can be easily treated. Between 30 and 50% of patients undergoing RYGB has a vitamin B12 deficiency postoperatively [37, 38]. Up to 50% of patients suffer from iron deficiency [38, 39]. Calcium and vitamin D deficiencies are also described as significant in post-bariatric patient groups and can cause osteoporosis-associated fractures [39]. Studies show that even simple countermeasures such as daily intake of multivitamin preparations can prevent deficiencies. Recent data suggest that micronutrient deficiencies after bariatric surgeries are increasing, while there is a decrease in monitoring patients at follow-up [40].
Marginal ulcerations have been described in 1% to 16% of patients after bypass surgery [41]. A recent study by Rodrigo et al. [42] names chronic use of non-steroidal anti-inflammatory drugs as the most common cause of these ulcerations after RYGB. Other causes are nicotine abuse and alcohol dependence [42]. Again, a Complication that can often be traced back directly to the patients’ compliance and can be managed well if there is sufficient adherence to all the medical requirements following bariatric surgery
The importance of clinical follow-up attendance has been shown by several studies, stating that a high attendance correlates with long-term weight loss and resolution of co-morbidities. [37, 40, 43-47] Long-term follow-up attendance is therefore recommended for bariatric patients to achieve good long-term outcomes [20, 48, 49]. Therefore, appropriate countermeasures for patients with insufficient preoperative weight loss could be taken, e.g., emphasizing more on the importance of followup visits or implementing additional dates for follow-up. Patients that regularly get checked and also get held accountable by a bariatric physician might have less sudden pain or discomfort. The attendance of regular follow-up visits might even contribute to a lower number of unscheduled appearances in bariatric centers or the outpatient clinic. A thesis that needs to be investigated in further studies.
Another important point that needs to be addressed is that even though our study showed a correlation between high preoperative weight loss and good adherence to follow-up visits, no positive impact on the other investigated perioperative outcome parameters was detected. Blood loss, operative times, need for revision, and weight loss one year after surgery weren’t significantly different in groups of low, medium, or high preoperative weight loss. Taking into account that some clinical centers and insurance companies are demanding obligatory weight loss before surgery, this practice can be seen as hindering and counterproductive.
Another finding of our study was that patients who underwent a RYGB attended the follow-ups significantly more often than patients who had a SG. One possible explanation could be that patients might perceive a bypass operation as more surgically complex and with changed anatomy, more drastic and life-changing, explaining the greater desire of patients for regular medical check-ups.
Concerning the limitations of our study, the first one must be its retrospective nature. Also, it would be beneficial to compare a group of patients who were told to lose weight preoperatively, to a group of patients who were not given such instructions. By mischance, preoperative weight loss has been required of our patients for numerous years, so a comparison with a non-dieting control group was not possible for this single center analysis.
Another limitation is the short length of 12 months. Only a study with a long-term follow-up can assess whether achieved weight-loss is sustainable.
The bariatric surgeons have also taken the abdominal girth into consideration when deciding on the appropriate procedure. If a patient’s abdominal girth was too high, the mechanical conditions for laparoscopy could suffer. In these cases, our surgeons preferred to perform SG. Because higher abdominal girth is mostly correlated with a higher weight, there was an unavoidable difference in initial weights in our SG and RYGB group.
Perioperative blood loss was calculated using the formulas of Nadler and Gross and remains just an estimation. Nonetheless, all patients’ haematocrit was equally identified one day before and two days after the bariatric surgery and hence should be comparable.
Also, we found a significantly higher occurrence of provisional operations in the first four weeks after sleeve gastrostomy for patients with a medium excess weight loss before surgery. However, the number of revisions was low in total and there was no such finding in the RYGB group, making it prone to error.
A prospective randomized trial with a long-term follow-up is needed to evaluate the rationale of preoperative weight loss prior to bariatric surgery as well as the benefits of a high attendance of clinical follow-ups after bariatric surgery.
Conclusions
Preoperative weight loss showed to be a good predictor of follow up adherence after bariatric surgery. Patients that lost higher amounts of EWL tended to participate more often in follow-up visits. Patients that underwent RYGB also showed higher adherence to follow-up visits than patients that underwent SG. Blood loss, operative times, need for revision, and weight loss after one year did not differ significantly between groups that lost a high, medium, or low amount of excess weight preoperatively.
Perhaps, the previous approach of looking for the raison d'être of preoperative weight loss in differences in operative times, blood loss, perioperative complications, and achieved postoperative weight loss was wrong. A more important factor could be the possible prediction of patients’ compliance, which, as explained above, appears to be crucial for the long-term success of bariatric interventions.
References
- Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B et al (2007) Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357:741-52.
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W et al (2004) Bariatric surgery: a systematic review and meta-analysis. Jama 292:1724-37.
- Mechanick JI, Apovian C, Brethauer S, Garvey WT, Joffe AM et al (2020) Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures-2019 update cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anaesthesiologists. Surg Obes Relat Dis 16:175-247.
- Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ et al (2014) the effectiveness and risks of bariatric surgery an updated systematic review and meta-analysis, 2003-2012. JAMA Surg 149:275-87.
- Busetto L, Dicker D, Azran C, Batterham RL, Farpour-Lambert N et al (2017) Practical Recommendations of the Obesity Management Task Force of the European Association for the Study of Obesity for the Post-Bariatric Surgery Medical Management. Obes Facts 10:597-632.
- Tewksbury C, Williams NN, Dumon KR, Sarwer DB (2017) Preoperative Medical Weight Management in Bariatric Surgery: a Review and Reconsideration. Obes Surg 27:208-14.
- Bawden S, Stephenson M, Falcone Y, Lingaya M, Ciampi E (2017) et al Increased liver fat and glycogen stores after consumption of high versus low glycaemic index food: A randomized crossover study. Diabetes Obes Metab 19:70-7.
- Edholm D, Kullberg J, Haenni A, Karlsson FA, Ahlstrom A (2011) et al Preoperative 4-week low-calorie diet reduces liver volume and intrahepatic fat, and facilitates laparoscopic gastric bypass in morbidly obese. Obes Surg 21:345-50.
- Van Wissen J, Bakker N, Doodeman HJ, Jansma EP, Bonjer HJ et al (2016) Preoperative Methods to Reduce Liver Volume in Bariatric Surgery: a Systematic Review. Obes Surg 26:251-6.
- Quidley AM, Bland CM, Bookstaver PB, Kuper K (2014) Perioperative management of bariatric surgery patients. Am J Health Syst Pharm 71:1253-64.
- Lascano CA, Kaidar-Person O, Szomstein S, Rosenthal R, Wexner SD (2006) Challenges of laparoscopic colectomy in the obese patient: a review. Am J Surg 192:357-365.
- Alami RS, Morton JM, Schuster R, Lie J, Sanchez BR et al (2007) is there a benefit to preoperative weight loss in gastric bypass patients? A prospective randomized trial. Surg Obes Relat Dis 3:141-5. Discussion 145-146.
- Cassie S, Menezes C, Birch DW, Shi X, Karmali S (2011) Effect of preoperative weight loss in bariatric surgical patients: a systematic review. Surg Obes Relat Dis 7:760-767.
- Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E et al (2009) does weight loss immediately before bariatric surgery improves outcomes: a systematic review. Surg Obes Relat Dis 5:713-721.
- Horwitz D, Saunders JK, Ude-Welcome A, Parikh M (2016) Insurance-mandated medical weight management before bariatric surgery. Surg Obes Relat Dis 12:496-499.
- Tan SYT, Loi PL, Lim CH, Ganguly S, Syn N et al (2020) Preoperative Weight Loss via Very Low Caloric Diet (VLCD) and Its Effect on Outcomes after Bariatric Surgery. Obes Surg. 30:2099-2107.
- Nadler SB, Hidalgo JH, Bloch T (1962) Prediction of blood volume in normal human adults. Surgery 51:224-324.
- Gross JB (1983) Estimating allowable blood loss: corrected for dilution. Anaesthesiology 58:277-280.
- Montero PN, Stefanidis D, Norton HJ, Gersin K, Kuwada T (2011) Reported excess weight loss after bariatric surgery could vary significantly depending on calculation method: a plea for standardization. Surg Obes Relat Dis 7:531-544.
- Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL et al (2013) Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient-2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring) 21 Suppl 1:S1-27.
- Gerber P, Anderin C, Thorell A (2015) Weight loss prior to bariatric surgery: an updated review of the literature. Scand J Surg 104:33-39.
- Solomon H, Liu GY, Alami R, Morton J, Curet MJ (2009) Benefits to Patients Choosing Preoperative Weight Loss in Gastric Bypass Surgery: New Results of a Randomized Trial. Journal of the American College of Surgeons 208:241-5.
- Giordano S, Victorzon M (2014) the Impact of Preoperative Weight Loss Before Laparoscopic Gastric Bypass. Obesity Surgery 24:669-674.
- Steinbeisser M, McCracken J, Kharbutli B (2017) Laparoscopic Sleeve Gastrostomy: Preoperative Weight Loss and Other Factors as Predictors of Postoperative Success. Obesity Surgery 27:1508-1513.
- Hutcheon DA, Hale AL, Ewing JA, Miller M, Couto F et al (2018) Short-Term Preoperative Weight Loss and Postoperative Outcomes in Bariatric Surgery. J Am Coll Surg 226:514-524.
- Ochner CN, Dambkowski CL, Yeomans BL, Teixeira J, Xavier Pi-Sunyer F (2012) Pre-bariatric surgery weight loss requirements and the effect of preoperative weight loss on postoperative outcome. Int J Obes (Lond) 36:1380-1387.
- Wiedemann AA, Baumgardt SS, Ivezaj V, Kerrigan SG, Lydecker JA et al (2021) getting a head start: identifying pre-treatment correlates associated with early weight loss for individuals participating in weight loss treatment. Transl Behav Med 11:236-243.
- Barnes RD, Ivezaj V, Pittman BP, Grilo CM (2018) Early weight loss predicts weight loss treatment response regardless of binge-eating disorder status and pre-treatment weight change. Int J Eat Disord 51:558-564.
- Unick JL, Neiberg RH, Hogan PE, Cheskin LJ, Dutton GR et al (2015) Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity (Silver Spring) 23:1353-1356.
- Unick JL, Pellegrini CA, Demos KE, Dorfman L (2017) Initial Weight Loss Response as an Indicator for Providing Early Rescue Efforts to Improve Long-term Treatment Outcomes. Curr Diab Rep 17:69.
- Yolsuriyanwong K, Thanavachirasin K, Sasso K, Zuro L, Bartfield J et al (2019) Effectiveness, Compliance, and Acceptability of Preoperative Weight Loss with a Liquid Very Low-Calorie Diet Before Bariatric Surgery in Real Practice. Obes Surg 29:54-60.
- Bergh I, Lundin Kvalem I, Risstad H, Sniehotta FF (2016) Preoperative predictors of adherence to dietary and physical activity recommendations and weight loss one year after surgery. Surg Obes Relat Dis 12:910-918.
- Cadena-Obando D, Ramirez-Renteria C, Ferreira-Hermosillo A, Albarran-Sanchez A, Sosa-Eroza E et al (2020) Are there really any predictive factors for a successful weight loss after bariatric surgery? BMC Endocr Disord 20:20.
- Swanson CM, Roust LR, Miller K, Madura JA (2012) what every hospitalist should know about the post-bariatric surgery patient. J Hosp Med 7:156-163.
- Erlinger S (2000) Gallstones in obesity and weight loss. Eur J Gastroenterol Hepatol 12:1347-1352.
- Sugerman HJ, Brewer WH, Shiffman ML, Brolin RE, Fobi MA et al (1995) A multicentre, placebo-controlled, randomized, double-blind, prospective trial of prophylactic ursodiol for the prevention of gallstone formation following gastric-bypass-induced rapid weight loss. Am J Surg 169:91-6.
- Brolin RE (2001) Gastric bypass. Surg Clin North Am 8:1077-95.
- Via MA, Mechanick JI (2017) Nutritional and Micronutrient Care of Bariatric Surgery Patients: Current Evidence Update. Curr Obes Rep 6:286-296.
- Bloomberg RD, Fleishman A, Nalle JE, Herron DM, Kini S (2005) Nutritional deficiencies following bariatric surgery: what have we learned? Obes Surg 15:145-541.
- Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA (2017) American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg Obes Relat Dis 13:727-741.
- Ying VW, Kim SH, Khan KJ, Farrokhyar F, D'Souza J et al (2015) Prophylactic PPI help reduce marginal ulcers after gastric bypass surgery: a systematic review and meta-analysis of cohort studies. Surg Endosc 29:1018-1023.
- Rodrigo DC, Jill S, Daniel M, Kimberly C, Maher EC (2020) Which Factors Correlate with Marginal Ulcer After Surgery for Obesity? Obes Surg 30:4821-4827.
- Compher CW, Hanlon A, Kang Y, Elkin L, Williams NN (2012) Attendance at Clinical Visits Predicts Weight Loss After Gastric Bypass Surgery. Obesity Surgery. 22:927-934.
- Gould JC, Beverstein G, Reinhardt S, Garren MJ (2007) Impact of routine and long-term follow-up on weight loss after laparoscopic gastric bypass. Surgery for Obesity and Related Diseases 3:627-630.
- Odom J, Zalesin KC, Washington TL, Miller WW, Hakmeh B et al (2010) Behavioural Predictors of Weight Regain after Bariatric Surgery. Obesity Surgery 20:349-356.
- Shen R, Dugay G, Rajaram K, Cabrera I, Siegel N et al (2004) Impact of patient follow-up on weight loss after bariatric surgery. Obes Surg 14:514-519.
- Pontiroli AE, Fossati A, Vedani P, Fiorilli M, Folli F et al (2007) Post-surgery adherence to scheduled visits and compliance, more than personality disorders, predict outcome of bariatric restrictive surgery in morbidly obese patients. Obes Surg 17:1492-1497.
- Gebhart M (2019) Medical Follow Up After Bariatric Surgery. There Umsch 76:154-160.
- Parretti HM, Hughes CA, Jones LL (2019) The rollercoaster of follow-up care' after bariatric surgery: a rapid review and qualitative synthesis. Obes Rev 20:88-107.
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Citation: Boussouf SH, Marzouk MM, Weiner S, Götze, Elshafei M (2022) Successful Weight Loss Before Bariatric Surgery as an Indicator for Perioperative Complications, Adherence, And Postoperative Weight Reduction? A Retrospective Analysis. J Obes Weight Loss Ther 12: 477. DOI: 10.4172/2165-7904.1000476
Copyright: © 2022 Boussouf SH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4264
- [From(publication date): 0-2022 - Dec 08, 2025]
- Breakdown by view type
- HTML page views: 3600
- PDF downloads: 664