Transforming Growth Factor (TGF-β) Levels in Tumour Microenvironment: An in-vitro Study
Received: 12-Oct-2018 / Accepted Date: 17-Oct-2018 / Published Date: 24-Oct-2018 DOI: 10.4172/2576-3881.1000123
Keywords: Tumour microenvironment; TGFβ; Immune suppression; Modulation of bone marrow MSC (BM-MSC)
Introduction
Mesenchymal stem cells (MSCs) are multipotent cells known for their immunomodulatory properties [1,2]. They play a pivotal role in tissue remodelling, wound healing and homeostasis [3,4]. Since these cells form an integral part of the stroma and are intimately in contact with the tissue undergoing tumorigenesis, their role in cancer development is taking centre stage. MSCs exert their effects either by direct contact or by paracrine effects with immune cells, induce migration of suppressor cells in the tumour microenvironment and create an area of immune suppression. Bone marrow derived mesenchymal stem cells (BM-MSC) can be mobilized into circulation, migrate towards the tumour and aid in tumour progression [5,6]. Clinical studies indicate migration of BM-MSC towards tumours to be a marker of poor prognosis [7].
Wound healing properties of MSCs are well documented and solid tumours are now regarded as chronic wounds, i.e. wounds which never heal [8]. It is now becoming clear that tumour progression does not involve only the tumour cells, but more likely similar to a "soil and seed" hypothesis, wherein the "seed/tumour cell" strongly interacts with the tumour microenvironment, comprising of stromal cells, BM-MSC, adipocytes and endothelial cells, which secrete growth factors and a wide array of mediators to aid in tumour progression [9].
Transforming Growth Factor (TGFβ) is a pleiotropic cytokine and is over expressed in the tumour microenvironment and suppresses the immune system and also mediates tumour progression [10-12]. One of the main escape mechanisms of neoplasm is the recruitment of Treg in the tumour microenvironment and subsequent increase in TGFβ levels which results in immune suppression [13]. Modulation of this immune suppression could lead to favourable response to cancer immunotherapy.
Autologous activated T cell based immunotherapy (ACT) involves infusion of (non-specifically activated) T cells into patients and demonstrated promise in several cancer conditions [14]. Effects of ACT on TGF β levels in the tumour microenvironment would help understand the outcomes of immune-therapy and treatment endpoints.
The study explores the interplay of HeLa cells and BM-MSC and Transforming Growth Factors Beta (TGF β) levels. Activated T cell supernatant derived from PHA activated T cells were also co-cultured to detect any change in TGF β levels.
Materials and Methods
Cell lines
HeLa cells (Human cervical cancer cells) were obtained from NCCS, Pune. The cells were maintained in DMEM (Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco). Human BM-MSCs were obtained from Lonza. BM-MSCs were maintained in DMEM supplemented with 20% FBS. BM-MSCs between passages 2-3 were used for the experiments. All cultures were incubated at 37˚C in 5% humidified CO2 incubator.
Preparation of T cells conditioned medium
15 ml human blood was collected after venous puncture (standard blood bank procedures) in healthy subject, in EDTA vacutainer. Institutional ethical clearance was obtained under reference number EC/363/17/09 for the study and informed consents were given prior blood collection. Mononuclear cells were isolated by density gradient using Ficoll-Hypaque and were cultured in DMEM supplemented with 10% FBS with mitogen, Phytohemagglutin (PHA, Gibco). After 72 h, the supernatant was collected and centrifuged at 1500rpm for 10 mins. The supernatant (T sup) was collected and preserved at -80˚C until used.
Cell culture in different microenvironment
HeLa cells and BM-MSCs were cultured and co-cultured on a 3D scaffold (patent application number: 201841037897). After 24 h, the complete medium was changed according to respective groups, as shown in Table 1. Further the cells were incubated at 37˚C in 5% humidified CO2 incubator for 5 days and the supernatant was collected for cytokine analysis.
| Group No | Name | Culture condition |
|---|---|---|
| 1 | HeLa | 0.1 × 106 HeLa cells in DMEM supplemented with 20% FBS. |
| 2 | HeLa + T sup | 0.1 × 106 HeLa cells in DMEM supplemented with 20% FBS and 20% T sup. |
| 3 | BM-MSC | 0.1 × 106 BM-MSCs in DMEM supplemented with 20% FBS. |
| 4 | BM-MSC + T sup | 0.1 × 106 BM-MSCs in DMEM supplemented with 20% FBS and 20% T sup. |
| 5 | HeLa + BM-MSC | 0.05 × 106 HeLa cells and 0.05 × 106 BM-MSCs in DMEM supplemented with 20% FBS. |
| 6 | HeLa + BM-MSC + T sup | 0.05 × 106 HeLa cells and 0.05 × 106 BM-MSCs in DMEM supplemented with 20% FBS and 20% T sup. |
Table 1: Groups showing different culture conditions used for the experiment. Group (1) HeLa cells, (2) HeLa cells supplemented with activated T cell Supernatant, (3) Bone Marrow Mesenchymal Stem cells, (4) Bone Marrow Mesenchymal Stem cells supplemented with activated T cell supernatant, (5) HeLa cells co-cultured with Bone Marrow Mesenchymal Stem cells, (6) HeLa cells co-cultured with Bone Marrow Mesenchymal Stem cells supplemented with activated T cell Supernatant.
Cytokines detection
TGF-beta levels in all the groups were determined by ELISA (RayBio ELISA Kit).
Statistical analysis
All values were expressed as mean ± standard error mean (SEM). Differences of the parameters between patients and controls were analyzed by using unpaired Student’s -test. P value less than 0.05 were considered to be statistically significant.
Results
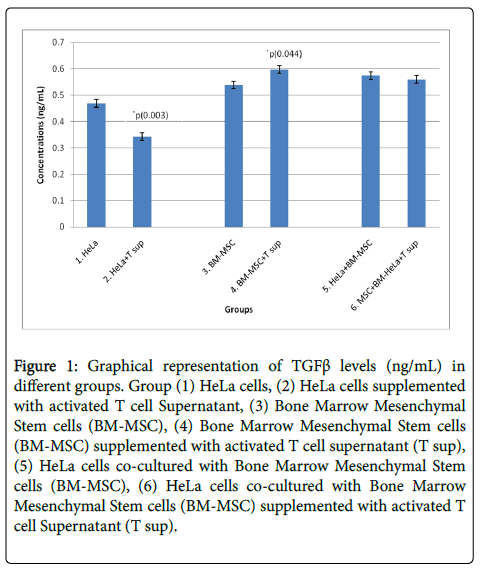
Three independent experiments were performed and TGFβ cytokine levels were measured in cell supernatant cultured in different 3D microenvironment by ELISA. Concentration of TGFβ measured is summarized in Table 2. Unpaired student test was done on group 1 and 2, 3 and 4, 5 and 6. Significant decrease in TGFβ levels were observed in HeLa cells supplemented with T sup when compared to HeLa cells with p<0.05. Significant increase in TGFβ levels were seen in BM-MSCs cells supplemented with T sup when compared to BM-MSCs with p<0.05. No significant changes were seen in co-cultured groups i.e., HeLa + BM-MSC and HeLa + BM-MSC + T sup as shown in Figure 1.
| Group No | Name | TGF Beta (ng/mL) |
|---|---|---|
| 1 | HeLa | 0.4685 ± 0.0142 |
| 2 | HeLa + T sup | 0.3426 ± 0.0142 |
| 3 | BM-MSC | 0.5385 ± 0.0142 |
| 4 | BM-MSC + T sup | 0.5967 ± 0.0142 |
| 5 | HeLa + BM-MSC | 0.5734 ± 0.0142 |
| 6 | HeLa + BM-MSC + T sup | 0.5594 ± 0.0142 |
Table 2: Summary of TGFβ levels (ng/mL) in different groups. Group (1) HeLa cells, (2) HeLa cells supplemented with activated T cell Supernatant, (3) Bone Marrow Mesenchymal Stem cells, (4) Bone Marrow Mesenchymal Stem cells supplemented with activated T cell supernatant, (5) HeLa cells co-cultured with Bone Marrow Mesenchymal Stem cells, (6) HeLa cells co-cultured with Bone Marrow Mesenchymal Stem cells supplemented with activated T cell Supernatant.
Figure 1: Graphical representation of TGFβ levels (ng/mL) in different groups. Group (1) HeLa cells, (2) HeLa cells supplemented with activated T cell Supernatant, (3) Bone Marrow Mesenchymal Stem cells (BM-MSC), (4) Bone Marrow Mesenchymal Stem cells (BM-MSC) supplemented with activated T cell supernatant (T sup), (5) HeLa cells co-cultured with Bone Marrow Mesenchymal Stem cells (BM-MSC), (6) HeLa cells co-cultured with Bone Marrow Mesenchymal Stem cells (BM-MSC) supplemented with activated T cell Supernatant (T sup).
Discussion
The tumour microenvironment appears to be an important component and one of the key factors which determines the final outcome of disease [15,16]. Tumour stroma comprise of MSC, endothelial cells, adipocytes, myo-fibroblasts, Cancer Associated Fibroblasts (CAF), as well as immune cells like T cells, NK cells macrophages and neutrophils [17]. CAF has been pronounced as an important if not singular component to promote epithelialmesenchymal transition (EMT) of tumour cells, aids in tumour progression, induces aggressive phenotype of tumour cells promote metastasis and angiogenesis [18-21]. The EMT transition could be due to the paracrine effects of TGF β signalling [21]. In vitro studies with MSC and tumour conditioned medium induced differentiation of MSC into CAF via TGF β signalling [22,23]. Due to their inherent plastic nature, fusion with tumour cells or their trans-differentiation due to soluble factors associated with the tumour, results in remodelling of tumour microenvironment leading to a pro tumourigenic stroma, as seen in lung cancer [24], human melanoma [25], breast cancer and ovarian adenocarcinoma cells [26]. These studies suggest a major role for MSC towards promoting a pro tumoural stroma. TGF β mediated immune suppression mediates a chronic inflammatory microenvironment encouraging other suppressor cells like MDSC to migrate in the region [27].
These results are contrary to previous studies by Ping-Jin [28], where they showed a polarization of the stroma towards a Th1 type phenotype on co culturing with TNFα and IFNγ, which are produced by activated TILs. Th1 polarization of tumour microenvironment is conducive to adoptive T cell therapy [29], vaccination [30] and immune checkpoint inhibitors [31,32]. A strong Th1 response is critical to induce tumour rejection, following immunotherapy [31,33-37]. Our study demonstrated no reduction of TGFβ levels in HeLa- BM MSC co culture group, implying involvement of additional factors not solely dependent on cytokine circuitry, within the tumour environment. Most importantly, it suggests that the optimal period for adoptive T cell immune-therapy could be preferably after surgical resection, as surgery results in massive reduction tumour mass and significant reduction in TGFβ levels in the tumour microenvironment.
Further studies to explore modalities to mitigate the TGFβ mediated immunosuppressive tumour microenvironment will be conducive to cellular immune-therapies. Also, elucidation of MSC in the tumour microenvironment and analysis of CAF associated molecular markers will help understand the implications of TGFβ induced tumour remodelling, cancer progression and metastasis. Analysis of Th1 versus Th2 cytokine profiles will help elucidate the larger picture at play in terms of our existing TGFβ data.
References
- English K (2013) Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol 91: 19-26.
- Jin P, Civini S, Zhao Y, De Giorgi V, Ren J, et al. (2014) Direct T cell-tumour interaction triggers TH1 phenotype activation through the modification of the mesenchymal stromal cells transcriptional programme. Br J Cancer 110: 2955-2964.
- Devine SM, Hoffman R (2000) Role of mesenchymal stem cells in hematopoietic stem cell transplantation. Curr Opin Hematol 7: 358-363.
- Sabine Neus, Eva Becher, Michael Woltie, Lothar Tietze, Willi Jahnen-Dechent (2013) Functional expression of HGF and HGF receptor/c-met in Adult Human Mesenchymal Stem Cells uggests a role in cell mobilization, tissue repair and wound healing. Gut 2013; 62: 550-560.
- Wu XZ, Chen D, Xie GR (2007) Bone marrow-derived cells: roles in solid tumor. Neoplasma 54: 1-6.
- Roorda BD, Ater Elst, Kamps WA, ES de Bont (2009) Bone marrow-derived cells and tumor growth: contribution of bone marrow-derived cells to tumor micro-environments with special focus on mesenchymal stem cells. Crit Rev Oncol Hematol 69: 187-198.
- De Boeck A, Pauwels P, Hensen K, Rummens JL, Westbroek W, et al. (2013)Â Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut 62: 550-560.
- Riss J, Khanna C, Koo S, Chandramouli GV, Yang HH, et al. (2006) Cancers as wounds that do not heal: differences and similarities between renal regeneration/repair and renal cell carcinoma. Cancer Res 66: 7216-7224.
- Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21: 309-322.
- Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA (2006) Transforming growth factor-b regulation of immune responses. Ann. Rev Immunol 24: 99-146.
- Turner M, Chantry D, Feldmann M (1990) TGFb induces production of IL-6 by human peripheral blood mononuclear cells. Cytokine 2: 211-216.
- Wiseman DM, Polverini PJ, Kamp DW, Leibovich SJ (1988) Transforming growth factor (TGF)-b is chemotactic for human monocytes and induces their expression of angiogenic activity. Biochem Biophys Res Commun 157: 793-800.
- Izabela Winkler, Barbara Wilczynska, Agnieszka Bojarska-Junak, Marek Gogacz, Aneta Adamiak, Krzysztof Postawski, et al (2015) Regulatory T lymphocytes and transforming growth factor beta in epithelial ovarian tumors-prognostic significance. J Ovarian Res 8: 39.
- Karlo Perica BS, Juan Carlos Varela, Mathias Oelke, Jonathan Schneck (2015) Adoptive T Cell Immunotherapy for Cancer. Rambam Maimonides Med J 6: e0004.
- Valerie Chew, Han Chong Toh, Jean-Pierre Abastado (2012) Immune Microenvironment in Tumor Progression: Characteristics and Challenges for Therapy. J Oncol 608406.
- Â Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19: 1423-1437.
- Pattabiraman DR, Weinberg RA (2014) Tackling the cancer stem cells- what challenges do they pose? Nat Rev Drug Discov 13: 497-512.
- Orimo A, Weinberg RA (2006) Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 5: 1597-1601.
- Erez N, Truitt M, Olson P, Arron ST, Hanahan D (2010) Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell 17: 135-147.
- Cirri P, Chiarugi P (2011) Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res 1: 482-497.
- Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, et al. (2014) Cancer associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br J Cancer 110: 724-732.
- Jotzu C, Alt E, Welte G, Li J, Hennessy BT, et al. (2011) Adipose tissue derived stem cells differentiate into carcinoma associated fibroblast-like cells under the influence of tumor derived factors. Anal Cell Pathol (Amst) 33: 61-79.
- Sugihara H, Ishimoto T, Yasuda T, Izumi D, Eto K, et al. (2015) Cancer-associated fibroblast-derived CXCL12 causes tumor progression in adenocarcinoma of the esophagogastric junction. Med Oncol 32:618.
- Wei HJ, Nickoloff JA, Chen WH, Liu HY, Lo WC, et al. (2014) FOXF1 mediates mesenchymal stem cell fusion-induced reprogramming of lung cancer cells. Oncotarget 5: 9514-9529.
- Kucerova L, Skolekova S, Demkova L, Bohovic R, Matuskova M (2014) Long-term efficiency of mesenchymal stromal cell-mediated CDMSC/ 5FC therapy in human melanoma xenograft model. Gene Ther 21: 874-887.
- Yang Y, Otte A, Hass R (2015) Human mesenchymal stroma/stem cells exchange membrane proteins and alter functionality during interaction with different tumor cell lines. Stem Cells Dev 24: 1205-1222.
- Gong D, Shi W, Yi S J, Chen H, Groffen J, et al. (2012) TGFb signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol 13: 31.
- Ping Jin, Yuanlong Zhao, Hui Liu, Jinguo Chen, Jiaqiang Ren, et al. (2016) Interferon-γ and Tumor Necrosis Factor-α Polarize Bone Marrow Stromal Cells Uniformly to a Th1 Phenotype. Scientific Reports 6: 26345.
- Bedognetti D, Spivey TL, Zhao Y, Uccellini L, Tomei S, et al. (2013) CXCR3/CCR5 pathways in metastatic melanoma patients treated with adoptive therapy and interleukin-2. Br J Cancer 109: 2412-2423.
- Ulloa-Montoya F, Louahed J, Dizier B, Gruselle O, Spiessens B, et al. (2013) Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol 31: 2388-2395.
- Ji R R, Chasalow SD, Wang L, Hamid O, Schmidt H, et al. (2012) An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 61: 1019-1031.
- Herbst R S, Soria JC, Kowanetz M, Fine GD, Hamid O, et al. (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515: 563-567.
- Wang E, Bedognetti D, Marincola FM (2013) Prediction of response to anticancer immunotherapy using gene signatures. J Clin Oncol 31: 2369-2371.
- Weiss G R, Grosh WW, Chianese-Bullock KA, Zhao Y, Liu H, et al. (2011) Molecular insights on the peripheral and intratumoral effects of systemic high-dose rIL-2 (aldesleukin) administration for the treatment of metastatic melanoma. Clin Cancer Res 17: 7440-7450.
- Carretero R, Wang E, Rodriguez AI, Reinboth J, Ascierto ML, et al. (2012) Regression of melanoma metastases after immunotherapy is associated with activation of antigen presentation and interferon-mediated rejection genes. Int J Cancer 131: 387-395
- Wang E, Miller LD, Ohnmacht GA, Mocellin S, Perez-Diez A, et al. (2002) Prospective molecular profiling of melanoma metastases suggests classifiers of immune responsiveness. Cancer Res 62: 3581-3586.
- Bedognetti D, Wang E, Sertoli MR, Marincola FM (2010) Gene-expression profiling in vaccine therapy and immunotherapy for cancer. Exp Rev Vaccines 9: 555-565.
Citation: Nagpal A, Umrao A, Purushothama H, Rao GA, Rao JA (2018) Transforming Growth Factor (TGF-β) Levels in Tumour Microenvironment: An in-vitro Study. J Cytokine Biol 3: 123. DOI: 10.4172/2576-3881.1000123
Copyright: © 2018 Nagpal A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7088
- [From(publication date): 0-2018 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 6124
- PDF downloads: 964