The Development of Autoimmune Membrano-Proliferative Glomerulonephritis (Type II) In a Female Patient with Serological Combined Autoantibodies against Complement 3b and Factor B: Case Report
Received: 09-Dec-2015 / Accepted Date: 26-Dec-2015 / Published Date: 02-Jan-2016 DOI: 10.4172/2161-0681.1000262
Abstract
Background: Most of membranoproliferative glomerulonephritis (MPGN) raise from the abnormalities in alternative complement pathways.
Case presentation: A 3-year-old girl manifested hematuria (HU), proteinuria (PU), and hypocomplementemia (HC) without a known cause; the first renal biopsy indicated MPGN that resembled type I MPGN. After treatment with Methylprednisolone and Cyclophosphamide (CTx), the nephritic syndrome (HU plus PU) withdrew but often relapsed. At the age of 7 years, the clinical situation suddenly worsened, PU and the activity of the urinary sediment increased, and the glomerular filtration rate rapidly decreased; simultaneously, auto-antibodies against Complement 3b (C3b) and Factor B were detected with high titers. The second renal biopsy exhibited a typical glomerular injury of type II MPGN with diffuse cellular crescents; the patient was treated with methylprednisolone plus plasmapheresis and Rituximab, but the renal failure still proceeded. She started peritoneal dialysis at the age of 9 years and received renal transplantation at the age of 11 years; after transplantation, she was given routine immunosuppression plus plasmapheresis and Rituximab (anti-CD 20 globulin), and presented clinical remission except for persistent HC. The nephritic syndrome relapsed at the age of 13 years, and the third renal biopsy confirmed a recurrence of type II MPGN; thereafter, Eculizumab (anti-C5 monoclonal antibody) was administered, which significantly ameliorated the clinical condition.
Conclusion: The pathological appearance of type II MPGN may be variable but not completely irrelevant according to the clinical conditions at the time of biopsy
Keywords: Autoimmune membranoproliferative glomerulonephritis type II; Serologic autoimmunoantibodies; Complement 3; Kidney transplantation
312755Abbreviations
HU: Hematuria; PU: Proteinuria; HC: Hypocomplementemia; MPGN: Membranoproliferative glomerulonephritis; CTx: Cyclophosphamide; C3: Complement 3; C1q: Complement 1q; Rituximab: anti-CD 20 globulin; Eculizumab: anti-C5 monoclonal antibody; LM: Light Microscopy; GBM: Glomerular Basement Membrane; CFHR1: Complement Factor H-related proteins 1; CFHR3: Complement Factor H-related proteins 3; Scr: Serum creatinine; IF: Immunofluorescence microscopy; EM: Electron Microscopy; EDD: Electron-Dense Deposits; MMF: Mycophenolate Mofetil; PSGN: Poststreptococcal glomerulonephritis; IgAN: immunoglobulin A nephropathy; LN: Lupus Nephritis.
Introduction
Membrano-proliferative glomerulonephritis (MPGN), an uncommon form of chronic nephritis, is principally diagnosed by renal biopsy at the light microscopy (LM) level on two pathological patterns of glomerular injury: (a) mesangial and endothelial hypercellularity; and (b) thickening of the glomerular capillary walls due to mesangial interposition and double contours of the glomerular basement membrane (GBM) [1]. MPGN may be idiopathic or secondary in etiology; most of the idiopathic and secondary MPGN exhibit similar pathological appearances [2]. Idiopathic MPGN can be further divided into I, II, and III types based on the ultrastructural details because the LM features are mostly indistinguishable among 3 types [3]. Regardless of the types, most of MPGN raise from the abnormalities in alternative complement pathways because of systemic C3 activation and deposition of C3 cleavage products along the GBM [4]. The excessive activation of C3 convertase can induce a permanent activation that complements and enhances the alternative complement cascade [5]. C3b and Factor B, two individual components of the C3 convertase, can enhance C3 convertase activity after C3b and Factor B are motivated by genetic or acquired pathogenetic mechanisms [6]. In 2011, Chen et al reported two patients that had the serologic combined autoantibodies of C3b and Factor B [7]; the younger patient was detected with the heterozygous deficiency of genes encoding complement factor H-related proteins 1 (CFHR1) and 3 (CFHR3); she clinically manifested persistent hypocomplementemia (HC) and nephritc syndrome (hematuria HU plus proteinuria PU), eventually proceeded into uremia, and received allograft transplantation. During the development of type II MPGN, she underwent three renal biopsies when the clinical situation changed: (i) the first episode of the nephritc syndrome; (ii) rapid progression of the renal insufficiency; and (iii) relapse of nephritic syndrome posttransplantation. The type II MPGN is a rare form of MPGN and has evidently poorer prognostic significance in comparison with types I and III [8]. We reviewed these biopsies and analyzed the clinic-pathologic features associated with the development of autoimmune MPGN type II.
Case Presentation: Clinical and Laboratory Evidences and Pathological Features
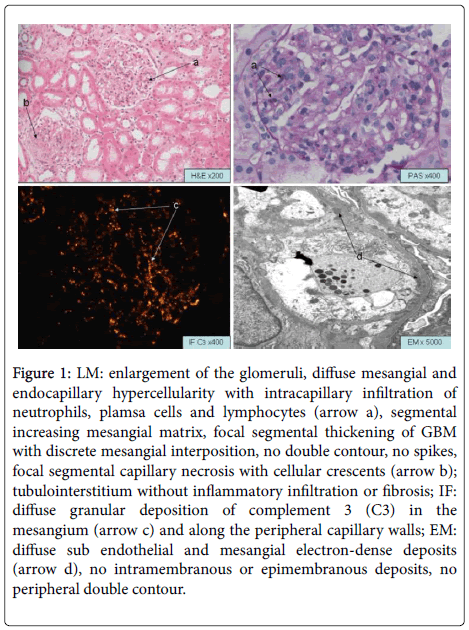
The 3-year-old girl noticed that her urine was a deep red color in absence of any clinical causes, i.e., previous respiratory/urinary tract infection, skin blood spots, or medicine intake, and the laboratory examination showed HU 3+ and PU 3+, low serum C3 concentration, C4 and serum creatinine (Scr) concentration were at normal values. Also, antinuclear antibodies and antibodies against double-stranded DNA were negative. The renal biopsy at that time (Figure 1) exhibited diffuse endocapillary hypercellularity, intracapillary neutrophil infiltration, interposition of mesangial cells, and focal segmental capillary disruption with cellular crescents (involving 3 of 36 glomeruli). The immunofluorescence microscopy (IF) displayed focal subendothelial and mesangial granular deposits that stained 3+ for C3 and 1+ for C1q. Electron microscopy (EM) found focal granular subendothelial and mesangial electron-dense deposits (EDD), intramembranous mesangial interposition, but intramembranous or subepithelial areas were free of the EDD. The LM appearance resembled the post streptococcal glomerulonephritis, but the ultrastructural findings suggested the beginning of MPGN. The clinical improvement could be seen with the methylprednisolone therapy plus cyclophosphamide, while the HU and PU often relapsed, accompanying with persistent and often profound depression of C3 (lowest C3c level was 33 mg/dL) in the following 4 years.
Figure 1: LM: enlargement of the glomeruli, diffuse mesangial and endocapillary hypercellularity with intracapillary infiltration of neutrophils, plamsa cells and lymphocytes (arrow a), segmental increasing mesangial matrix, focal segmental thickening of GBM with discrete mesangial interposition, no double contour, no spikes, focal segmental capillary necrosis with cellular crescents (arrow b); tubulointerstitium without inflammatory infiltration or fibrosis; IF: diffuse granular deposition of complement 3 (C3) in the mesangium (arrow c) and along the peripheral capillary walls; EM: diffuse sub endothelial and mesangial electron-dense deposits (arrow d), no intramembranous or epimembranous deposits, no peripheral double contour.
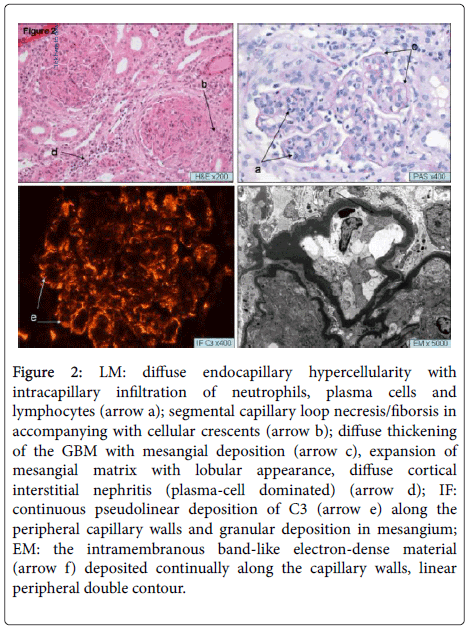
At the age of 7 years, the clinical situation substantially worsened with the active urinary sediment, heavy PU (maximal at 3.17 g/L), rapidly elevating the serum creatinine concentration and the glomerular filtration rate (GFR) decreased. In addition, the combined autoantibodies of Complement 3b (C3b) and Factor B were detected with high titers in circulation, leading to extreme low C3 level. The second kidney biopsy (Figure 2) showed the prominent mesangial hypercellularity, dense linear transformation of peripheral GBM with double contours and interposition of mesangial cells, and diffuses peripheral linear subendothelial and intramembranous staining of 3+ for C3, 1+ for C1q. The EM showed the typical appearance of MPGN type II with intramembranous linear band-shaped EDD and mesangial granular EDD. In addition, the segmental capillary disruption and extracapillary proliferation were seen in 50% glomeruli, which presented the convincing evidence to explain the acute renal failure. The girl was treated with 2 methylprednisolone pulses, 3 fresh plasma infusions, and 15 plasmapheresis. In addition, Rituximab (anti-CD 20 globulin) was also administrated at a dose of 375 mg/m2. Simultaneously, the secondary complications of renal failure including hypertension, anemia, and hyperkalemia were well controlled. However, the profound renal failure progressed; she began the peritoneal dialysis at the age of 9 years.
Figure 2: LM: diffuse endocapillary hypercellularity with intracapillary infiltration of neutrophils, plasma cells and lymphocytes (arrow a); segmental capillary loop necresis/fiborsis in accompanying with cellular crescents (arrow b); diffuse thickening of the GBM with mesangial deposition (arrow c), expansion of mesangial matrix with lobular appearance, diffuse cortical interstitial nephritis (plasma-cell dominated) (arrow d); IF: continuous pseudolinear deposition of C3 (arrow e) along the peripheral capillary walls and granular deposition in mesangium; EM: the intramembranous band-like electron-dense material (arrow f) deposited continually along the capillary walls, linear peripheral double contour.
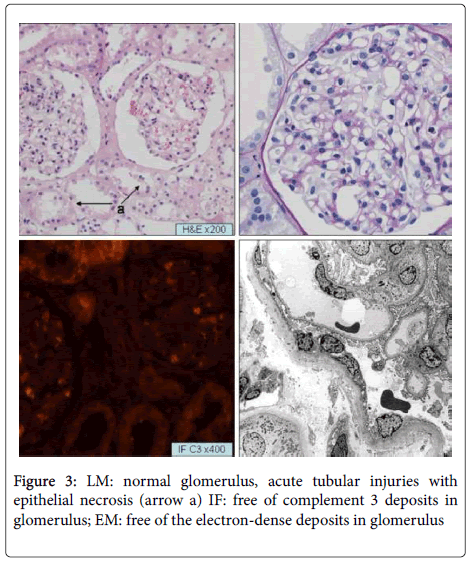
The patient received cadaveric kidney transplantation at the age of 11 years. The biopsy at zero-time of operation showed severe acute tubular ischemic-reperfusion injury only (Figure 3). The immunosuppressive protocol began with Tacrolimus at a dose of 0.25 mg/kg, mycophenolate mofetil (MMF) at a dose of 1g/m2, and methylprednisolone at a dose of 1 mg/kg. The doses of Tacrolimus and MMF were adjusted according to whole blood trough levels. In addition, the plasmapheresis was performed every other day before and for 3 weeks; they then continued once per week from week 4 through week 12 and then every other week from week 13 through week 16. She exhibited a complete clinical remission shortly after transplantation. Until 18 months after the transplantation, the serum creatinine concentration varied between 61.8 and 79.4 μmol/L, and the PU was negative since 1 week after transplantation. The serological C3c level varied between 50 and 60 mg/dL, but the autoantibody level of Factor B was negative (lower than 0.2 Unit/L).
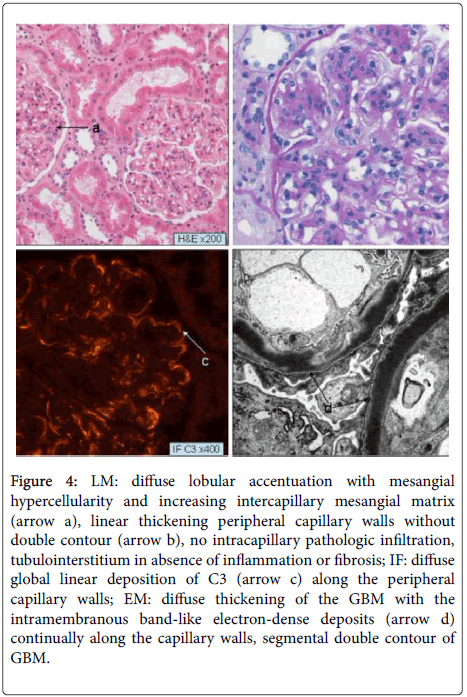
At the beginning of 2013, the PU output obviously increased accompanied with active urine sediment and extreme low C3c levels. The third kidney biopsy was performed and showed (Figure 4) diffuse glomerular hypercellularity, intercapillary expansion of mesangial matrix, thickening of GBM with diffuse double contour, focal segmental capillary sclerosis (involving 1 of 21 glomeruli), and diffuse peripheral linear subendothelial deposits that stained 3+ for C3 and 1+ for C1q. There was also diffuse thickening of capillary walls with segmental double contours and intramembranous band-like EDD, visceral podocytes activation, and segmental foot-process effacement. The recurrent type II MPGN was confirmed, and the patient was treated with Eculizumab : 900 mg/week for 4 weeks, then 1200 mg/14 days. After the administration of Eculizumab, the PU was evidently reduced, the urine sediment was remissed; and the Scr and blood pressure maintained in a normal reference range, although the HC remained.
Figure 4: LM: diffuse lobular accentuation with mesangial hypercellularity and increasing intercapillary mesangial matrix (arrow a), linear thickening peripheral capillary walls without double contour (arrow b), no intracapillary pathologic infiltration, tubulointerstitium in absence of inflammation or fibrosis; IF: diffuse global linear deposition of C3 (arrow c) along the peripheral capillary walls; EM: diffuse thickening of the GBM with the intramembranous band-like electron-dense deposits (arrow d) continually along the capillary walls, segmental double contour of GBM.
Discussion
We reviewed a young patient who had serologic combined autoantibodies of C3b and Factor B, manifested nephritic syndrome and even progressive renal failure, and who underwent three renal biopsies that showed not only the characteristic features of MPGN but also other pathological findings, according to the clinical conditions at the time of biopsy, i.e., diffuse glomerular hyper cellularity , capillary disruption, cellular crescents, and sclerosis [9]. Similarly, some other kidney diseases could exhibit the membranoproliferative patterns of glomerular injury resembling MPGN, which should be distinguished from MPGN through the complete clinical history and serological evidence. The first biopsy showed that diffuse endocapillary hypercellularity and intracapillary exudation of neutrophils mimic poststreptococcal glomerulonephritis (PSGN) at the LM level; however, the mesangial and subendothelial EDD were commonly seen in type I MPGN in contrast to the well-demarcated subepithelial humps in PSGN. In addition, the strong C3 immunostaining in absence of immunoglobulin deposits was also an ominous clue representing MPGN [10-11]. Some cases of immunoglobulin A nephropathy (IgAN) exhibited the glomerular membranoproliferative changes, and the dominant or co-dominant immunostaining of IgA is readily distinguished from MPGN. Note that the persistent HC is not often present in IgAN patients [12]. Lupus nephritis (LN) should be considered in any patient with an active nephritis on the renal biopsy and laboratory evidence of hypocomplementemia. However, the presence of “full house” immunoglobulin staining, strong C1q immunostaining, and positive lupus serologies are helpful to distinguish LN from MPGN [13]. In addition to the fast deterioration of the renal function, the second biopsy showed an extensive disruption of the glomerular tufts and extracapillary proliferation. The histological findings of the second biopsy showed extracapillary proliferation of podocytes and formation of cellular crescents, which reached the criteria of crescentic glomerulonephritis, but the convincing C3 immunostaining and negativity of antineutrophil cytoplasm antibody (ANCA) were distinguishable factors from the ANCA-associated crescentic glomerulonephritis [14].
Traditionally, MPGN is classified into three subtypes based on the pattern and location of EDD [9]. For example, the presence of subepithelial EDD make it type III; type 2 is also called dense deposit disease since the EDD deposits in the GBM; and the subendothelial/ mesangial EDD without subepithelial EDD is found in type I [9,15-16]. However, the EM-based classification has an obvious limitation when the location of EDD changes or overlaps in three subtypes. In our study, the first biopsy showed subendothelial and mesangial EDD resembling type I MPGN, but the second and third biopsies exhibited evidently type II MPGN. Thus, the diagnostic value of IF findings for the classification should be emphasized because the patterns of complement activation differ in the three types [17]. In most cases, type II MPGN shows C3 immunostaining but is free of the immunoglobulins deposition. This patient had combined C3b and factor B autoantibodies, which enhanced the activation of the alternative pathway and led to the complement-complex deposition. In contrast, the combined staining of C3 and IgG were more frequently seen in types I and III MPGN [18].
MPGN mainly affects children and 50% of affected children progress into end stage renal disease (ESRD) within 10 years and normally become candidates for renal transplantation [19]; the recurrence rate of type II MPGN approaches 80-100%, which is much higher than that of type I or type III [20-22]. The recurrent MPGN tends to be clinically mild, but the allograft survival appears to be significantly diminished [23]. In addition, although clinical presentations are similar for the 3 types MPGN, they manifest somewhat different mechanisms of complement activation and predisposition to recur in renal transplants; the existence of serologic autoimmune monoclonal antibodies and severe HC appear to be associated with early recurrent, aggressive progression and poor graft outcome [24].
So far, there are still few studies of the effectiveness of therapeutic intervention to consistently improve the clinical outcomes for patients with either original or recurrent type II MPGN [25-26]. The girl didn’t benefit from the plasmapheresis and Rituximab therapy in conjunction with a steroid. Recently, Eculizumab has been used in patients with dense deposit disease to control over the persistent HC or to regain the renal function [27-28]. The girl began the Eculizumab therapy after the confirmation of the recurrent type II MPGN in the allograft; she responded well with it, and the PU has been evidently ameliorated.
In conclusion, the patient presents type II MPGN in child; the diagnosis requires renal biopsy and intramembranous deposits by electron microscopy; the uremia develops within 5 years of diagnosis; the recurrence occurs in renal allograft at 2 years post transplantation; in etiology, the autoantibodies against complement trigger uncontrolled systemic activation of the complement cascade; most treatments for type II MPGN are ineffective, whether local control of the complement cascade can prevent ongoing renal failure is so far still not clear.
Consent
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Author’s Contribution
Dr. Rudolph, Birgit: participated in evaluation of pathologic slices and writing;
Dr. Wu, Kaiyin: participated in the performance of the case report and writing the paper.
Competing Interest
None of the authors has any competing financial interests to declare. There was no support/funding for this study.
References
- West CD (1992) Idiopathic membranoproliferative glomerulonephritis in childhood. PediatrNephrol 6: 96-103.
- Rennke HG (1995) Secondary membranoproliferative glomerulonephritis. Kidney Int 47: 643-656.
- Cameron JS, Turner DR, Heaton J, Williams DG, Ogg CS, et al. (1983) Idiopathic mesangiocapillary glomerulonephritis. Comparison of types I and II in children and adults and long-term prognosis. Am J Med 74: 175-192.
- Sethi S, Nester CM, Smith RJ (2012) Membranoproliferative glomerulonephritis and C3 glomerulopathy: resolving the confusion. Kidney Int 81: 434-441.
- Hawfield A, Iskandar SS, Smith RJ (2013) Alternative pathway dysfunction in kidney disease: a case report and review of dense deposit disease and C3 glomerulopathy. Am J Kidney Dis 61: 828-831.
- Rooijakkers SH, Wu J, Ruyken M, van Domselaar R, Planken KL, et al. (2009) Structural and functional implications of the alternative complement pathway C3 convertase stabilized by a staphylococcal inhibitor. Nat Immunol 10: 721-727.
- Chen Q, Müller D, Rudolph B, Hartmann A, Kuwertz-Bröking E, et al. (2011) Combined C3b and factor B autoantibodies and MPGN type II. N Engl J Med 365: 2340-2342.
- Sethi S, Fervenza FC (2011) Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. SeminNephrol 31: 341-348.
- Appel GB, Cook HT, Hageman G, Jennette JC, Kashgarian M, et al. (2005) Membranoproliferative glomerulonephritis type II (dense deposit disease): an update. J Am SocNephrol 16: 1392-1403.
- Sethi S, Fervenza FC, Zhang Y, Zand L, Vrana JA, et al. (2012) C3 glomerulonephritis: clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int 82: 465-473.
- Pickering MC, D'Agati VD, Nester CM, Smith RJ, Haas M, et al. (2013) C3 glomerulopathy: consensus report. Kidney Int 84: 1079-1089.
- Emancipator SN (1994) IgA nephropathy: morphologic expression and pathogenesis. Am J Kidney Dis 23: 451-462.
- Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, et al. (2004) The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am SocNephrol 15: 241-250.
- Neild GH, Cameron JS, Ogg CS, Turner DR, Williams DG, et al. (1983) Rapidly progressive glomerulonephritis with extensive glomerular crescent formation. Q J Med 52: 395-416.
- Levy M, Gubler MC, Sich M, Beziau A, Habib R (1978) Immunopathology of membranoproliferative glomerulonephritis with subendothelial deposits (Type I MPGN) ClinImmunolImmunopathol 10: 477-492.
- Sethi S, Nester CM, Smith RJ (2012) Membranoproliferative glomerulonephritis and C3 glomerulopathy: resolving the confusion. Kidney Int 81: 434-441.
- Sethi S, Fervenza FC (2012) Membranoproliferative glomerulonephritis--a new look at an old entity. N Engl J Med 366: 1119-1131.
- Fakhouri F, Frémeaux-Bacchi V, Noël LH, Cook HT, Pickering MC (2010) C3 glomerulopathy: a new classification. Nat Rev Nephrol 6: 494-499.
- Karakayali FY, Ozdemir H, Kivrakdal S, Colak T, EmiroÄŸlu R, et al. (2006) Recurrent glomerular diseases after renal transplantation. Transplant Proc 38: 470-472.
- Moritz MJ1, Burke JF, Jarrell BE, Carabasi RA (1987) The incidence of membranoproliferative glomerulonephritis in renal allografts. Transplant Proc 19: 2206-2207.
- Habib R, Antignac C, Hinglais N, Gagnadoux MF, Broyer M (1987) Glomerular lesions in the transplanted kidney in children. Am J Kidney Dis 10: 198-207.
- Eddy A, Sibley R, Mauer SM, Kim Y (1984) Renal allograft failure due to recurrent dense intramembranous deposit disease. ClinNephrol 21: 305-313.
- Vangelista A, Frascà GM, Martella D, Bonomini V (1990) Glomerulonephritis in renal transplantation. Nephrol Dial Transplant 5 Suppl 1: 42-46.
- Lorenz EC, Sethi S, Leung N, Disenzieri A, Fervenza FC, et al. (2010) Recurrent membranoproliferative glomerulonephritis after kidney transplantation. Kidney Int 77: 721-728.
- Tarshish P, Bernstein J, Tobin JN, Edelmann CM. (1992) Treatment of mesangiocapillary glomerulonephritis with alternatie-day prednisone-a report of the international study of kidney disease in children. PediatrNephrol 6: 123-130.
- Sethi S, Fervenza FC, Zhang Y, Nasr SH, Leung N, et al. (2011) Proliferative glomerulonephritis secondary to dysfunction of the alternative pathway of complement. Clin J Am SocNephrol 6: 1009-1017.
- Daina E, Noris M, Remuzzi G (2012) Eculizumab in a patient with dense-deposit disease. N Engl J Med 366: 1161-1163.
- Bomback AS, Smith RJ, Barile GR, Zhang Y, Heher EC, et al. (2012) Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am SocNephrol 7: 748-756.
Citation: Rudolph B, Wu KY (2016) The Development of Autoimmune Membrano-Proliferative Glomerulonephritis (Type II) In a Female Patient with Serological Combined Autoantibodies against Complement 3b and Factor B: Case Report. J Clin Exp Pathol 6:262. DOI: 10.4172/2161-0681.1000262
Copyright: © 2016, Rudolph B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 11647
- [From(publication date): 2-2016 - Aug 20, 2025]
- Breakdown by view type
- HTML page views: 10681
- PDF downloads: 966