The Value of MRI in Diagnosis of Solid Pancreatic Tumors
Received: 27-Sep-2017 / Accepted Date: 08-Dec-2017 / Published Date: 13-Dec-2017 DOI: 10.4172/2167-0846.1000307
Abstract
Aim: To investigate the value of 3.0 T MRI in the diagnosis of solid pancreatic tumor.
Materials and Methods: Aggregate of 36 patients were enlisted with suspected or proven as solid pancreatic tumors were collected from the first affiliated Hospital of Anhui Medical University Teaching Hospital during the period of January 2012 to August 2015, contained 20 cases of pancreatic adenocarcinoma, 7 cases of pancreatic neuroendocrine tumors (PNETs), 5 cases of pancreatic metastases, 4 cases of solid pseudopapillary tumors (SPTP). All the patients performed with plain scan and multiphasic contrast enhanced imaging on 3.0 T MR, 23 cases performed with MRCP. The data of clinic and imaging were retrospectively analyzed.
Results: Pancreatic adenocarcinoma was irregular in shape, lesion was hypo intense in 10 cases, slightly hypo intense in 9 cases and isointense in one case on T1WI. 10 tumor showed slightly enhancement in arterial phase, and all of tumors showed moderate enhancement during portal and delayed phase; 19 cases showed double duct sign, and one case showed distal pancreatic duct dilation. Of the 7 cases with PNETs, the margin was well defined in six well differentiated lesions and ill-defined in one pancreatic neuroendocrine carcinoma; six tumors were marked enhancement in arterial phase and one tumor was moderate enhancement in pancreatic parenchyma in all of three phases. The mean diameter of SPTP was 5.2 cm. The solid part of tumors was hyperintense on T1WI and slightly hyper intense on T2WI, with progressively enhancement. 4 SPTPs had capsule which were hyperintense on T1WI and T2WI exhibited moderate enhancement. Of the 5 pancreatic metastases, one cases had only one lesion, two cases had multiple lesions and two cases showed diffuse pancreatic enlargement, one case showed heterogenous slightly enhancement, two case showed peripheral rim enhancement, two case showed marked enhancement in arterial, then wash-out in delayed and portal phases.
Conclusion: MRI is valuable for diagnosis and distinguishing solid pancreatic tumors.
Keywords: Magnetic resonance imaging; Solid pancreatic tumors; Pancreatic ductal adenocarcinoma; Neuroendocrine tumors; Metastases
Introduction
The most, timely affirmation of pancreatic tumor has been credited to the eighteenth century, Italian specialist Giovanni Battista Morgagni, and the historical father of modern-day anatomic pathology, who asserted to have followed a few instances of growth in the pancreas. Numerous eighteenth and nineteenth century doctors were doubtful about the presence of the ailment, given the comparative appearance of pancreatitis. Some case reports were circulated in the 1830s, and a certifiable histopathologic determination was in the long run recorded by the American clinician Jacob Mendes Da Costa, who likewise questioned the unwavering quality of Morgagni's understandings. The begin of the twentieth century; tumor of the head of the pancreas had turned into an entrenched [1].
The pancreas is a non-encapsulated organ found retroperitoneally. It is known to be influenced by an expansive range of dangerous and in addition amiable conditions. Pancreatic masses are regularly found by chance in asymptomatic patients, and if the mass can't be grouped further, clinical administration can be troublesome. Routine imaging modalities for surveying the pancreas incorporate transabdominal ultrasound, endo-sonography, endoscopic retrograde cholangiopancreatography (ERCP) and computed tomography. However, these examination apparatuses are restricted in their capacity to delineate the delicate tissue contrasts in detail. Accordingly, today, pre-and Post-Gadolinium magnetic resonance imaging. MRI is the technique for decision for the assessment of pancreatic pathologies. Morphology and histologic example of solid pancreatic tumors, for example, adenocarcinoma is all around portrayed. In this article, we introduce a choice of uncommon dangerous and conceivably solid pancreatic neoplasm.
With the approach of more current imaging methods, radiologists can now pick among a few noninvasive imaging modalities for assessing patients with suspected pancreatic issue. Pancreatic imaging is a basic instrument in achieving early determination of pancreatic illnesses, especially pancreatic adenocarcinoma, where early finding may prompt to enhanced survival among patients whose visualization would somehow or another is grim. Preoperative imaging based arranging has two noteworthy restricting elements [2]. The powerlessness to precisely judge the level of tumor or encasement of vascular structures and [3]. The occasionally unobtrusive augmentation of the tumor into encompassing per pancreatic tissues and intrusion of non-expanded lymph nodes. In this presentation, the MR imaging discoveries of pancreatic adenocarcinoma and other pancreatic neoplasms, for example, Pancreatic Neuroendocrine Tumors, Pancreatic metastatic, papillary tumor of the pancreas are delineated. Moreover, the imaging qualities of provocative procedures, for example, incessant and intense pancreatitis, are likewise highlighted.
Pancreatic adenocarcinoma
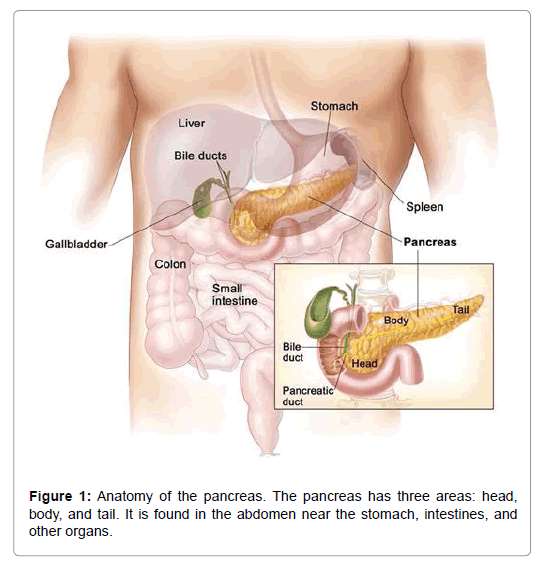
The most surely understood kind of development of the pancreas is an adenocarcinoma of the pancreas. 85% of each and every harmful tumor of the pancreas is adenocarcinomas. Pancreatic adenocarcinoma is the fourth driving explanation behind malignancy determined in men and women in the United States. The American Malignancy Society assesses that consistently 29,000 American are resolved to have adenocarcinoma of the pancreas and around 28,000 die of pancreatic cancer. Around 20-40% of patients with pancreatic disease seem to have the tumor contained altogether inside the pancreas at the season of the finding. Surgical expulsion of the tumor is suggested in this gathering of patients and this gives the best alternative to long haul survival [4]. Surgery for pancreatic disease is an exceedingly concentrated operation and subsequently the patient ought to be assessed by a specialist who is exceptionally experienced in treating this issue. Around 60-80% of the patients are found to have a privately propelled malignancy since it is attacking into the encompassing tissues outside the pancreas or where the growth has metastasized (Figure 1).
Pancreatic adenocarcinoma conveys a prognosis, with a general 5-year survival rate of under 5%, chiefly in light of the fact that the analysis is made late. Computed tomography (CT) is the referencestandard imaging system for diagnosing pancreatic adenocarcinoma yet shows a few constraints, most eminently isoattenuation in 5% to 20% of every pancreatic adenocarcinoma, an example conceivably connected with better survival [5,6]. Magnetic resonance imaging (MRI) gives great difference determination and has profited in the course of the most recent couple of years from specialized advances connected with checked changes in execution. Diffusion-weighted MR imaging (DWI), specifically, has been accumulating extensive intrigue and has delivered empowering preparatory affectability and specificity [7,8]. By the way, in a late review, DWI neglected to portray 47% of pancreatic adenocarcinomas, in light of hyper-intensity of the pancreatic parenchyma distal to the tumor [9], identified with upstream obstructive pancreatitis. The vast majority of the reviews looking at tumor perceptibility crosswise over MRI sequences were performed several years ago [10,11].
Pancreatic neuroendocrine tumor
Neuroendocrine tumor with respect to the acknowledgment of PNETs, the likelihood of growth of the islet cells was at first recommended in 1888. The main instance of hyper-insulinism because of a tumor of this sort was accounted for in 1927. Zollinger and Ellison, who gave their names to Zollinger-Ellison disorder, subsequent to proposing the presence of a gastrin-discharging pancreatic tumor in a report of two instances of abnormally serious peptic ulcers distributed in 1955. In 2010, the WHO prescribed that PNETs be alluded to as "neuroendocrine" instead of "endocrine" tumors [12].
Neuroendocrine tumors (or neuroendocrine neoplasms, NET) of the pancreas emerge from the pancreatic endocrine cells. They more often than not happen in patients more than 50 years, with a slight male predominance. The tumor evaluating proposed by The World Health Organization (WHO) depends on mitotic number and Ki67 record. Neoplasms can be evaluated as neuroendocrine tumor (NET) G1 (carcinoid) or G2 and neuroendocrine carcinoma (NEC), G3 [13]. NET can give common clinical discoveries because of hormone hypersecretion ("working" or "syndromic" NET) or they can be nonworking (or "non-syndromic"). Contingent upon the sort of hormone they emit the most and the clinical presentation, syndromic tumors can be further named gastrinoma, insulinoma, somatostatinoma, VIPoma and glucagonoma and additionally uncommon sorts, for example, ACTHoma.
On MRI, neuroendocrine tumors show up hypo-intense on fat suppressed T1WI contrasted with the encompassing solid pancreas while they generally appear hyper-intense contrasted with the typical pancreas on T2WI [14]. Particularly bigger tumors may contain cystic or necrotic parts because of degeneration, which brings about a heterogeneous appearance. On post-contrast images, both working and non-working tumors demonstrate noteworthy difference upgrade. Upgrade may seem homogeneous, diffuse heterogeneous, ring-or target-like, contingent upon the arrangement of the mass. Cystic or necrotic parts do not improve and show up hypo-intense contrasted with whatever remains of the mass on post-contrast T1WI. Indeed, even in particularly cystic deteriorated masses, very much vascularized tissue can be discovered, indicating the determination of NET [15]. On element differentiate upgraded contemplates, NET improve prior and more seriously than the sound pancreatic parenchyma, accordingly show up hyper-intense amid the arterial phase [16].
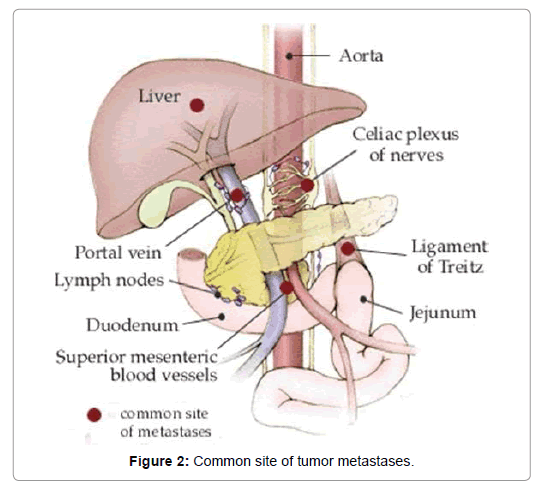
Metastases, for instance in the liver, are hyper-vascular also and in this manner their complexity conduct is practically identical to that of the essential injuries. Once in a while, tumors contain a ton of stringy tissue subsequently demonstrate low signal intensity on T2WI and less complexity upgrade. DWI may be useful in recognizing small neuroendocrine tumors because of its higher image differentiate; ADCvalues have a tendency to be lower than in the encompassing solid pancreas (Figure 2) [17].
Solid pseudo papillary tumor of pancreas
SPT of the pancreas are phenomenal (1-2% of exocrine pancreatic tumors at for the most part associations). Franz [18] initially depicted this tumor in 1959 as a "papillary tumor of the pancreas, considerate or harmful. In 1996, the World Health Organization (WHO) renamed this tumor as SPT for the worldwide histologic grouping of tumor of the exocrine pancreas [19].
Solid pseudo papillary tumors (SPTs) is an exceptional tumor with low quality unsafe potential, whose pathogenesis is not totally gotten on. Proportional words are solid pseudopapillary neoplasm (SPN), papillary and solid epithelial neoplasm, papillary cystic and (Gruber-) Frantz tumor. SPN generally happen in young women (2nd - 3rd decades of life). As a rule, SPTs are of poor quality threat yet can create metastases in up to 15% of cases. The injuries are normally single and frequently situated in the pancreatic body or tail. SPTs can achieve a momentous size and regularly contain necrotic or hemorrhagic regions. As far as MR imaging discoveries, a few attributes vary between smaller (<3 cm) and mass. As a rule, MR imaging demonstrates a very much outlined, adjusted solid mass with low signal on T1WI and high to middle signal on T2WI. Cystic segments can be available and are auxiliary to tumor degeneration. In the event that mass is transcendently cystic, signal is nearer to liquid on T2WI. SPTs generally show homogeneously.
Substantial SPTs frequently seem heterogeneous as they contain hemorrhagic, cystic or necrotic parts, calcifications or a T2 hypo-intense or differentiate upgrading case. In the event that drain, additionally optional to degeneration, is available, T1 motion inside the mass can be expanded. On gadolinium-upgraded scan, SPT demonstrate heterogeneous improvement with a progressive focal filling, which is useful for separating SPT from injuries with early blood vessel upgrade. In the event that a container is available, it tends to demonstrate late upgrade. As to administration, finish surgical extraction is suggested as SPT are conceivably reparable by resection of the essential tumor.
Pancreatic metastases
As the pancreas is a non-encapsulated organ, metastases of different malignant tumors can be found in the pancreas. Essential tumors from the liver kidney, lung, gastrointestinal tract and additionally lymphoma are the most widely recognized substances metastasizing to the pancreas [20]. Other incessant substances are tumors of the breast, thyroid organ, bones (osteosarcoma) and skin (melanoma). Still, the occurrence of metastases to the pancreas and the peripancreatic lymph nodes is just 3-12% of patients with across the board metastatic ailment in dissection studies and 2-5% in clinical reviews, underlining their irregularity. Regularly, pancreatic metastases are connected with other extra-pancreatic metastases since pancreatic metastases have a tendency to grow somewhat death of malignant disease [21,22].
Metastases to the pancreas can be discovered anyplace inside the pancreas (head/body/tail) [23]. Hints that may separate metastases from essential pancreatic tumors incorporate an earlier death, variety of injuries and obviously far reaching metastatic ailment. Pancreatic metastases are generally little and all around characterized masses [24]. Because of the assortment of substances, appearance on MRI is different. We might want to show the MR appearance of one of the elements: pancreatic metastases from liver. Pancreatic liver metastases demonstrate a low signal on T1WI, while intensity is high on T2WI.
On element differentiate upgraded thinks about they demonstrate a solid improvement in the early stage, which may show up ring-like in bigger injuries. As masses are T1-hyperintense, subtraction imaging can be useful in distinguishing contrast upgrade.
A noteworthy concern when diagnosing a pancreatic tumor is regardless of whether the disease has effectively spread (metastasized) outside of the pancreas. The area of the metastases will figure out if the patient has locoregional or metastatic disease. The area of the metastases will likewise figure out if the tumor can be surgically evacuated or not able to be surgically expelled. There are sure destinations that pancreatic malignancy may spread to that typically, however not generally, dispense with surgery as a treatment choice. They are:
Risk factors for pancreatic cancer
• Age, sexual orientation, and ethnicity; the danger of creating pancreatic tumor increments with age. Most cases happen after age 65, [25] while cases before age 40 are uncommon. The disease is somewhat more regular in men than women, and in the United States is more than 1.5 circumstances more basic in African Americans, however occurrence in Africa is low.
• Cigarette smoking is the best-settled avoidable danger figure for pancreatic malignancy, around increasing risk among whole deal smokers, the peril extending with the amounts of cigarettes smoked and the times of smoking. The risk diminishes bit by bit in the wake of smoking end, taking some place in the scope of 20 years to return to almost that of non-smokers [26].
• Obesity; a BMI greater than 35 increases relative risk by about half.
• Family history; 5-10% of pancreatic illness cases have a gained fragment, where people have a family history of pancreatic malignancy. The risk rises colossally if more than one firstdegree relative had the disease, and more subtly in case they made it before the age of 50. Most of the qualities included have not been perceived. Natural pancreatitis gives a massively extended lifetime risk of pancreatic disease of 30–40% to the age of 70. Screening for early pancreatic tumor may be offered to individuals with acquired pancreatitis on an investigation start [27]. A few people may have their pancreas surgically expelled to avoid malignancy creating later on.
• Chronic pancreatitis appears to practically triple hazard, and as with diabetes, new-onset pancreatitis might be a side effect of a tumor. The danger of pancreatic disease in people with familial pancreatitis is especially high.
• Diabetes mellitus is a risk factor for pancreatic cancer and newonset diabetes may also be an early sign of the disease. People who have been diagnosed with Type 2 diabetes for longer than ten years may have a 50% increased risk, as compared with nondiabetics.
• Specific sorts of sustenance (as particular from obesity) have not been obviously appeared to expand the danger of pancreatic disease. Dietary elements for which there is some proof of marginally expanded hazard incorporate prepared meat, red meat, and meat cooked at high temperatures (e.g. by cooking or grilling) [28].
Materials and Methods
Research objects
We led a solitary focus review contemplate via searching our foundation's database down patients who had pancreatic resection. Among these patients, we identified those with an analysis of pancreatic carcinoma and a preoperative MRI assessment performed inside 2 months before surgery. MRI is performed in all patients whose tumor is viewed as resectable on account of its higher conspicuity for identification of pancreatic adenocarcinoma and its higher affectability for discovery of liver metastases. Aggregate of 36 patients were enlisted with suspected or proven as solid pancreatic tumors were collected from the first affiliated Hospital of Anhui Medical University Teaching Hospital during the period of January 2012 to August 2015, contained 20 cases of pancreatic adenocarcinoma, 7 cases of pancreatic neuroendocrine tumors (PNETs), 5 cases of pancreatic metastases, 4 cases of solid pseudopapillary tumor (SPTP). All the patients performed with plain scan and multiphasic contrast enhanced imaging on 3.0 T MR.
Image capture of MRI
MRI was performed on GE Discovery 750 W 3.0 T, fasting for solids and liquids for 6 hours before examination. First, unenhanced T1weighted imaging (LAVA and FSPGR), T2 weighted imaging (FSE) and Magnetic resonance cholangiopancreatography (MRCP) were performed. Then multiphasic contrast enhanced imaging was performed, contrast agent was injected into the elbow vein, gadopentetate dimeglumine was injected at a dose of 0.1 mmol per kilogram of body weight and the injection of normal saline disodium at a dose of 25 ml, the flow rate was 2.0 ml per second, we could acquire the images of arterial phase, portal vein phase and delayed phase. Regarding all sequence parameters are summarized (Table 1).
| Parameters | TIWI | T2WI | MRCP | Multiphasic CE-MRI | |
|---|---|---|---|---|---|
| Sequence | LAVA | FSPGR | FSE | - | LAVA |
| TR (ms) | 2.7 | 265 | 6316 | 6000 | 2.7 |
| TE (ms) | 1.3 | in-phase 2.4 out-phase 5.8 | 90 | 1000 | 1.3 |
| TI (ms) | 5 | - | - | - | 5 |
| FOV (cm × cm) | 38 × 34.2 | 40 × 32 | 40 × 28 | 30 × 30 | 38 × 30.4 |
| Slice thickness (mm) | 5 | 6 | 6 | 40~60 | 5 |
| Interlayer spacing (mm) | -2.5 | 2 | 2 | 0 | -2.5 |
| Imaging matrix | 270 × 224 | 384 × 160 | 320 × 224 | 320 × 288 | 270 × 224 |
| NEX | 0.72 | 0.5 | 2 | 1 | 0.72 |
| Delayed time (s) | - | - | - | - | arterial phase 15~20 portal vein phase 55~75 delayed phase 120 |
Table 1: Parameters of the MR sequences.
MRI protocol
MR imaging convention has been created to highlight pancreas. Ideal convention ought to get evident difference between pancreatic parenchyma and injuries. On the off chance that neuroendocrine tumor is suspected or clinicians need to know whether arterial phase is important. Another favorable position of pancreatic stage is that peripancreatic vessels, including veins, can be improved. This can encourage appraisal of vascular invasion. Customary MR imaging convention for pancreas ought to incorporate T1-weighted images, T2-weighted images, Magnetic resonance cholangiopancreatography (MRCP), and element upgrade with blood vessel stage, port vein stage, and delayed stage utilizing fast gradient recall sequence.
Fat-suppress T1 weighted imaging is recommended because it can improve the dynamic contrast of pancreatic parenchyma. MRCP is routinely utilized as a part of assessing pancreatic adenocarcinoma to depicture morphological changes of biliary and pancreatic channels, which has substituted diagnostic ERCP.
Analysis of MRI images
Two radiological doctors with work experience at least five years analyzed the images, the difference was reached an agreement through discussion. Comparing to normal pancreatic parenchyma, they analyzed the features of lesion, including position, size, boundary, signal, schedule of enhancement, dilation of bile duct and pancreatic duct (diameter of bile duct more than 1.0 cm, diameter of pancreatic duct more than 0.3 cm).
Patient preparation
Prior to the MRI test is performed, patients are re-quested to change into a doctor's facility outfit and evacuate all adornments and other metal articles, such as Jewellery, Hairpins, Eyeglasses, Watches, Wigs, Dentures, Hearing aids, Underwire bras which can might affect the magnetic imaging.
Result
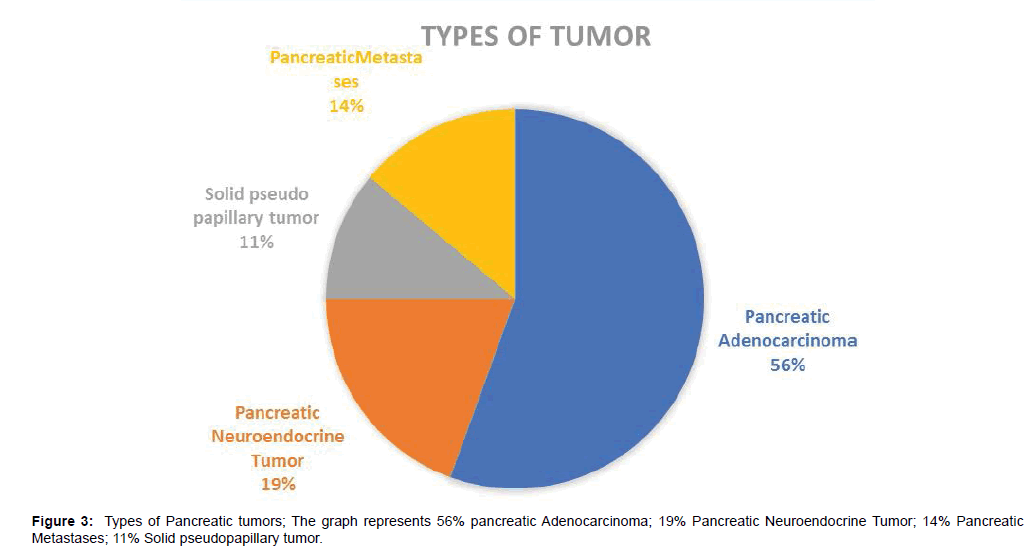
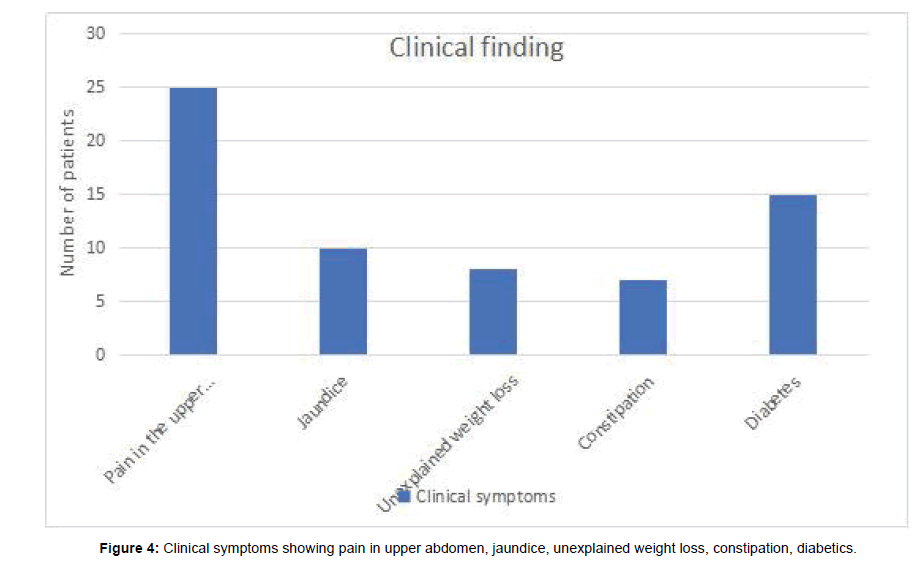
Our study enlisted 36 patients with suspected or demonstrated Pancreatic cancer. There were 12 females and 24 males. Among them 25 were presented with pain in the upper abdomen, 15 with diabetes, 10 with jaundice, 8 with unexplained weight loss and 7 patients with constipation. The tumor occurred between the ages of 42-78 years with a mean age falling in the 5th decade. Of 36 patients, 20 cases were diagnosed pancreatic adenocarcinoma, 7 cases were diagnosed pancreatic neuroendocrine tumors (PNETs), 5 cases were diagnosed pancreatic metastases, 4 cases were diagnosed solid pseudopapillary tumors (SPTPs) (Tables 2-5).
| Case | Location | Diagnosis | Size (cm2) | Shape/ Margin | Signal intensity | Double duct sign | Arterial Phase | Portal Phase | Delayed Phase | |
|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | |||||||||
| 1 | Head | Adenocarcinoma | 1.8 × 1.6 | Irreg/Well-defined | Hypo intense | Hyper intense | + | slightly enh | Moderate enh | Moderate enh |
| 2 | Head | Adenocarcinoma | 1.2 × 1.4 | Irreg/Well-defined | Hypo intense | Hyper intense | + | slightly enh | Moderate enh | Moderate enh |
| 3 | Head | Adenocarcinoma | 2.4 × 1.8 | Irreg/Well-defined | Hypo intense | Hyper intense | + | slightly enh | Moderate enh | Moderate enh |
| 4 | Head | Adenocarcinoma | 3.2 × 1.2 | Irreg/Well-defined | Hypo intense | Hyper intense | + | slightly enh | Moderate enh | Delayed enh |
| 5 | Posterior to head | Adenocarcinoma | 1.6 × 2.1 | Irreg/Well-defined | Hypo intense | Hyper intense | + | slightly enh | Moderate enh | Moderate enh |
| 6 | Head | Adenocarcinoma | 2.6 × 2.4 | Irreg/Well-defined | Hypo intense | Hyper intense | + | slightly enh | Moderate enh | Moderate enh |
| 7 | Tail | Adenocarcinoma | 0.6 × 1.1 | Irreg/Well-defined | Hypo intense | Hyper intense | + | slightly enh | Moderate enh | Moderate enh |
| 8 | Uncinate process | Adenocarcinoma | 1.5 × 1.8 | Irreg/Well-defined | Hypo intense | Hyper intense | + | slightly enh | Moderate enh | Moderate enh |
| 9 | Body | Adenocarcinoma | 1.7 × 1.9 | Irreg/Well-defined | Hypo intense | Hyper intense | + | slightly enh | Moderate enh | Moderate enh |
| 10 | Head | Adenocarc inoma |
2.4 × 3.2 | Irreg/Well-defined | Hypo intense | Hyper intense | + | slightly enh | Moderate enh | Moderate enh |
| 11 | Head | Adenocarcinoma | 2.7 × 1.6 | Irreg/Well-defined | Slight Hypo intense | Hyper intense | + | slightly enh | Moderate enh | Moderate enh |
| 12 | Head | Adenocarcinoma | 0.8 × 1.2 | Irreg/Well-defined | Slight Hypo intense | Hyper intense | + | slightly enh | Moderate enh | Moderate enh |
| 13 | Tail | Adenocarcinoma | 2.8 × 1.6 | Irreg/Well-defined | Slight Hypo intense | Hyper intense | + | slightly enh | Moderate enh | Moderate enh |
| 14 | Body | Adenocarcinoma | 1.8 × 1.0 | Irreg/Well-defined | Slight Hypo intense | Hyper intense | + | delayed enh | delayed enh | delayed enh |
| 15 | Tail | Adenocarcinoma | 2.1 × 1.5 | Irreg/Well-defined | Slight Hypo intense | Hyper intense | + | slightly enh | Moderate enh | Moderate enh |
| 16 | Head | Adenocarcinoma | 0.9 × 1.8 | Irreg/Well-defined | Slight Hypo intense | Iso intense | + | slightly enh | Moderate enh | Moderate enh |
| 17 | Body | Adenocarcinoma | 2.6 × 1.8 | Irreg/Well-defined | Slight Hypo intense | Iso intense | + | slightly enh | Moderate enh | Moderate enh |
| 18 | Tail | Adenocarcinoma | 1.7 × 1.0 | Irreg/Well-defined | Slight Hypo intense | Iso intense | + | slightly enh | Moderate enh | Moderate enh |
| 19 | Head | Adenocarcinoma | 3.2 × 2.1 | Irreg/Well-defined | Slight Hypo intense | Iso intense | + | slightly enh | Moderate enh | Moderate enh |
| 20 | Head | Adenocarcinoma | 1.1 × 1.6 | Irreg/Well-defined | Iso -intense | Iso intense | - | slightly enh | Moderate enh | Moderate enh |
| Irreg: Irregular; +: positive; -: negative; enh: enhancement | ||||||||||
Table 2: Showing location, size, signal intensity and their enhancement pattern for pancreatic adenocarcinoma.
| Case | Location | Diagnosis | Size (cm2) | Shape/Margin | Signal intensity | Arterial Phase | Portal Phase | Delayed Phase | |
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | ||||||||
| 1 | Tail | PNET | 1.2 × 1.0 | well-defined | Low intensity | slightly hyper intense | marked enh | Moderate enh | Moderate enh |
| 2 | Tail | PNET | 2.1 × 1.9 | well-defined | Low intensity | slightly hyper intense | marked enh | Moderate enh | Moderate enh |
| 3 | Head | PNET | 2.0 × 1.5 | well-defined | Low intensity | slightly hyper intense | avid enh | avid enh | avid enh |
| 4 | Tail | PNET | 3.5 × 2.5 | well-defined | Low intensity | slightly hyper intense | marked enh | Moderate enh | Moderate enh |
| 5 | Tail | PNET | 2.5 × 2.1 | well-defined | Low intensity | slightly hyper intense | marked enh | Moderate enh | Moderate enh |
| 6 | Body | PNET | 1.1 × 0.8 | well-defined | Low intensity | slightly hyper intense | avid enh | Moderate enha | Moderate enh |
| 7 | Central | PNET | 1.7 × 2.1 | ill-defined | Low intensity | slightly hyper intense | Marked enh | Moderate enh | Moderate enh |
| PNET: Pancreatic neuroendocrine tumor; enh: enhancement | |||||||||
Table 3: Showing location, size, signal intensity and their enhancement pattern for neuroendocrine tumor.
| Case | Location | Diagnosis | Size (cm2) | Shape/Margin | Signal intensity | Arterial Phase | Portal Phase | Delayed Phase |
|---|---|---|---|---|---|---|---|---|
| 1 | Head | SPTP | 6.8 × 6.2 | Irreg/Well-defined | Heterogeneous | slight enhancement | moderate enhancement | moderate enhancement |
| 2 | Tail | SPTP | 5.2 × 6.0 | Irreg/Well-defined | Heterogeneous | slight enhancement | moderate enhancement | moderate enhancement |
| 3 | Tail | SPTP | 3.3 × 5.1 | Irreg/Well-defined | Heterogeneous | slight enhancement | moderate enhancement | moderate enhancement |
| 4 | Head | SPTP | 4.4 × 7.0 | Irreg/Well-defined | Heterogeneous | slight enhancement | moderate enhancement | moderate enhancement |
| SPTP: Pseudopapillary tumor of the pancreas; Irreg: Irregular | ||||||||
Table 4: Showing location, size, signal intensity and their enhancement pattern for Pseudopapillary tumor of the pancreas.
| Case | Location | Diagnosis | Size (cm2) | Shape/Margin | Signal intensity | Arterial Phase | Portal Phase | Delayed Phase | |
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | ||||||||
| 1 | Head | pancreatic metastases | 2.8 × 1.2 | Irreg/Well-defined | hypointensity | iso-intense | Mild enh | Moderate enh | Moderate enh |
| 2 | Body | pancreatic metastases | 2.2 × 1.0 | Irreg/Well-defined | hypointensity | iso-intense /high | Rim enh | Rim enh | Rim enh |
| 3 | Body | pancreatic metastases | 1.3 × 2.1 | Irreg/Well-defined | hypointensity | iso-intensity | Mild enh | Moderate | Moderate enh |
| 4 | Head | pancreatic metastases | 2.4 × 1.0 | Irreg/Well-defined | hypointensity | iso-intense | Mild enh | Moderate enh | Moderate enh |
| 5 | Body | pancreatic metastases | 1.1 × 1.0 | Irreg/Well-defined | hypointensity | iso-intense | Rim enh | Rim enh | Rim enh |
| enh: Enhancement; Irreg: Irregular | |||||||||
Table 5: Showing location, size, signal intensity and their enhancement pattern for pancreatic metastases.
The specific proportions of distribution of types and symptoms are depicted in Figures 3 and 4 respectively. Pancreatic adenocarcinoma was irregular in shape, lesion was hypo-intense in 10 cases, slightly hypo-intense in 9 cases and iso-intense in one case on T1WI. 10 tumor showed slightly enhancement in arterial phase, and all of tumors showed moderate enhancement during portal and delayed phase; 19 cases showed double duct sign, and one case showed distal pancreatic duct dilation.
Of the 7 cases with PNETs, the margin was well-defined in six welldifferentiated lesions and ill-defined in one pancreatic neuroendocrine carcinoma; six tumors were marked enhancement in arterial phase and one tumor was moderate enhancement than pancreatic parenchyma in all of three phases (Figures 5-8).
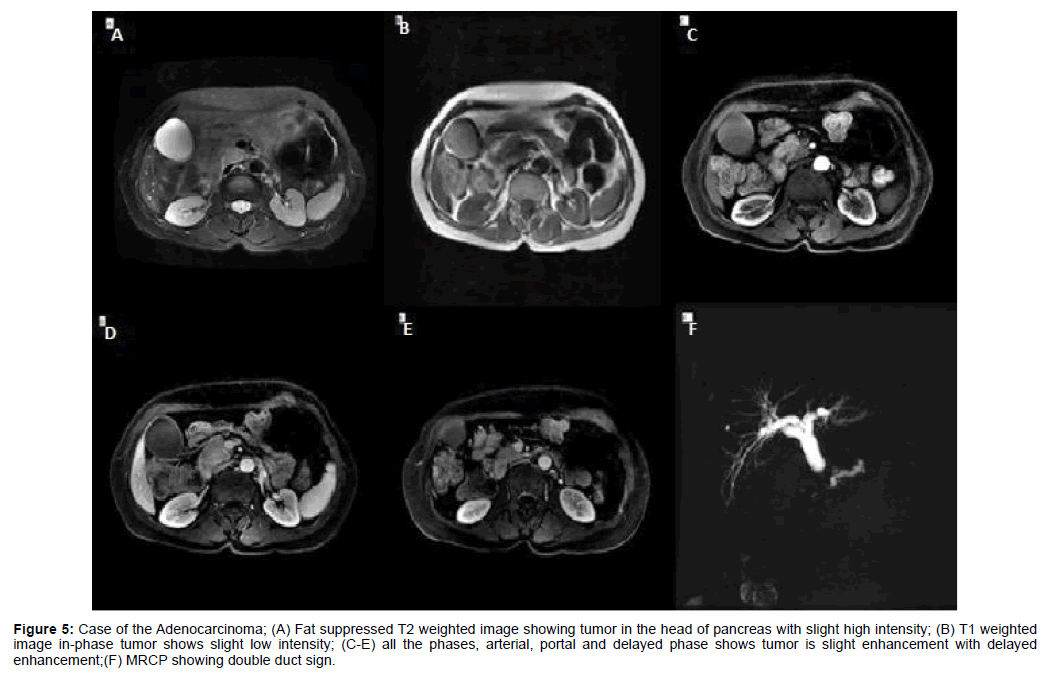
Figure 5: Case of the Adenocarcinoma; (A) Fat suppressed T2 weighted image showing tumor in the head of pancreas with slight high intensity; (B) T1 weighted image in-phase tumor shows slight low intensity; (C-E) all the phases, arterial, portal and delayed phase shows tumor is slight enhancement with delayed enhancement;(F) MRCP showing double duct sign.
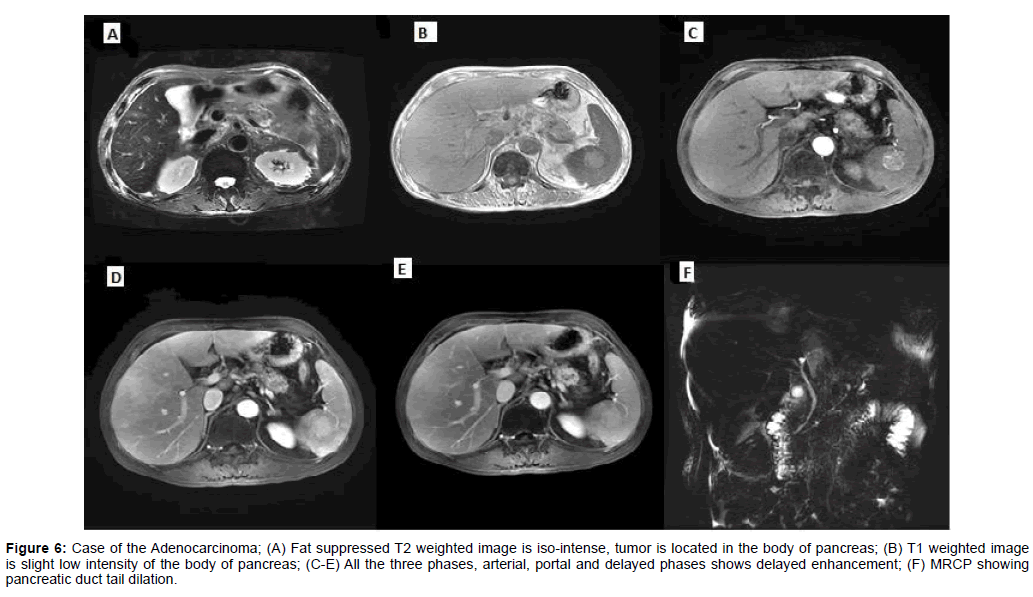
Figure 6: Case of the Adenocarcinoma; (A) Fat suppressed T2 weighted image is iso-intense, tumor is located in the body of pancreas; (B) T1 weighted image is slight low intensity of the body of pancreas; (C-E) All the three phases, arterial, portal and delayed phases shows delayed enhancement; (F) MRCP showing pancreatic duct tail dilation.
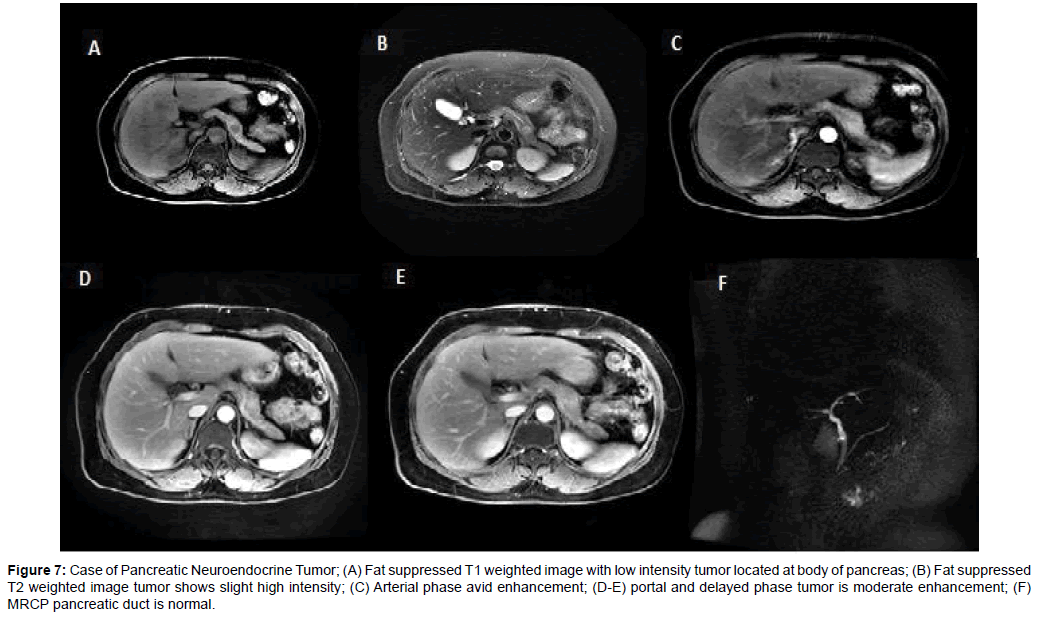
Figure 7: Case of Pancreatic Neuroendocrine Tumor; (A) Fat suppressed T1 weighted image with low intensity tumor located at body of pancreas; (B) Fat suppressed T2 weighted image tumor shows slight high intensity; (C) Arterial phase avid enhancement; (D-E) portal and delayed phase tumor is moderate enhancement; (F) MRCP pancreatic duct is normal.
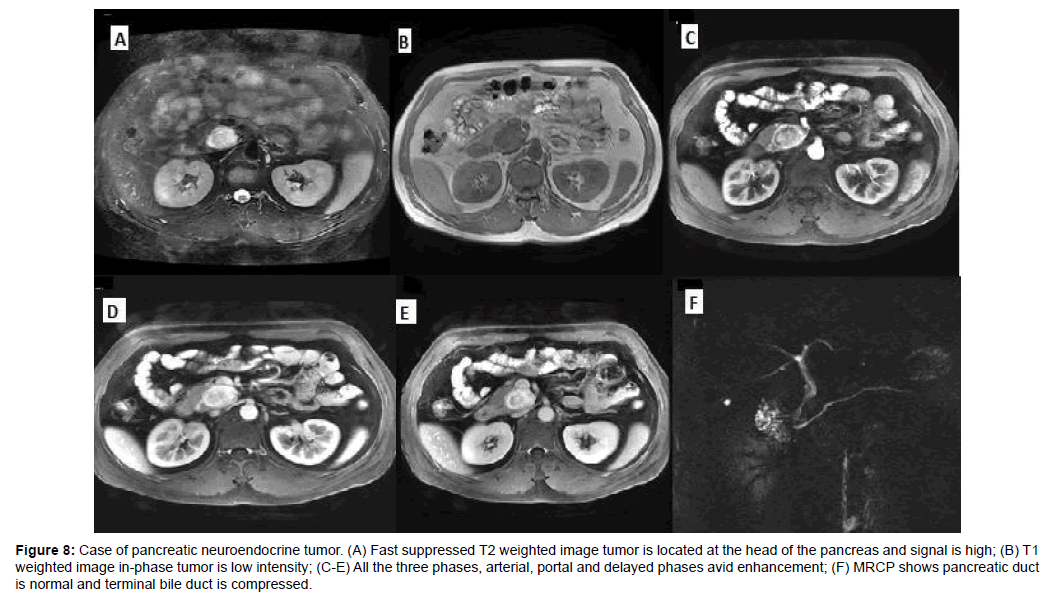
Figure 8: Case of pancreatic neuroendocrine tumor. (A) Fast suppressed T2 weighted image tumor is located at the head of the pancreas and signal is high; (B) T1 weighted image in-phase tumor is low intensity; (C-E) All the three phases, arterial, portal and delayed phases avid enhancement; (F) MRCP shows pancreatic duct is normal and terminal bile duct is compressed.
The mean diameter of SPTP was 5.2 cm. The solid part of tumors was slightly hyper-intense on T1WI and slightly hyper-intense on T2WI, with progressively enhancement. 4 SPTPs had capsule which were hypo-intense on T1WI and T2WI, and exhibited moderate enhancement (Figures 9 and 10).
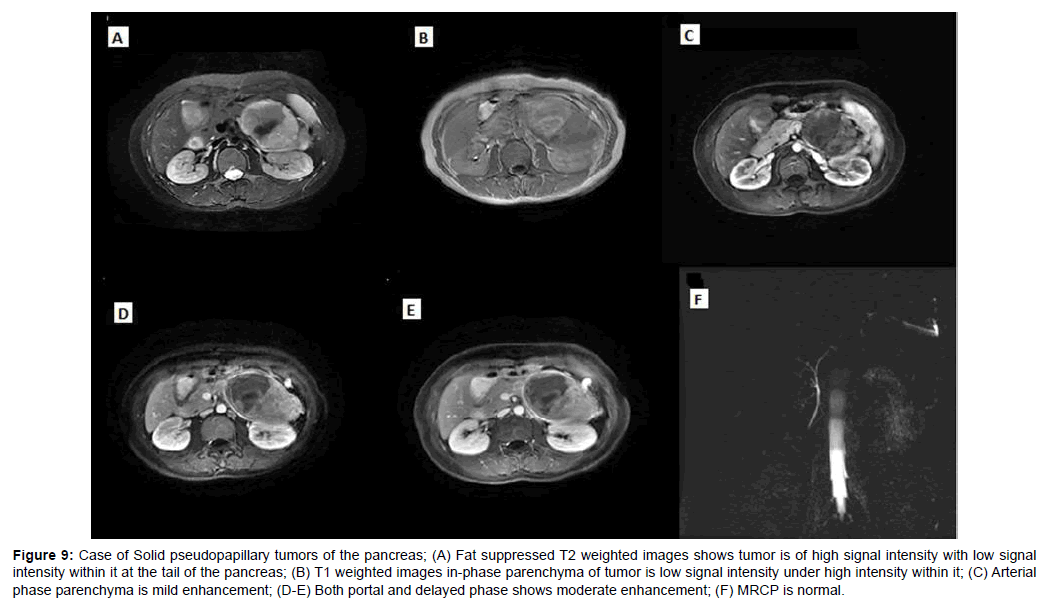
Figure 9: Case of Solid pseudopapillary tumors of the pancreas; (A) Fat suppressed T2 weighted images shows tumor is of high signal intensity with low signal intensity within it at the tail of the pancreas; (B) T1 weighted images in-phase parenchyma of tumor is low signal intensity under high intensity within it; (C) Arterial phase parenchyma is mild enhancement; (D-E) Both portal and delayed phase shows moderate enhancement; (F) MRCP is normal.
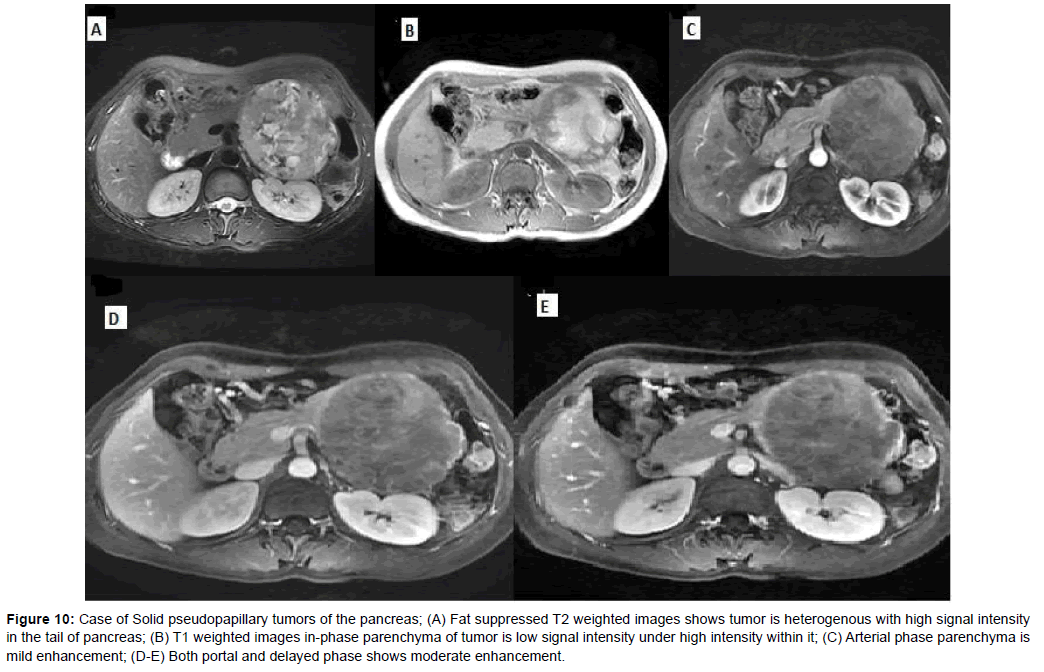
Figure 10: Case of Solid pseudopapillary tumors of the pancreas; (A) Fat suppressed T2 weighted images shows tumor is heterogenous with high signal intensity in the tail of pancreas; (B) T1 weighted images in-phase parenchyma of tumor is low signal intensity under high intensity within it; (C) Arterial phase parenchyma is mild enhancement; (D-E) Both portal and delayed phase shows moderate enhancement.
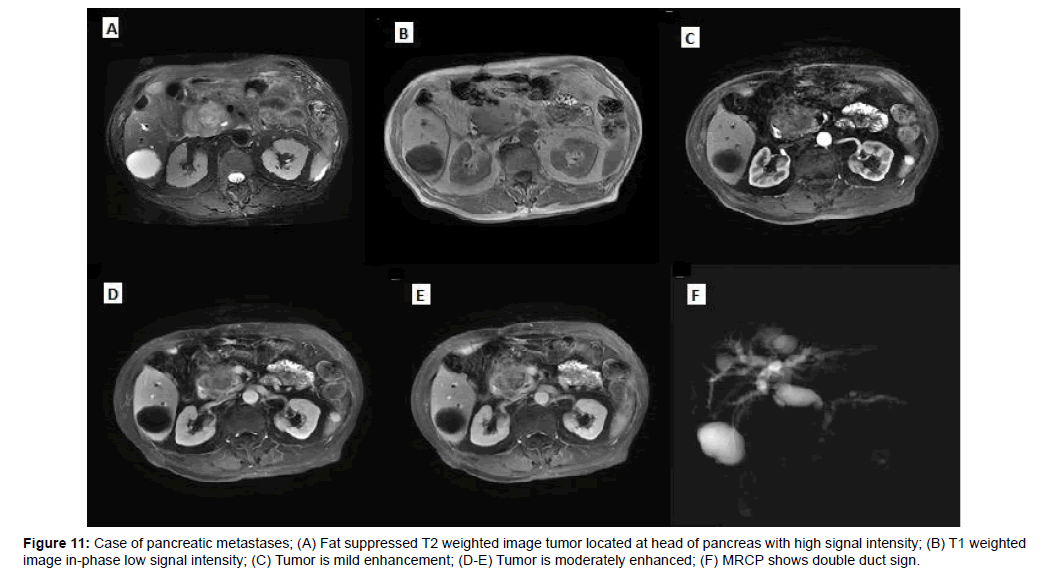
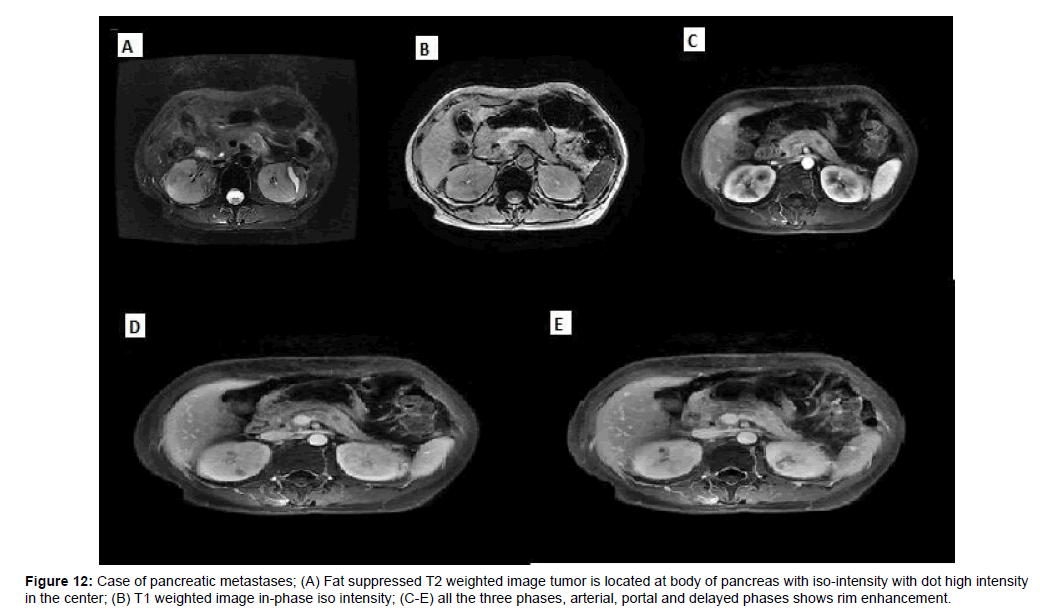
Of the 5 pancreatic metastases, one cases had only one lesion, two cases had multiple lesions and two cases showed diffuse pancreatic enlargement, one case showed heterogenous slightly enhancement, two case showed peripheral rim enhancement, two case showed marked enhancement in arterial, then wash-out in delayed and portal phases (Figures 11 and 12).
Figure 12: Case of pancreatic metastases; (A) Fat suppressed T2 weighted image tumor is located at body of pancreas with iso-intensity with dot high intensity in the center; (B) T1 weighted image in-phase iso intensity; (C-E) all the three phases, arterial, portal and delayed phases shows rim enhancement.
Discussion
Pancreatic carcinoma is a malignant tumor emerging from the epithelial components of the pancreas. It is an extremely regular neoplasm. As of now, somewhere around 3% and 7% of all cancer death can be ascribed to pancreatic carcinoma. The pinnacle number of patients happens somewhere around 42 and 78 years old. There is a male predominance in a proportion fluctuating somewhere around 2:1 and 1.1:1. The expansive larger part of carcinomas of the pancreas are adenocarcinoma and its variations; pancreatic neuroendocrine tumors (PNETs), solid pseudopapillary tumors (SPTs), pancreatic metastases These histological types together constitute more than 96% of all carcinomas of the pancreas, though adenocarcinoma alone speaks to more than 92% of these tumors. This description will therefore deal mainly with adenocarcinoma, PNETs, SPTP, pancreatic metastases.
Magnetic resonance imaging
Adenocarcinoma: On MRI, pancreatic adenocarcinoma will be obvious as a zone of signal intensity on T1-weighted images Therefore, especially in greasy penetrated pancreas the tumor may turn out to be great noticeable on these successions. Taking after intravenous organization of gadolinium many-sided quality medium, the tumor locale of low signal may turn out to be considerably more obvious. On T2-weighted sequences, pancreatic adenocarcinoma may present as area of higher signal intensity depending on the degree of intra tumor necrosis. Köhler et al. [29] reported that the incidence of associated acute pancreatitis is higher when the head of the pancreas is involved with pancreatic carcinoma.
The role of MRI in the conclusion of pancreatic adenocarcinoma is still under discussion. In examination with CT its analytic esteem is very constrained due to a few specialized components, for example, bring down spatial determination and volume averaging because of respiratory developments. In any case, instance rapid image acquisition may change this circumstance later on. Adenocarcinoma of the head of the pancreas spreads by direct invas hilum will much of the time result in segmental infraction of the spleen.
In spite of the fact that barium studies may likewise show tumor invasion of the gastrointestinal structures [30,31], coordinate confirmation of the tumor augmentation is frequently striking on CT. The combined administration of oral water with intravenous iodinated contrast medium and with a spasmolytic agent has turned out to be especially useful. Looking at the patient in various positions (prone/supine versus left or right lateral decubitus) may show whether the gastrointestinal structures move with respect to the tumor [32]. Perivascular intrusion is demonstrated by thickening and an expansion in constriction of the delicate tissue encompassing the proximal segments of the coeliac, hepatic or splenic artery and the superior mesenteric artery and vein. In any case, decimation of this perivascular fat sheath can be seen in pancreatitis, and in addition in patients with lymphoma or metastasis [33]. Perivascular encasement, narrowing or impediment of the peripancreatic vessels by tumor are promptly evident after intravenous contrast enhancement. Augmented lymph nodes because of metastatic contribution will be seen on CT as strange nodular structures of more than 1 cm in breadth, arranged in the periaortic and pericaval regions and in the supra-pancreatic region. Inaccessible metastases happen much of the time in the liver and will be apparent as non-enhancing low attenuation areas. Peritoneal spread will be suspected when ascites is available in spite of the fact that the metastatic inserts themselves are just seldom obvious. Ascites in patients with carcinoma of the pancreas may likewise be because of tumor attack and occlusion of the splenic vein.
However, essential point is the conceivable nearness of peritumoral fibrosis, which is regular in the distal pancreas in case of pancreatic duct obstruction and upstream pancreatitis [34]. Delayed tumor enhancement might be less unmistakably obvious in patients with delayed improvement of the distal pancreas, as happens in chronic pancreatitis. Accordingly, the delayed hyper-intensity of pancreatic adenocarcinoma might be connected to the stringy tumor segment, as well as to the nonattendance of peritumoral fibrosis. A review intended to figure out which tumor part (fibrosis, cellularity, necrosis, or mucin) was in charge of dispersion irregularities in pancreatic adenocarcinoma indicated the collagen fibers. In a study of 20 patients with pancreatic adenocarcinoma and distal pancreas were significantly higher on the arterial sequence. The T1WI arterial sequence provided the best detectability of pancreatic adenocarcinoma but consistently underestimated tumor size. The arterial grouping has been accounted for to give the best differentiation between pancreatic tumors and whatever remains of the pancreatic parenchyma. In this manner, adenocarcinoma as are viewed as a hypo-intense zone that is obvious unmistakably inside the firmly improved pancreas. Our review affirms this point.
Pancreatic neuroendocrine tumors (PNETs): It represents 1-2% of all pancreatic tumors. They ordinarily happen in adults, with a pinnacle rate between 30 to 60 years, however cases have been described in all ages. pancreatic neuroendocrine tumors are separated into functional and nonfunctional tumors [35]. The greater part of tumors is utilitarian and as a rule deliver an overabundance of hormone, and hence their nearness gets to be distinctly apparent as a consequence of the clinical impacts of the hormone. The minority of tumors are nonfunctional and don't create any hormone or peptide in abundance. Patients with nonfunctional pancreatic endocrine neoplasms normally give nonspecific side effects identified with the nearness of the mass [36], although biliary obstruction and jaundice may happen with tumors in the pancreatic head. Nonfunctional tumors will probably be harmful and nonresectable when they are detected [16].
In a study of 7 patients with pancreatic neuroendocrine tumor and distal pancreas were significantly avid on the arterial sequence and moderately enhanced in both portal and delayed phase. While the relationship of pancreatitis with exocrine pancreatic malignancy is all around perceived, its relationship with neuroendocrine tumors of the pancreas is extraordinary with even less cases being accounted for in relationship with nonfunctional tumors. The determination of the tumor is typically delayed when an endocrine pancreatic tumor presents with intense pancreatitis. Besides, patients with endocrine tumors as a rule have different operations for pancreatitis or pseudocyst before the endocrine tumor is perceived. The general frequency of pseudocyst in patients with pancreatic islet cell tumors was 33%. The course of our case was classic stereotype. Our patient gave scenes of acute attack, trailed by pancreatic pseudocyst arrangement that justified waste. Subsequent to expelling the warranted drainage, in any case, the radiologic discoveries for our situation, the unremarkable pancreatic head yet extended, strange pancreatic tail with expanded tube, are exemplary for obstructive constant pancreatitis connected with islet cell tumors.
The results of delayed diagnosis for islet cell tumors might be huge, in light of the fact that many cases end up being malignant islet cell tumors. Moreover, the related entering abscess as a longterm inconvenience of delayed analysis required fractional gastric wall resection for our situation. Radiologic identification I might be troublesome in little not well characterized injuries particularly in a foundation of interminable pancreatitis with fibrosis. In the greater part of reported instances of endocrine tumors introducing as pancreatitis or pancreatic tube stricture, a pancreatic mass was not recognized preoperatively by stomach ultrasound (US) or CT. Then again, endoscopic ultra sonography (EUS) has been appeared to be exceptionally valuable when a pancreatic endocrine tumor is suspected. Endoscopic ultrasound guided-fine needle aspiration (EUSFNA) might be useful for getting a preoperative tissue diagnosis. For pathologists, cautious examination of resection examples is critical to abstain from missing little lesions, particularly in the background of chronic obstructive pancreatitis. Luckily, regardless of the noteworthy deferral in the finding of our case, the tumor was still resectable without separation metastasis. Chronic pancreatitis is a hazard for pancreatic neuroendocrine tumor [12]. Ductal changes, including twisting, dilatation, and pancreatic ductal hypertension in the setting of Chronic pancreatitis, instigate genomic harm and expanded cell turnover [14].
Furthermore, signaling components that assume a part in the improvement of embryonic pancreas are restored. Therefore, assuming a part in repair, recovery, and change. This, thusly, may bring about dysregulation of the cell cycle and in the long run prompts to pancreatic cancer. A late distribution archived that hazard elements for sporadic pancreatic endocrine tumors included family history of any malignancy, chronic pancreatitis, high alcohol intake, and recentonsite diabetes. To keep away from over-finding of grouped islet cells in chronic pancreatitis as islet cell tumors, pathologists must know about neuroendocrine cell (islet cell) [37] pseudo hyperplasia because of atrophy of exocrine organs. On microscopic examination, hyperplastic endocrine cells keep up their lobulated setup with encompassing atrophic exocrine acini and expansion of pancreatic ducts. Likewise, in pseudo-hyperplastic islets, immunohistochemistry will demonstrate mixed populace of neuroendocrine cells (creating mixed hormones), like in a typical Langhans islands, as opposed to clonal expansion saw in endocrine tumors [38].
In summary, constant obstructive pancreatitis made by pancreatic islet tumors is to a great degree uncommon however ought to be considered as a conceivable basic pathology in patients that present with seething, unabating as well as intermittent unending pancreatitis. Whenever intense or intermittent pancreatitis is available without an unmistakable cause, particularly if standard imaging strategies don't uncover an obvious etiology, neuroendocrine tumors should be recognized as a rare but possible cause. Clinicians should know about this uncommon presentation to evade delayed finding and superfluous various operations. Amid earning examination, pathologists ought to deliberately search for conceivable small endocrine tumors bringing about chronic obstructive pancreatitis. What's more, they should know about pseudo hyperplasia of endocrine cells as a copy of endocrine tumor keeping in mind the end goal to maintain a strategic distance from over analysis of the last mentioned.
Solid pseudo papillary tumor: This speaks to 2.7% of every single pancreatic tumor; this is relating to worldwide rates. Clinical elements are generally vague and nonspecific including stomach pain, dyspepsia, early satiety, and vomiting because of massive tumor packing the upper abdomen. Up to 20% of patients found unintentionally either on imaging or at operation for inconsequential pathology. The pathogenesis still stays obscure, despite the fact that its propensity to influence young women has recommended that sex hormones may contribute in the origin of SPT. In the immune-histochemical staining for sex hormone-receptor proteins and clinicopathological qualities, no distinction has been inferable from sexual orientation alone [39]. Sun et al. reported that 62.5% of SPT patients are contaminated with hepatitis B infection (HBV), which can prompt over-articulation of β-catenin in tumor cells, demonstrating that HBV disease might be included in the pathogenesis of SPTP [40].
Since the report of five cases by Kloppel et al. [41], the quantity of revealed cases has extended. According to Lam et al. [42] an aggregate of 452 cases have been accounted for in the English writing. Equivalent words incorporate solid and cystic tumor, solid and papillary epithelial neoplasm, papillary-cystic neoplasm, papillary cystic epithelial neoplasm, papillary-cystic tumor, and Franz tumor.
In a study of 4 patients with Solid pseudo papillary tumor and distal pancreas were significantly mild on the arterial sequence and moderately enhanced in both portal and delayed phase. Radiological discoveries incorporate the nearness of a typified mass with two segments solid and cystic on either CT output or MRI, with MRI prominently better for distinguishing proof of uncommon tumor qualities, for example, presence of a capsule, hemorrhage, necrosis or cystic degeneration [43]. Pathologically, SPTs are distinguished also outlined from nearby pancreatic tissues by fibrous capsule. Microscopic examination yields blended strong and cystic parts with districts of rot and discharge. Trademark discoveries incorporate the nearness of solid regions substituting with pseudopapillary arrangements, frothy histiocytes, atomic furrows and cytoplasmic globules [44]. As the cause of this tumor remains dim, the immunohistochemistry is required for correct finding. SPT is the interesting invulnerable histochemical highlights with articulation of CD56 and CD10 have not achieved an understanding in a current review, likewise SPT cells may uncover central immunoreactivity for cytokeratin (CK) and synaptophysin (Syn), unusual atomic area of catenin, and nearness of progesterone receptors (PR), and may express galectin-3, all of which are valuable in separating SPT from endocrine pancreatic tumor [45]. Furthermore, SPT infrequently creates outside the pancreas and up to 95% are confined to it at the season of conclusion. Additional pancreatic SPT cases have been narrated for in retroperitoneum, liver, omentum and mesocolon. Some of them were calculated to emerge from an ectopic pancreas [46].
Despite the way that they have low malignant potential, they can attack locally and when distant metastasis introduces normally considered as synchronous disease to the liver as well as peritoneum. Any patient with a solid and partly cystic mass of the pancreas particularly in females under 35 years old SPT ought to be added to the differential finding which ought to incorporate microcystic adenoma, mucinous cystic neoplasm, nonfunctioning islet cell tumor, pancreatic adenocarcinoma, pancreaticoblastoma, cystic degeneration of solid neoplasm and calcified hemorrhagic pseudocyst. The prognosis of SPTs is great, even with nearby repeat, and in addition metastases or invasion. More than 95% of patients with SPTs constrained to the pancreas are cured by entire surgical extraction. The general 5-year survival was assessed to be 95% in a survey of 718 patients reported in the English literature. The part of adjuvant treatment in treatment of SPT is hazy, with few reviews exhibited a part for gemcitabine and radiotherapy to scale down vast tumors or treat the uncommon unrespectable cases.
Metastases: In a study of 5 patients with Metastases from liver might be found at the season of essential tumor finding or amid followup after surgery [47,48]. In any case, pancreatic metastases from HCC are unordinary. Tumors are mild to moderate and rim enhancement. In post-mortem examination arrangement of patients with HCC, the reported occurrence extended from 1 to 3% [49]. On the off chance that pancreatic metastases are found at the development, pancreas not unfrequently is the main metastatic site and moderately frequently has a singular metastasis [50]. Truth be told, HCC has been accounted for to be the most widely recognized essential tumor prompting to single pancreatic metastasis. No relationship has yet been found between the site of an isolated pancreatic metastasis and the site of the essential hepato-cellular carcinoma [51]. Be that as it may, multifocality of pancreatic metastases from HCC is not surprising, running from 20% to 45% [52]. It doesn't really identify with a more regrettable result and it doesn't speak to a contraindication to surgery. Regardless of the unior multifocality, when there are no perceptible metastases in different organs there is a good sign for a radical surgery, with a reported mean survival of 4 years. Truth be told surgery is viewed as the gold standard therapy for localized disease [53]. In our arrangement, both on CT and MRI the metastatic foci showed up as round or ovoid masses, for the most part all around depicted and with smooth fringes.
As reported, metastases came about isodense or hypodense in contrast with ordinary parenchyma on unenhanced CT, enthusiastically upgraded in the pancreatic blood vessel stage and showed a huge washout, In the delayed stage masses showed up now and then difficult to perceive, moving toward isodensity with the encompassing pancreatic parenchyma obtaining a blood vessel stage is in this way obligatory at whatever point we are managing a patient with history of HCC, whatever the clinical sign. It is truly difficult to separate HCC metastases from endocrine tumors of the pancreas. Additionally, multifocality inside the pancreas, generally basic within the sight of repeat, is not normal for essential pancreatic carcinoma. Another purpose of separation could be the confirmation of encasement or invasion of the peripancreatic supply artery and veins.
Radiographic features: Carcinoma of the pancreas just creates changes unmistakable on upper gastrointestinal tract barium contemplates when the tumor is as of now huge. Tumors beginning in the body of the pancreas may bring about dislodging of the body of the stomach by pressure or inconsistency of its border because of direct tumor invasion. Tumors beginning in the head of the pancreas will prompt to broadening of the duodenal loop and unpredictable inside borders.
Conclusion
The MRI succession is ideal for recognizing pancreatic adenocarcinoma, pancreatic neuroendocrine tumors (PNETs), solid pseudopapillary tumor (SPTP), pancreatic metastases. However, reliably underestimates tumor size. An imminent review in a substantial number of patients would be valuable in looking over the execution of MRI for surveying the neighborhood spread of solid pancreatic tumors. Solid pancreatic tumor is a pancreatic tumor with non-particular presentation. The precision of conclusion might be enhanced by immune-histochemical staining in that capacity tumors. At whatever point conceivable, surgical resection is the treatment of decision with great long-term survival. Additionally, concentrates required for estimate the rules for treatment and illuminate the part of chemotherapy or radiotherapy.
References
- Busnardo AC, DiDio LJ, Tidrick RT, Thomford NR (1983) "History of the pancreas". Am J Surg 146: 539-550.
- Amano Y, Oishi T, Takahashi M (2001) Non enhanced magnetic resonance imaging of mild acute pancreatitis. Abdom Imaging 26: 59-63.
- Arslan A, Geitung JT, Viktil E, Abdelnoor M, Osnes M (2000) Comparison of 2D single-shot turbo spinecho MR cholangiopancreatography with endoscopic retrograde cholangiopancreatography. Acta Radiologica 41: 621-626.
- Crippa S, Angelini C, Mussi C, Bonardi C, Romano F, et al. (2006) Surgical treatment of metastatic tumors to the pancreas: A single center experience and review of the literature. World J Surg 30: 1536-1542.
- Kim JH, Park SH, Yu ES, Kim MH, Kim J, et al. (2010) Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology 257: 87-96.
- Yoon SH, Lee JM, Cho JY, Lee KB, Kim JE, et al. (2011) Small (£20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology 259: 442-452.
- Ichikawa T, Erturk SM, Motosugi U, Sou H, Iino H, et al. (2007) High-b value diffusion-weighted MRI for detecting pancreatic adenocarcinoma: Preliminary results. Am J Roentgenol 188: 409-414.
- Kartalis N, Lindholm TL, Aspelin P, Permert J, Albiin N (2009) Diffusion-weighted magnetic resonance imaging of pancreas tumours. Eur Radiol 19: 1981-1990.
- Fukukura Y, Takumi K, Kamimura K, Shindo T, Kumagae Y, et al. (2012) Pancreatic adenocarcinoma: Variability of diffusion-weighted MR imaging findings. Radiology 263: 732-740.
- Gabata T, Matsui O, Kadoya M, Yoshikawa J, Miyayama S, et al. (1994)Â Small pancreatic adenocarcinomas: Efficacy of MR imaging with fat suppression and gadolinium enhancement. Radiology 193: 683-688.
- Obuz F, Dicle O, Coker A, Sagol O, Karademir S (2001) Pancreatic adenocarcinoma: Detection and staging with dynamic MR imaging. Eur J Radiol 38: 146-150.
- Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S (2010) The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 39: 707-712.
- Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO classification of tumours of the digestive system, Fourth Edition. Intern Agency Research Canc 3: 1-417.
- Balci NC, Semelka RC (2001) Radiologic features of cystic, endocrine and other pancreatic neoplasms Eur J Radiol, 38: 113-119.
- Kalb B, Sarmiento JM, Kooby DA, Adsay NV, Martin DR (2009) MR imaging of cystic lesions of the pancreas. Radiographics 29: 1749-1765.
- Low G, Panu A, Millo N, Leen E (2011) Multimodality imaging of neoplastic and non-neoplastic solid lesions of the pancreas. Radiographics 31: 993-1015.
- Brenner R, Metens T, Bali M, Demetter P, Matos C (2012) Pancreatic neuroendocrine tumor: added value of fusion of T2-weighted imaging and high b-value diffusion-weighted imaging for tumor detection. Eur J Radiol, 81: e746-e749.
- Franz VK (1959) Tumors of the pancreas. Atlas of tumor pathology: Fascicles 27 and 28. Armed Forces Institute of Pathology, Washington, DC 7: 32-33.
- Kloppel G, Solcia E, Longnecker DS, Capella C, Sobin LH (1996) Histological typing of tumours of the exocrine pancreas, Berlin, Germany. World Health Organization 2: 242-246.
- Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, et al. (2004) Secondary tumors of the pancreas: An analysis of a surgical and autopsy database and review of the literature Virchows Arch 444: 527-535.
- Robbins EG, Franceschi D, Barkin JS (1996) Solitary metastatic tumors to the pancreas: a case report and review of the literature. Am J Gastroenterol 91: 2414-2417.
- Ballarin R, Spaggiari M, Cautero N, Ruvo ND, Montalti R, et al. (2011) Pancreatic metastases from renal cell carcinoma: The state of the art World J Gastroenterol, 17: 4747-4756.
- Mayo SC, Herman JM, Cosgrove D, Bhagat N, Kamel I, et al. (2013) Emerging approaches in the management of patients with neuroendocrine liver metastasis: role of liver-directed and systemic therapies. J Am Coll Surg 216: 123-134.
- Bhosale PR, Menias CO, Balachandran A, Tamm EP, Charnsangavej C, et al. (2013) Vascular pancreatic lesions: Spectrum of imaging findings of malignant masses and mimics with pathologic correlation Abdom Imaging, 38: 802-817.
- Bosetti C, Lucenteforte E, Silverman DT, Petersen G, Bracci PM, et al. (2012) Cigarette smoking and pancreatic cancer: An analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann Oncol Annals of Oncology 23: 1880-1888.
- Grocock C, Harcus M, Neoptolemos J (2009) Screening of high-risk families for pancreatic cancer. Pancreatology 9: 215-222.
- Larsson SC, Wolk A (2012) Red and processed meat consumption and risk of pancreatic cancer: Meta-analysis of prospective studies. Br J Cancer 106: 603-607.
- Köhler H, Lankisch PG (1987) Acute pancreatitis and hyperamylasaemia in pancreatic carcinoma. Pancreas 2: 117-119.
- Wang Y, Miller FH, Chen ZE, Merrick L, Mortele KJ, et al. (2011) Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas. Radiographics 31: E47-E64.
- Re TJ, Lemke A, Klauss M, Laun FB, Simon D (2011) Enhancing pancreatic adenocarcinoma delineation in diffusion derived intravoxel incoherent motion f-maps through automatic vessel and duct segmentation. Magn Reson Med 66: 1327-1332.
- Yoshikawa T, Kawamitsu H, Mitchell DG, Ohno Y, Ku Y, et al. (2006) ADC measurement of abdominal organs and lesions using parallel imaging technique. Am J Roentgenol 187: 1521-1530.
- Ogawa K, Karasawa K, Ito Y, Ogawa Y, Jingu K, et al. (2010) Intraoperative radiotherapy for resected pancreatic cancer: A multi-institutional retrospective analysis of 210 patients. Int J Radiat Oncol Biol Phys 77: 734-742.
- Sultana A, Smith CT, Cunningham D, Starling N, Neoptolemos JP, et al. (2008) Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer: Results of secondary end points analyses. Br J Cancer 99: 6-13.
- Kulke MH, Siu LL, Tepper JE, Fisher G, Jaffe D, et al. (2011) Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol 29: 934-943.
- Nikfarjam M, Warshaw AL, Axelrod L, Deshpande V, Thayer SP, et al. (2008) Improved contemporary surgical management of insulinomas: A 25-year experience at the Massachusetts General Hospital. Ann Surg 247: 165-172.Â
- Scarpa A, Mantovani, W, Capelli P, Beghelli S, Boninsegna L, et al. (2010) Pancreatic endocrine tumors: Improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol 23: 824-833.
- Pape UF, Berndt U, Müller-Nordhorn J, Böhmig M, Roll S, et al. (2008) Prognostic factors of long-term outcome in gastroentero pancreatic neuroendocrine tumors. Endocr Relat Cancer 15: 1083-1097.
- Tien YW, Ser KH, Hu RH, Lee CY, Jeng YM, et al. (2005) Solid pseudopapillary neoplasms of the pancreas: Is there a pathologic basis for the observed gender differences in incidence? Surgery 137: 591-596.
- Sun GQ, Chen CQ, Yao JY, Shi HP, He YL, et al. (2010) Diagnosis and treatment of solid pseudopapillary tumor of pancreas: A report of 8 cases with review of domestic literature. Chinese J General Surg 17: 902-907.
- Kloppel G, Morohoshi T, John HD, Oehmichen W, Opitz K, et al. (1981) Solid and cystic acinar cell tumor of the pancreas: A tumor in young women with favourable prognosis. Virchows Arch A 392:171-183.
- Lam KY, Lo CY, Fan ST (1999) Pancreatic solid-cystic-papillary tumor: Clinicopathologic features in eight patients from Hong Kong and review of the literature. World J Surg 23: 1045-1050.
- Zhang H, Liang T, Wang W, Shen Y, Ren GP, et al. (2006) Diagnosis and treatment of solid pseudopapillary tumor of the pancreas. Pancreas 5: 454-458.
- Martin R, Klimstra D, Brennan M, Conlon K (2002) Solid pseudopapillary tumor of the pancreas: A surgical enigma? Ann Surg Oncol 9: 35-40.
- Geers C, Moulin P, Gigot JF, Weynand B, Deprez P, et al. (2006) Solid and pseudopapillary tumor of the pancreas-Review and new insights into pathogenesis. Am J Surg Pathol 30: 1243-1249.
- Ishikawa O, Ishiguro S, Ohhigashi H, Sasaki Y, Yasuda T, et al. (1990) Solid and papillary neoplasm arising from an ectopic pancreas in the mesocolon. The American journal of gastroenterology 85: 597-601
- Zech CJ, Herrmann KA, Reiser MF, Schoenberg SO (2007) MR imaging in patients with suspected liver metastases: Value of liver-specific contrast agent Gd-EOB-DTPA. Magn Reson Med Sci 6: 43-52.
- Imam K, Bluemke DA (2000) MR imaging in the evaluation of hepatic metastases. Magn Reson Imaging Clin N Am 8: 741-756.
- Michalski CW, Erkan M, Huser N, Muller MW, Hartel M, et al. (2008) Resection of primary pancreatic cancer and liver metastasis: a systematic review. Dig Surg 25: 473-480.
- Tsitouridis I, Diamantopoulou A, Michaelides M, Arvanity M, Papaioannou S (2010) Pancreatic metastases: CT and MRI findings. Diagn Interv Radiol 16: 45-51.
- Bydder S, Spry NA, Christie DR, Roos D, Burmeister BH, et al. (2003) A prospective trial of short-fractionation radiotherapy for the palliation of liver metastases. Australas Radiol 47: 284-288.
- Moss AC, Morris E, Mathuna PM (2006) Palliative biliary stents for obstructing pancreatic carcinoma. Cochrane Database Syst Rev 2: CD004200.
- Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, et al. (2007) Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: A new paradigm for combination therapy in solid cancers. Cancer Res 67: 2187-2196.
Citation: Bayar SN, Mahaseth P, Feng QY (2018) The Value of MRI in Diagnosis of Solid Pancreatic Tumors. J Pain Relief 7: 307. DOI: 10.4172/2167-0846.1000307
Copyright: ©2018 Bayar SN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 14011
- [From(publication date): 0-2018 - Dec 03, 2025]
- Breakdown by view type
- HTML page views: 12661
- PDF downloads: 1350