The Combination of Aluminum Adjuvant and CpG Adjuvant can Enhance the Immunogenicity of Recombinant HPV Vaccines
Received: 06-Apr-2022 / Manuscript No. JIDT-22-59794 / Editor assigned: 08-Apr-2022 / PreQC No. JIDT-22-59794 (PQ) / Reviewed: 22-Apr-2022 / QC No. JIDT-22-59794 / Revised: 29-Apr-2022 / Manuscript No. JIDT-22-59794 (R) / Published Date: 06-May-2022 DOI: 10.4172/2332-0877.1000495
Abstract
Prophylactic vaccination and early detection of cervical cancers can effectively prevent Human Papilloma Virus (HPV) infection. HPV major capsid protein (HPV L1), a coat protein assembles into Virus-Like Particles (VLPs), acts as a potent immunogen. In the present investigational study, we evaluated the role of the combination effect of the aluminum adjuvant and TLR-9 agonist Cytosine phosphoguanine (CpG) on the immune response and the antigen dose-sparing effect.
HPV L1 VLPs for types 16/18/52 and 58 have been produced by a gene expressed in Hansenula polymorpha. Bivalent (16,18) and tetravalent (16,18,52,58) vaccines with mono (Al+) and biadjuvanted (Al+CpG) were formulated by adsorbing purified VLPs onto aluminum hydroxide and CpG. Sera collected from immunized BALB/c mice showed that biadjuvanted vaccines elicits significantly higher anti-HPV neutralizing antibodies than monoadjuvanted vaccines. Full-dose biadjuvant vaccines (4 μg) elicits 2.5 times higher neutralizing antibodies than half-dose vaccine and no significant differences were observed between the full-dose monoadjuvant vaccine (Al+) and the half-dose biadjuvant vaccine (Al+CpG). Immunised mice have distinct levels of secreted IgG antibodies and the vaccines formulated with a aluminium and CpG, a TLR-9 agonist had skewed towards high Th1 biased immune response with an elevated IgG2a/IgG1 ratio.
In conclusion, recombinant HPV vaccines formulated with an immune modulating CpG agonist and an adjuvant can induce a greater immune response. This combination of CpG and alum effectively reduces the dose of the HPV L1 vaccine by 2.5 times and makes the availability of vaccines at an affordable price.
Keywords: Cervical cancer; Human papilloma virus; Virus like particles; CpG adjuvant; Biadjuvanted vaccines; Antigen dose sparing
Introduction
Human Papilloma Virus (HPV) is an important cause of cervical cancer and other tumour diseases. Every year, nearly 600,000 new cervical cancer cases are diagnosed and nearly 341,831 people died with HPV associated cancers in the world. In recent years HPV incidence in China is 10.7% with a mortality of 5.3% accounting for 18% of the total global burden [1,2]. So far, more than 200 HPV serotypes have been identified, of which type 16 and 18 contributes the mostly with 70% cervical cancers, followed by type 52 and 58 with approximately another 15% of cancers in China [3].
The development of HPV prophylactic vaccine with good immunogenicity and early detection of the cancers can prevent HPV infections to a large extent. The key hurdles in vaccination rollout programs mainly are preparation of purified antigen and the requirement of high-dose antigen for eliciting the immune response. Low antigenic vaccine dosages with a high efficacy will have a significant impact on prevention of HPV infections and protection of women's health through mass vaccination.
HPV prophylactic vaccine is generally made of virus capsid protein L1 or L2. Virus capsid protein, L1 protein having a molecular weight of about 55 kD, can be self-assembled into hollow Virus Like Particles (VLPs) in cells [4]. It has the same antigen spatial epitope as the intact virus, which can stimulate the body's CD4+ lymphocyte mediated humoral immune response to produce protective neutralization antibody [5,6].
The formulation of a vaccine often involves the addition of an adjuvant for augmenting the immune response. Aluminum based adjuvants are most widely used adjuvants in human vaccines and exposure to aluminum is burgeoning with significant implications, the HPV vaccine approved in the Chinese mainland are composed of either aluminum adjuvant or modified aluminum adjuvant [7]. Alternately, CpG adjuvant, a synthetic oligonucleotide containing CpG Oligodeoxynucleotides (CpGODN), which mainly activates B cells and shows a series of immune activity effects, can be used as a co-adjuvant along with aluminum-based adjuvants. Currently, there are no bi- adjuvanted HPV preventive vaccines in the combination of aluminum and CpG adjuvant which elicits higher immunogenicity at a low vaccine dose.
The present study uses a robust protein expression host, Hansenula polymorpha, to produce HPVL1 VLPs for 16/18/52 and 58 and was purified by series of downstream processes using cell lysis, two-step ion exchange chromatography, and final dialysis. Bivalent (HPV L1 VLPs 16/18) and tetravalent (HPV L1 VLPs 16/18) mono (Al+) and biadjuvant (Al++CpG) vaccines were formulated for the first time in combination with aluminum adjuvant (Aluminum hydroxide- Al(OH)3) and CpG adjuvant (synthesized by Guangzhou RiboBio Co., Ltd, CHN) and were administered to BALB/c mice in the 0, 2, 4-week regime. Blood sera collected after the fourth week and sixth week of administration and neutralizing antibody titers were evaluated by pseudovirus neutralization test. The investigational bi adjuvanted vaccine formulation approach is to develop a vaccine with lower antigen content and better immune effect.
Materials and Methods
Strains, cells and plasmids
Strain: Hansenula polymorpha strain (URA- constructed by TheraVac) used for the expression of the HPV L1 protein.
Cells: HEK293FT (ATCC: CCL-41) were used for neutralization assay and were maintained at 37°C under 5% CO2, in humidified environment.
Plasmids: The high copy strain of pMAUR (S.C) KARS1-HPVL1 (16, 18, 52, 58) Hansenula polymorpha was constructed and used to investigate the immune response. Expression plasmids (for HPV L1:p16 shell, p18 L1, p52 shell p58 shell) were extracted from E.coli by standard protocols. The report plasmids (Enhanced Green Fluorescent Protein (EGFP), Red Fluorescent Protein (RFP), Crimson Fluorescent Protein (CFP) prepared as described previously [8].
Animal use
Four to six week old BALB/c female mice (Shanghai SLAC Laboratory Animal Co., Ltd, CHN) were used for the experiments after obtaining the necessary animal ethics approvals.
Fermentation and purification of the L1 protein of HPV 16/18/52/58
Serotype specific (HPV16/HPV18/HPV52/HPV58) high copy strains of pMAUR (S.C) KARS1-HPVL1 Hansenula polymorpha constructed using standard protocols. Hansenula polymorpha strains were cultured in baffled flasks with YPD media and induced by methanol for sufficient protein expression. After achieving the OD of (30-60) the harvested cells were centrifuged at 8000 g and the cells were resuspended in lysis buffer (200 mM MOPS, 100 mM NaCl, 20 mM EDTA and 0.05% Tween 80 at pH 7.0) and lysed at 1500 bar for six times using a high-pressure nano homogenizer. Cell lysate wad centrifuged at 15000 g/30 minutes and crude protein (Pellet). The cell lysate pellet was resuspended in column equilibration buffer, filtered and subjected to sequential chromatographic purification by POROS 50 HS (Thermofisher: 1335906) and CHT II (Bio-Rad: 1574000). Finally, The purified protein fractions were diafiltered (Pellicon XL 30KD- PXB030A50) with PBS solution (Hyclone: SH30256.01) and concentrated to approximately 0.5-1.0 mg/ml using a 30 kD Amicon Ultra-15 centrifugal ultrafiltration device (UFC903008: Millipore).
Protein characterization
A) Estimation and VLPs quantification: The protein concentration was determined using the Pierce BCA Protein Assay Kit (Thermofisher: Catalog number:23225).
B) SDS-PAGE and western blotting: The relative purity of the protein was detected by SDS-PAGE using the ChemiDoc MP System (Bio-Rad) and characterized by Western blot with specific antisera. In short, purified HPV L1 protein fractions were resolved on 10% SDS PAGE and stained using Coomassie Blue for visualizing the protein. HPV L1 proteins resolved on SDS-PAGE gel were also transferred to the polyvinylidene fluoride membrane (PVDF, Merck Millipore, 0.45 μm). The membrane was probed using anti-HPV L1 specific monoclonal antibody (1:1000; BioDragon, CHN) [9]. The purified VLPS were further characterized by electron microscopy.
Preparation of bivalent and tetravalent vaccines
First, purified HPV 16/18/52/58 L1 VLPs were diluted with final formulation buffer (PBS) and adjuvanted with on 500 μg of aluminum hydroxide at a final concentration of 40 μg/mL of VLPs. Biadjuvanted vaccine formulations prepared by formulating the aluminum adjuvanted vaccine were with CpG adjuvant (10 mg/ml) at a final concentration of 150 μg/mL mixed well. The mono and biadjuvanted vaccine formulations were stored at 2°C-8°C until further use.
Immunization of mice
BALB/c mice from 4 to 6 weeks of age were used as an animal model to assess the immunogenicity of the formulated vaccines. The animals were subdivided into two sets representing six groups (A to E-6 animals/ group). The groups were further classified according to mono adjuvated (A:Al+ adjuvanted and B:CpG adjuvanted) and biadjuvanted formulation (Al++CpG) at three different doses of antigen full dose (C:4.0 μg) half dose (D:2.0 μg) and 1/10th dose (E:0.4 μg), non-adjuvanted (F) and negative control (PBS alone) as described in Tables 1 and 2. Three doses of formulated vaccines were administered intramuscularly at 0, 2 and 4 weeks regimens (Tables 1 and 2). Serum samples were collected at an interval of 2 weeks (2,4,6) and stored at -20°C for further analysis.
| Group | Components | ||||
|---|---|---|---|---|---|
| 16 | 18 | Al(OH)3 | CpG | PBS | |
| A | 4 μg | 4 μg | 50 μg | - | + |
| B | 4 μg | 4 μg | - | 15 μg | + |
| C | 4 μg | 4 μg | 50 μg | 15 μg | + |
| D | 2 μg | 2 μg | 25 μg | 7.5 μg | + |
| E | 0.4 μg | 0.4 μg | 5 μg | 1.5 μg | + |
| F | 4 μg | 4 μg | - | - | + |
| G | - | - | - | - | + |
Table 1: Details of animal groups for bivalent vaccine immunization (HPVL1 16/18).
| Group | Components | ||||||
|---|---|---|---|---|---|---|---|
| 16 | 18 | 52 | 58 | Al(OH)3 | CpG | PBS | |
| A | 4 μg | 4 μg | 4 μg | 4 μg | 50 μg | - | + |
| B | 4 μg | 4 μg | 4 μg | 4 μg | - | 15 μg | + |
| C | 4 μg | 4 μg | 4 μg | 4 μg | 50 μg | 15 μg | + |
| D | 2 μg | 2 μg | 2 μg | 2 μg | 25 μg | 7.5 μg | + |
| E | 0.4 μg | 0.4 μg | 0.4 μg | 0.4 μg | 5 μg | 1.5 μg | + |
| F | 4 μg | 4 μg | 4 μg | 4 μg | - | - | + |
| G | - | - | - | - | - | - | + |
Table 2: Details of animal groups for tetravalent vaccine immunization (HPVL1 16/18/52/58).
Preparation of pseudovirus
The pseudovirus for HPV 16/18/52/58 serotypes were produced by co- transfection of 293 FT cells with expression plasmids (p16shell/ p18shell/ p52shell/ p58shell) and report plasmids (EGFP/RFP/CFP) as described earlier [10,11]. In summary, first, 293 FT cells were cultured at 37°C and 5% CO2 overnight. Plasmid DNA (HPV types specific) was extracted and quantified using standard protocols (Qiagen kit-Cat. No./ID:12143). The 293 FT cells were co-transfected with 11.5 μg of a plasmid containing codon- optimized HPV capsid genes for L1 along with 11.5 μg of pseudo reporter genome plasmid (for ex:pEF-GFP) using lipofectamine (Invitrogen). Three to four days after transfections Cells were trypsinized, washed with PBS, and resuspended in fresh buffer aliquoted into 1.5 ml sterile centrifuge tubes, which were subsequently stored at -80°C for further assay [10-12].
Pseudovirus neutralization test
In the present study, the neutralizing antibodies were detected by Pseudo virion-based Neutralization Test (PNT). In short, 293 FT cells were seeded on pre plated in 96-well flat-bottom plates at a concentration of 15,000 cells/well with DMEM media and incubated at 37℃ and 5% CO2 for 24 hrs. Sera collected from BALB/c mice from all groups were diluted 500 times with Complete Medium, and serially diluted 2-fold at a final volume of 100 μL in 96-well round bottom plates (Corning Inc.). Pre- prepared type-specific pseudovirus (200 TCID50 /ml with DMEM/100 μL) was added to each well containing a serum sample and incubated at 4°C for an hour. The serum samples with pseudovirus were then transferred to 293 FT cells and further incubated at 37°C and 5% CO2 for 72 hours. After incubation, the plate was placed into the CTL fluorescence spot counter cells were counted at 10 ms/well using excitation/emission wavelengths of 460/535 nm. Finally, serum neutralization titers were calculated with the Reed-Muench Method. Sera with at least 70% neutralizing activity were regarded as neutralization positive [10,11].
ELISA for immunoglobulins
Sera from all mice were collected at different time points after immunization and evaluated for HPV specific IgG antibodies using ELISA. Sera collected at week 6 after vaccinations were also tested for HPV specific IgG1 and IgG2a antibodies using ELISA. Briefly, ELISA plates (Nunc- Immuno plates, Thermo Fisher Scientific) were coated with 100 ng of the HPV16-L1, HPV18-L1, HPV52-L1, HPV58-L1 protein per well overnight at 4°C in 50 μL coating buffer (Carbonate - Bicarbonate, Sigma, C3041- 100CAP) and then blocked with PBS-T/5% skim milk (blocking buffer, cell signaling, 9999s) for 1 h at 37℃. The plates were subsequently incubated for 1 h at 37℃ with 3-fold serial dilutions of the mouse sera in PBS-T/2% skim milk (dilution buffer, cell signaling, and 9999s). The plates were washed and bound specific IgG, IgG1 and IgG2a was detected with HRP-conjugated goat anti-mouse IgG (Sigma, A2554, 1:20000), IgG1 (Southernbiotech, 1070-05, 1:20000) and IgG2a (Southernbiotech, 1080-05, 1:5000) antibody, respectively. Color development was performed by the addition of 50 μL of TMB (Thermo Fisher Scientific, 34029). After 10 min, the enzyme reaction was stopped with 50 μL of 2 M sulfuric acid per well, and the absorbance was measured in a spark Model 450 microplate reader (Tecan, spark). The antibody titer represents the last reciprocal serum dilution above blank.
Statistical analysis
Geometric mean titers were compared to assess immune and neutralization responses. GraphPad Prism 5.0 software was used to perform graphic and statistical analysis of data. A P-value less than 0.05 are considered statistically significant.
Results
Fermentation and protein purification
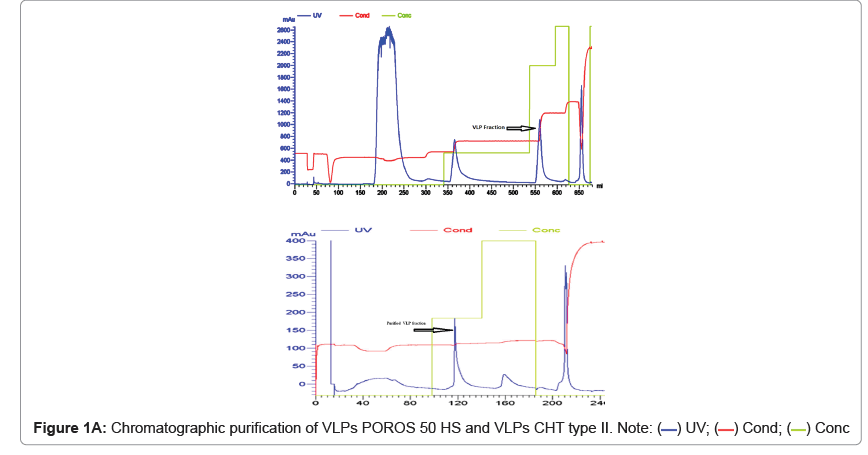
The type specific Hansenula polymorpha fermented on YPD media and induced by methanol. The final harvest culture was subjected to centrifugation and cell pellet of 41.2 g, 39.2 g, 36.4 g and 37.54 g per litre HPV 16, 18, 52 and 58 were collected and stored at -80°C for further use. Twenty grams of cell pellet was subjected to sequential purification steps including dual chromatography for VLPs purification (Figures 1A and 1B). The purified VLPs were finally concentrated to 1242 μg/ mL, 522 μg/mL, 3956.36 μg/mL and 2547.27 μg/mL respectively for the HPV16, 18, 52 and 58 serotypes and stored at 2°C-8°C.
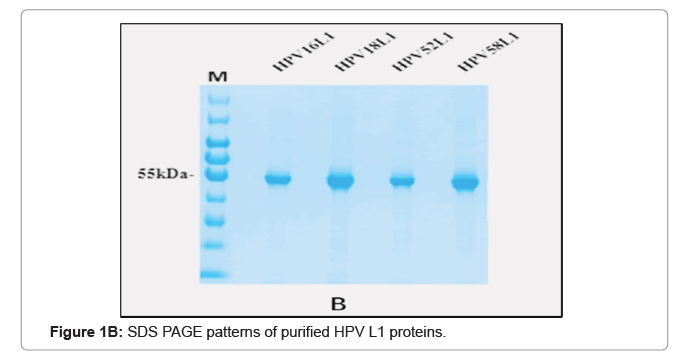
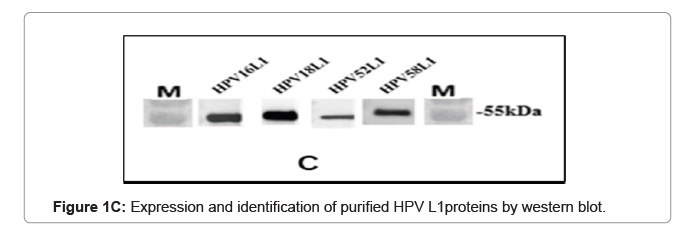
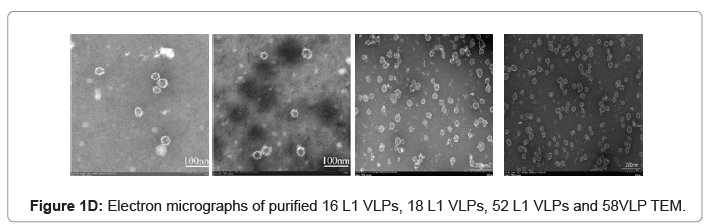
The purified VLP fractions were resolved on SDS-PAGE, stained with Coomassie brilliant blue, showing a protein band of approximately 55 kDa (Figure 1C). The purified protein samples were also characterized by Western blot with type specific HPV L1 monoclonal antibodies (HPV16 5A6, HPV18 3A2, HPV52 36B9, HPV58 2F7-Creative diagnostics). A protein band of approximately 55 kDa molecular weight was observed in the blot, confirming the presence of the HPV major capsid proteins L1 and purified VLPs are clearly visualised under cryo-TEM micrograph (Figure 1D). Process-related impurities such as residual HCP, residual DNA, endotoxin contents were estimated in final formulated samples and found to be <0.001%/dose, <10 ng/dose, <5 EU/dose respectively.
Determination of pseudovirus titer
The expression plasmids and the report plasmids were mixed at 1:1 mass ratio (11.5 μg each) and PEI was added to make the mass ratio of 1:3. Four different pseudo viruses, HPV16-EGFP, HPV18-RFP, HPV52-EGFP, and HPV58-CFP were obtained by co-transfection of the plasmid into 293 FT cells with a TCID50 of 106.415 TU/100 μL, 106.067 TU/100 μL, 106.212 TU/100 μL and 106.245 TU/100 μL respectively. The total titer of the pseudovirus was 105-107 TU/100 μL.
Immune response of HPV16/18 bivalent vaccine
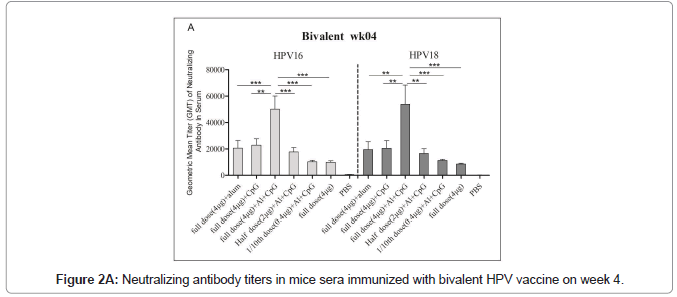
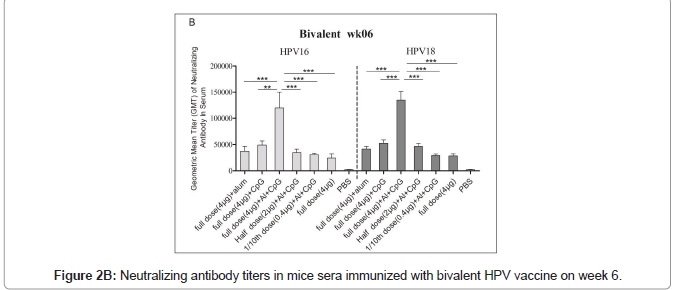
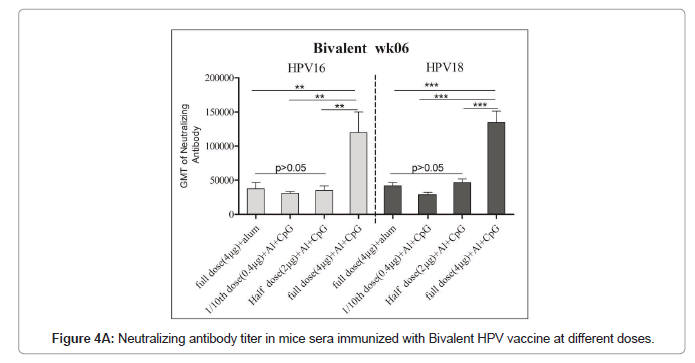
Immunized mice sera used for the pseudovirus neutralization test, and to obtain the neutralization antibody titer of mouse serum the number of fluorescence spots was read through the CTL fluorescence spot counter, and the IC50 was calculated using the Reed Muench method. The neutralizing antibodies titers against HPV16 and HPV18 in bivalent bi adjuvanted vaccine were 120082 ± 73839 and 134762 ± 40965 respectively and are significantly higher than other groups. The titer of biadjuvant vaccine group is higher than that of single adjuvant Al(OH)3 and CpG group, and the difference is very significant (P<0.01); the titer of full dose biadjuvant group is significantly higher (P<0.01) than that of half dose biadjuvant group. The titer of the double adjuvant vaccine group was higher than that of the Al(OH)3 and CpG single adjuvant group, and the difference was very significant (P<0.01), indicating that the double adjuvant vaccine can stimulate a stronger immune response. The titer of the full dose double adjuvant group was higher than that of the half dose double adjuvant group, and the difference was very significant (P<0.01), indicating that the vaccine with high antigen content can induce the body to produce higher titer and antibodies (Figures 2A and 2B).
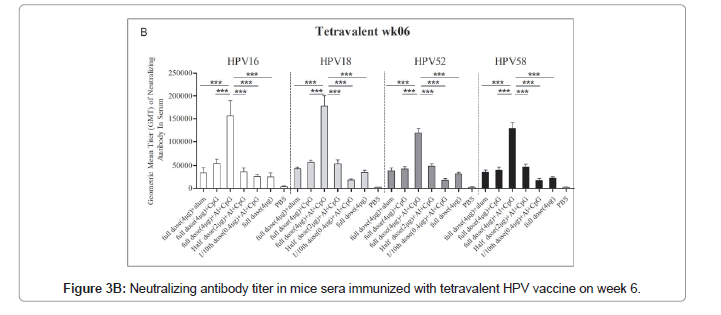
Immune response of HPV16/18/52/58 tetravalent vaccine
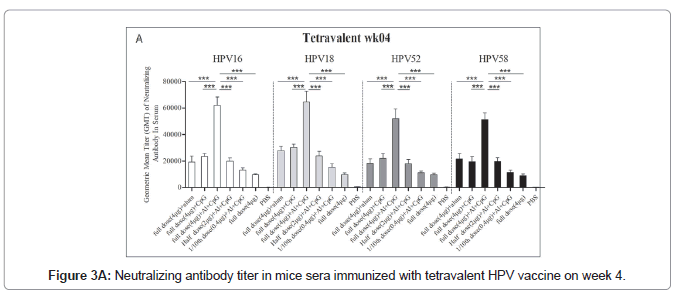
The immune response of the HPV16/18/52/58 tetravalent vaccine to mice is shown in Figure 3. In general, the neutralizing antibodies titers against HPV16, HPV18, HPV52, and HPV58 in the double adjuvant vaccine group were higher than those in the other groups, reaching 152648 ± 35964, 174635 ± 23658, 120645 ± 12453 and 135426 ± 13652, respectively (Figures 3A and 3B).
Dose response of bi adjuvanted vaccine
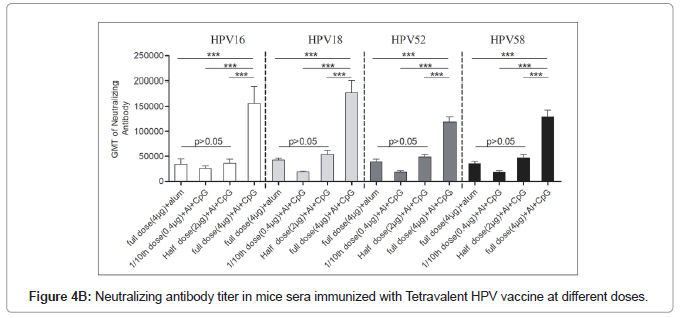
The neutralization antibody titters between half dose Al+CpG biadjuvanted (2 μg VLPs/dose) and full dose (4 μg VLPs/dose) monovalent Al+ adjuvanted vaccines had no significant differences (P>0.05) indicating similar or almost equal immune response (Figures 4A and 4B). The full dose of the bivalent or tetravalent biadjuvanted vaccine had elicited more than 2.5 folds of the neutralization of antibody titer with all four HPV serotypes (Table 3). Our earlier results showed that the immunogenicity of the full-dose alum monoadjuvant vaccine is similar to that of the commercially available vaccine (Gardasil).
| Dose | Bivalent | Tetravalent | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 4 | Week 6 | Week 4 | Week 6 | |||||||||
| 16 | 18 | 16 | 18 | 16 | 18 | 52 | 58 | 16 | 18 | 52 | 58 | |
| Full dose* vs. ½ dose+ | 2.8 | 3.2 | 3.4 | 2.9 | 3.1 | 2.7 | 2.9 | 2.6 | 4.3 | 3.3 | 2.4 | 2.8 |
| Full dose vs. 1/10th dose& | 4.8 | 4.7 | 3.9 | 4.7 | 4.7 | 4.2 | 4.7 | 4.5 | 6.1 | 9.5 | 6.6 | 7.3 |
| ½ dose vs. 1/10th dose | 1.7 | 1.5 | 1.1 | 1.6 | 1.5 | 1.6 | 1.6 | 1.7 | 1.4 | 2.9 | 2.7 | 2.6 |
*=Full dose:4 µg/dose; + =½ dose:2 µg/dose and &=1/10th dose:0.4 µg/dose
Table 3: Fold of neutralizing antibody titre in sera of mice immunized with bi/tetra valent HPV vaccine at different doses.
Assessment of Th1/Th2 response
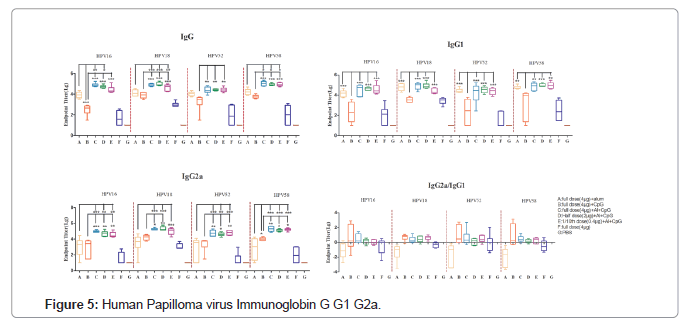
Further, the balance of Th1 and Th2 cells was evaluated by comparing the levels of type specific immunoglobulins (IgG, IgG1and IgG2a) that are surrogates of Th1 and Th2 responses. Interestingly, HPV+CpG combinational groups induced a high IgG2a/IgG1 ratio indicative of a Th1-biased response. In contrast, mice immunized with mono adjuvanted vaccine (HPV+Alum) or vaccine without any adjuvant formulations induces Th2-biased responses with a low IgG2a/ IgG1 ratio and consistent with the production of high levels of IgG1 shows a clear skew toward a Th2 phenotype (Figure 5).
Discussion
Cervical cancer is the most common cause of cancer incidence and mortality in women worldwide, with almost 0.6 million cases and 0.3 million deaths per year [13]. Considering the highly preventable nature of cervical cancer, the World Health Organization (WHO) in 2018 called for action to reduce the global burden of cervical cancer and eliminate the disease [14]. China works as an important collaborator in the elimination of cervical cancer by supporting various Human Papilloma Virus (HPV) vaccine development and cancer screening programs [15]. At present in China, four HPV vaccines are licensed, and more than a dozen vaccines are in clinical trials (with bi/tetra/ nine valent vaccines).
The present study carried out on vaccines prepared using L1 VLPs for four HPV serotypes (16/18/52/58) produced with Hansenula polymorpha. The downstream purification of HPV L1 plays an critical role in maintaining the activity of HPV L1. In this study, L1 VLPS were purified using sequential two-step ion exchange chromatography followed by desalination and concentration. Purified VLPs were adjuvanted and administered to BALb/c mice. The neutralizing antibody titers were assessed by Pseudovirus neutralization assay using in-house prepared Pseudovirus. The Pseudovirus were prepared by standard protocols by wrapping the serotype specific VLP structure’s gene sequence and reporter genes into a plasmid. The prepared pseudovirus has the same infection characteristics as real virus particles and don’t have the ability to infect [11,16].
Adjuvant usage is a complementary approach to enhance vaccine immunogenicity by activating Pattern Recognition Receptor (PRR) receptors of the innate immune system or by modulating antigen pharmacokinetics. Adjuvant formulations of aluminum salts and PRR agonists have been shown to enhance vaccine immune responses compared to aluminum salts or PRR agonists alone [17]. Currently available HPV vaccines in China (Cervarix®-GSK or Gardasil® 4 or 9-Merck, Cecolin®-Xiamen Innovax Biotech Co. Ltd.) are formulated with mono adjuvants of alum based (ASO4 or Aluminum hydroxy phosphate or Al(OH)3) elicits a strong safety record, for a better immune response or antigen sparing still there is an ongoing need for alternate or combinational adjuvants [18-20].
Combinations of aluminum salts and PRR agonists represent a promising adjuvant platform to enhance antigen immunogenicity. Oligodeoxynucleotides (ODNs) with unmethylated CpG motifs are Toll-Like Receptor 9 agonists (TLR-9) and provide an innate immune response characterized by Th1 and proinflammatory cytokines production. This CpG ODN acts as a potent vaccine adjuvant by generating Th1-biased responses, thus an alternate choice as an excipient to enhance the immune response. In the present study mice immunized with alum adjuvanted vaccine skewed towards Th2 phenotypic response with a low IgG2a/IgG1 ratio and consistent high levels of IgG1 antibodies. In contrast, HPV vaccines in combination with CPG adjuvant shows high IgG2a/IgG1 ratio indicating a Th1- biased response (Figure 4).
Earlier studies with CpG ODNs as adjuvant had represented a function of professional antigen-presenting cells and boost the generation of humoral and cellular vaccine-specific immune responses [21]. CpG ODN that has already been used as an adjuvant in the licensed hepatitis B vaccine HEPLISAV-B showed a stronger immune response with a faster onset of protection compared to 3 doses of alum- adjuvanted Engerix-B [22]. Our earlier studies on combination of CpG ODN+alum adjuvants with hepatis vaccine (HBsAg, HBcAg) had showed a significant anti-HBsAg specific antibodies production and exhibiting CpG and Alum’s synergistic effect [23,24].
The mice immunised with alum and CpG combinational vaccines (bi or tetra valent) had elicited higher anti-HPV neutralizing antibody titers compared to mono adjuvanted vaccines. At the same time, the full dose (4 μg) biadjuvanted vaccine had shown 2.5 folds higher neutralization antibody titers than half dose vaccine (2 μg). Also, the half dose biadjuvanted vaccine (Al+CpG+2 μg) and full dose mono adjuvanted vaccine (Al++4 μg dose) had similar antibody titers (Figures 2 and 3). The results of these dose studies indicate that the Al:CpG combination dramatically enhanced vaccine immune responses compared to vaccines adjuvanted with aluminum alone in mice. Similar results were demonstrated with the SARS-CoV-2 receptor-binding domain vaccine, in which an AH:CpG adjuvant formulation induces potent antiRBD responses in young and aged mice and overcomes both the poor immunogenicity of the antigen and impaired immune responses in the aged [25]. Earlier interim analysis reports also suggested that the biadjuvanted SARS-CoV-19 vaccine had a good safety profile and elicits strong immunogenicity with significant higher levels of neutralizing antibody [26]. These studies have shown an alternative path for vaccine presentation in further enhancing the immune response at low dose of antigen content.
Conclusion
In conclusion, for effective HPV vaccination, the dosage regime and quantity of antigen per dose will play a substantial obstacle on vaccine roll-out. The present study had shown a significant enhancement in vaccine immunogenicity at dose sparing thus would increase the availability of vaccine dose manifold. This investigational study with biadjuvanted vaccine at half the dose of mono adjuvanted vaccines will support manufacturing aspects and the availability of vaccine at an affordable price. Finally, usage of combinational adjuvants for improvement of vaccine efficiency needed without compromising tolerability or safety.
Author Contributions
Design, conceptualization, supervision, Analysis, investigation, and writing. All authors have read and agreed to the published version of the manuscript. Jun Ge, Xiuying Pu, Juyin Li contributed equally to the research work design/ methodology and manuscript preparation.
Declaration of interests
The authors report there are no competing interests to declare.
References
- Bruni L, Albero G, Serrano B, Mena M, Collado JJ, et al. Human papillomavirus and related diseases report, ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre), 2021.
- Zhang X-R, Li Z-Q, Sun L-X, Liu P, Li Z-H, et al. (2021) Cohort profile: Chinese cervical cancer clinical study. Front Oncol 11:690275.
[Crossref] [Google Scholar] [PubMed]
- Human papillomavirus and related cancers, Fact sheet 2021, ICO/IARC Information Centre on HPV and Cancer, 2021.
- Campo MS, Roden RBS (2010) Papillomavirus prophylactic vaccines: established successes, new approaches. J Virol 84:1214-1220.
[Crossref] [Google Scholar] [PubMed]
- Jarrett WF, Smith KT, O'Neil BW, Gaukroger JM, Chandrachud LM, et al. (1991) Studies on vaccination against papillomaviruses: Prophylactic and therapeutic vaccination with recombinant structural proteins. Virology 184:33-42.
[Crossref] [Google Scholar] [PubMed]
- Kirnbauer R, Taub J, Greenstone H, Roden R, Dürst M, et al. (1993) Efficient self-assembly of human papillomavirus type 16 L1and L1-L2 into virus-like particles. J Virol 67:6929-6936.
[Crossref] [Google Scholar] [PubMed]
- Shardlow E, Mold M, Exley C (2018) Unraveling the enigma: Elucidating the relationship between the physicochemical properties of aluminium-based adjuvants and their immunological mechanisms of action. Allergy Asthma Clin Immunol 14:80.
[Crossref] [Google Scholar] [PubMed]
- Wu X-L, Zhang C-T, Zhu X-K, Wang Y-C (2010) Detection of HPV types and neutralizing antibodies in women with genital warts in Tianjin city. Virol Sin 25:8-17.
[Crossref] [Google Scholar] [PubMed]
- Naupu PN, van Zyl AR, Rybicki EP, Hitzeroth II (2020) Immunogenicity of plant-produced Human Papillomavirus (HPV) Virus-Like Particles (VLPs). Vaccines (Basel) 8:740.
[Crossref] [Google Scholar] [PubMed]
- Pastrana DV, Buck CB, Pang Y-YS, Thompson CD, Castl PE, et al. (2004) Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 321:205-216.
[Crossref] [Google Scholar] [PubMed]
- Buck CB, Pastrana DV, Lowy DR, Schiller JT (2005) Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med 119:445-462.
[Crossref] [Google Scholar] [PubMed]
- Touze A, Coursaget P (1998) In vitro gene transfer using human papillomavirus-like particles. Nucleic Acids Res 26:1317-1323.
[Crossref] [Google Scholar] [PubMed]
- Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, et al. (2020) Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 8:e191-e203.
[Crossref] [Google Scholar] [PubMed]
- WHO Director-General calls for all countries to take action to help end the suffering caused by cervical cancer, OPAS, 2018.
- Wei L, Xie X, Liu J, Qiao Y, Zhao F, et al. (2021) Elimination of cervical cancer: Challenges promoting the HPV vaccine in China. Indian J Gynecol Oncol 19:51.
- Muñoz N, Castellsagué X, de González AB, Gissmann L (2006) Chapter 1: HPV in the etiology of human cancer. Vaccine 24:1-10.
[Crossref] [Google Scholar] [PubMed]
- Sarkar I, Garg R, van den Hurk SDL (2019) Selection of adjuvants for vaccines targeting specific pathogens. Expert Rev Vaccines 18:505-521.
[Crossref] [Google Scholar] [PubMed]
- Gardasil, INN-Human Papillomavirus Vaccine [Types 6, 11, 16, 18] (Recombinant, adsorbed), Gardasil product information, 2006.
- Cervarix II-67 - EN PI (clean), Ceravarix product information, 2007.
- Cecolin®, World Health Organization, 2021.
- Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM (2011) CpG DNA as a vaccine adjuvant. Expert Rev Vaccines 10:499-511.
[Crossref] [Google Scholar] [PubMed]
- Champion CR (2021) Heplisav-B: A Hepatitis B vaccine with a novel adjuvant. Ann Pharmacother 55:783-791.
[Crossref] [Google Scholar] [PubMed]
- Xiaohan DS, Jun GE, Xiaoqian Z, Tong Z, Jianqiang L, et al. Hepatitis b vaccine, EP 2974740A1, 2016.
- Li J, Bao M, Ge J, Ren S, Zhou T, et al. (2017) Research progress of therapeutic vaccines for treating chronic hepatitis B. Hum Vaccin Immunother 13:986-997.
[Crossref] [Google Scholar] [PubMed]
- Nanishi E, Borriello F, O'Meara TR, McGrath ME, Saito Y, et al. (2022) An aluminum hydroxide: CpG adjuvant enhances protection elicited by a SARS-CoV-2 receptor-binding domain vaccine in aged mice. Sci Transl Med 14:eabj5305.
[Crossref] [Google Scholar] [PubMed]
- Hsieh S-M, Liu M-C, Chen Y-H, Lee W-S, Hwang S-J, et al. (2021) Safety and immunogenicity of CpG 1018 and aluminium hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: interim results of a large-scale, double-blind, randomised, placebo-controlledphase 2 trial in Taiwan. Lancet Respir Med 9:1396-1406.
[Crossref] [Google Scholar] [PubMed]
Citation: Ge J, Pu X, Li J, Xia X, Wang XD, et al. (2022) The Combination of Aluminum Adjuvant and CpG Adjuvant can Enhance the Immunogenicity of Recombinant HPV Vaccines. J Infect Dis Ther 10: 495. DOI: 10.4172/2332-0877.1000495
Copyright: © 2022 Ge J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3137
- [From(publication date): 0-2022 - Nov 09, 2025]
- Breakdown by view type
- HTML page views: 2424
- PDF downloads: 713