Tuberculosis of Central Nervous System: Report of Tunisian Cases and Literature Review
Received: 18-Jul-2020 / Accepted Date: 24-Aug-2020 / Published Date: 31-Aug-2020 DOI: 10.4172/2314-7326.1000302
Abstract
Tuberculosis (TB) of the central nervous system (CNS) is a highly devastating form of TB especially in endemic country such as Tunisia. We aimed to present four different cases of CNS TB to demonstrate the large spectrum of Neurotuberculosis (NTB) clinical manifestations in Tunisian population. Main features described were tuberculous meningitis, tuberculoma, and spinal tuberculous. There were currently no real diagnostic tools to distinguish NTB from differential diagnosis. NTB diagnosis was based on strong clinical and radiological evidence followed by an adequate response to anti tubercular treatment. Standardized diagnosis criteria need to be established for the different clinical categories in order to be uniformly applicable in NTB diagnosis and management.
Keywords: Tuberculosis; Central Nervous System; Meningitis
Introduction
Tuberculosis (TB) of the central nervous system (CNS) is a highly devastating form of TB, which, even in the setting of appropriate anti tubercular therapy, leads to unacceptable levels of morbidity and mortality [1]. Worldwide, CNS TB is noted in 5 to 10% of extra pulmonary TB cases, and accounts for approximately 1% of all TB cases [2]. In Tunisia, a middle-income country of 11 million populations, TB is of a public health concern. As Tunisia is at intermediate endemicity for TB with a reported incidence ranging from 23/ 100,000 population in 2005 to 31/100,000 population in 2012. TB burden has been steadily declining since the implementation of a national TB program in 1959. However, in the last two decades, Tunisia, experienced a substantial increase in extra pulmonary TB cases, reaching 68% of all new TB cases registered in 2017 [3]. Despite attention paid to the disease, diagnosis of neurological manifestations is still challenging. Given the clinical polymorphism and the lack of specificity of the biological and radiological signs, NTB as inaugural presentation is less diagnosed [4]. However, NTB has to be considered as differential diagnosis in a large number of cases with CNS involvement in endemic countries, like Tunisia. We aimed to present four different cases of CNS TB to demonstrate various manifestations of NTB in Tunisian population.
Case 1
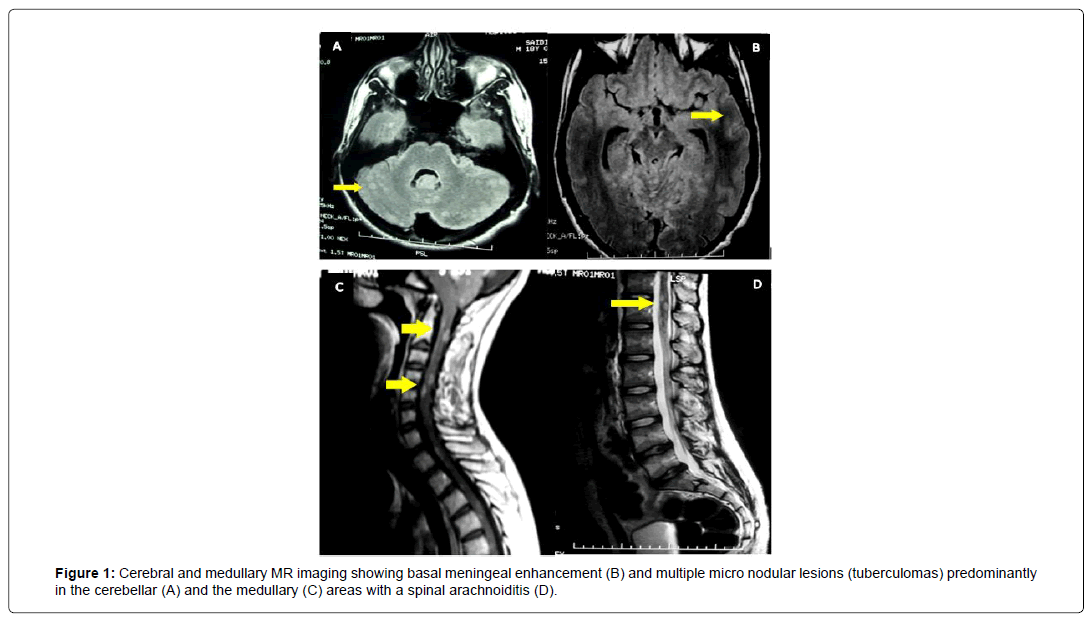
A 19 years old Tunisian boy from consanguineous marriage without medical family history presented a 9 month history of progressive gait ataxia. Four weeks before admission he had developed isolated occipital headache withoutintracranial hypertension syndrome, spinal pain and sphincter disorders. Neurological examination revealed dysmetria and ataxia are flexia of lower limb and posterior cordonal syndrome. General examination was normal. Lumbar puncture examination showed clear cerebrospinal fluid (CSF), 80 lymphocytes/mm3, 9 polymorphs/mm3, and protein of 1.72 g/L and glucose of 0.22 mmol/L. MR imaging showed basal meningeal enhancement and multiple micro nodular lesions predominantly in the cerebellar and the medullary areas with spinal arachnoiditis (Figure 1). Antituberculous treatment was expanded towardthe clinical, biological and radiological evidences. Clinical review revealed an improvement of cerebellar function and the patient becomes clinically well.
Case 2
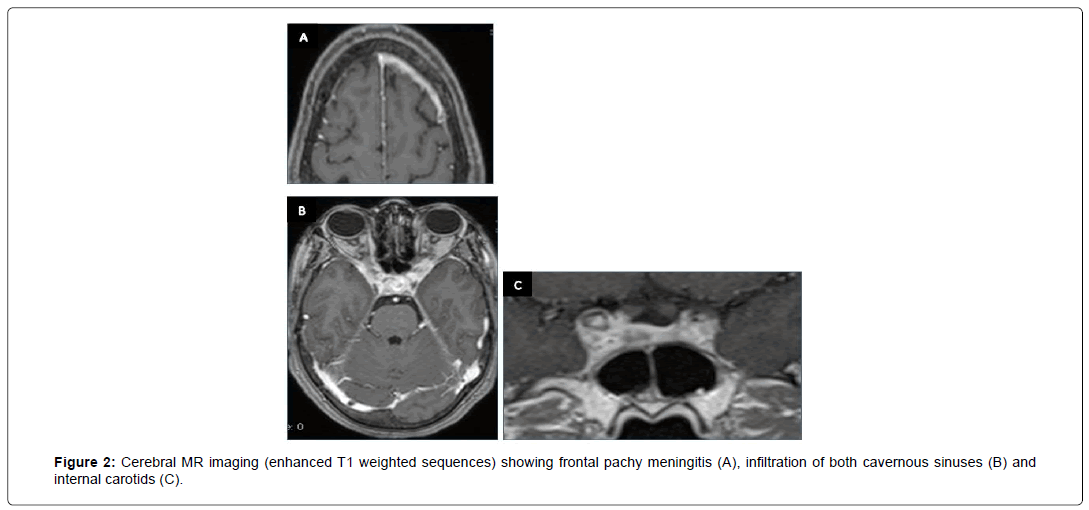
A 50-year-old patient, with medical history of diabetes, presented with intracranial Hypertension syndrome. Investigations revealed recent history of night sweats and a probable tuberculous contagion. The neurological examination found a quadric pyramidal syndrome with multiple cranial nerve palsies: bilateral involvement of second nerve, left fifth nerve (ophthalmic branchV1), and partial involvement of right third nerve. Fundus examination was normal. Bases on these clinical data, painful the cavernous sinus syndrome was suspected. CSF examination was normal and the tuberculin skin test reaction was phlyctenular. Biological blood tests have demonstrated lymphocytic hyper leukocytosis with high sedimentation rate (SR) at 120. Rapid sputum test for TB and polymerase chain reaction (PCR) (blood and CSF) were negative. Brain MRI showed the frontal pachy meningitis (Figure 2A) and diffused infiltration of both cavernous sinuses and internal carotids (Figures 2B and 2C). Based on clinical manifestations, contagion, biological and radiological aspects in an endemic country, the diagnosis of NTB was the most probable. The patient received anti tuberculous drugs and clinical improvement has been noted.
Case 3
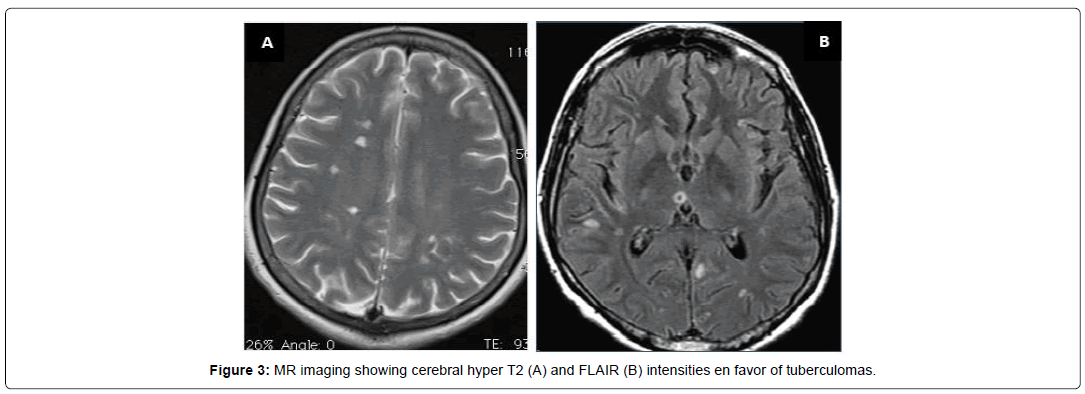
A 40-year-old patient who suffered from pulmonary TB 2 years ago treated and recovered, presented with a rapidly progressive symptomatology of headache, diplopia, left visual blur associated with weakness of the left upper limb. She also reported concomitant night sweats and fatigue. Neurological examination revealed a predominantly left quadric pyramidal syndrome with mono paresis of the left upper limb, involvement of the left third cranial nerve as well as a horizontalrotational nystagmus. CSF study showed normoglycorachia with hyper protein orachia (0.53) and 50white blood cells predominantly lymphocytes. Biological blood tests have shown lymphopenia at 730 and SR at 46. Rapid sputum test for TB and PCR tests (blood and CSF) were negative. Brain MRI showed cerebral tuberculomas (Figure 3). Clinical improvement has been noted under anti tuberculous drugs.
Case 4
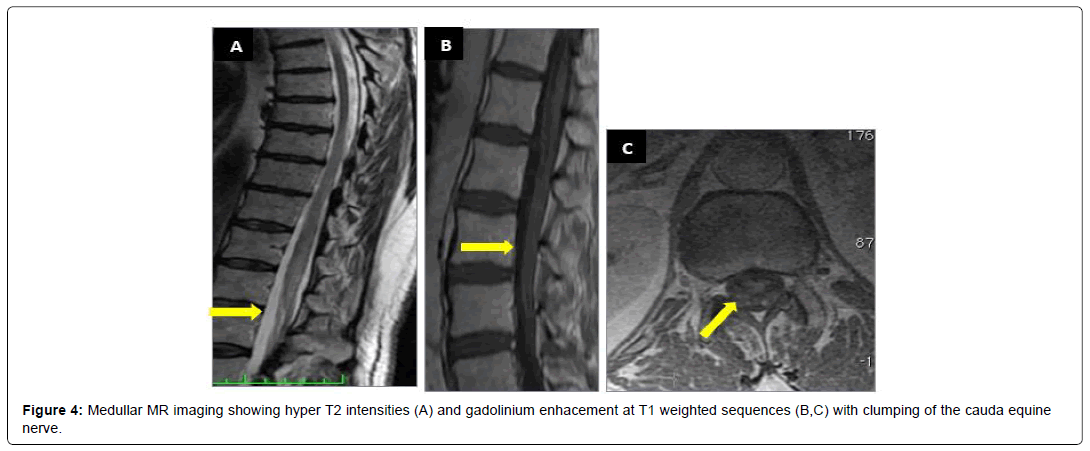
A 64-year-old patient, farmer, with medical history of diabetis and coronary artery disease, was suffering during 14 months from low back pain with left paraesthesia at the L5 and the S1 levelassociated with progressive weakness of the lower limbs. She has also reported bladder disturbances, asthenia, weight loss and fever. The neurological examination found cauda equina syndrome. Cerebral medullar MRI revealed hyperintensities in cauda equina (Figure 4). The CSF was clear with hyperproteinorachy at 2.21 g/l a normoglycorachie and 147 white blood cells predominantly lymphocytes (99% of lymphocytes), in favor of a lymphocytic meningoradiculitis. The chest X-ray was without abnormalities. Infectious serologies in the blood and CSF were negative. Tuberculin skin tests, Rapid sputum test for TB and PCR tests (blood and CSF) were negative. Given the causaequina syndrome, patient was candidate to surgical decompression. Anti tuberculous drugs were associated based on an infectious lymphocytic menigoradiculitis with negative serologic investigations in TB endemic country. Clinical improvement has been noted.
Discussion
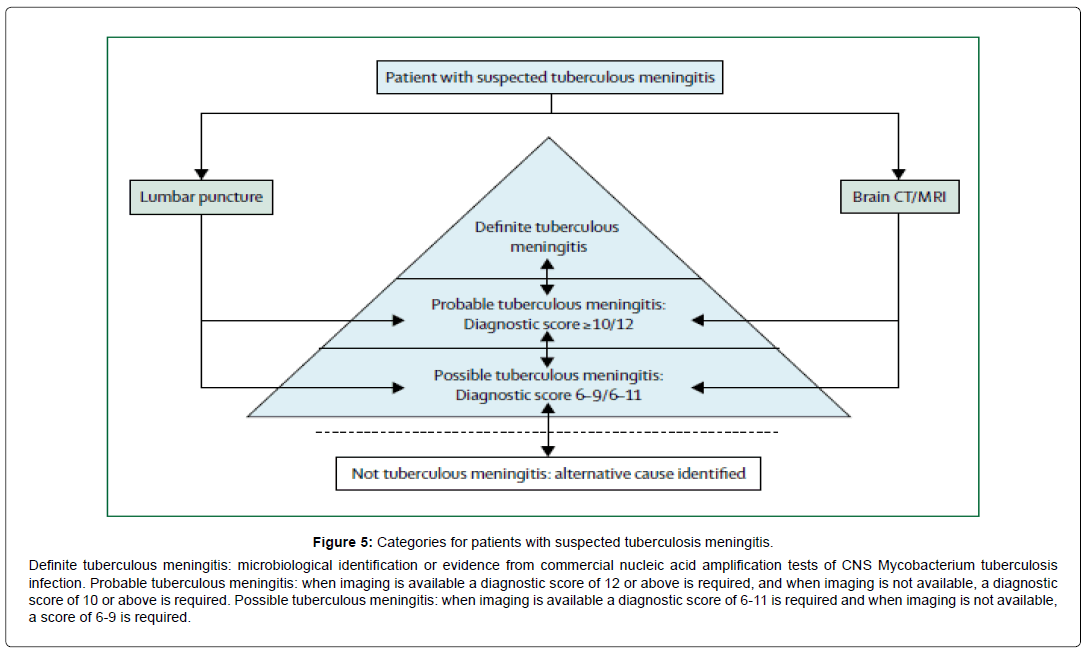
These cases aimed to highlight and to discuss the different clinical and radiological features of CNS TB. The development of CNS TB is now believed to be the consequence of haematogenous dissemination of Mycobacterium TB to the central nervous system, occurring either during the initial stages of the infection or later as a result of caseation at the primary site or other sites [5]. Only the case 3 had medical history of pulmonary TB. In fact, a prior history of TB is obtained in approximately 10% of adult cases of CNS TB. Symptoms and signs are dependent on the site of the lesions and their rate of expansion [2,4]. The most frequent clinical manifestation reported in our study was tuberculous meningitis due to inoculation of the CSF with Mycobacterium TB [6]. Typically, but inconstantly tuberculous meningitis is preceded by a period of 2 to 8 weeks of nonspecific symptoms that include malaise, anorexia, fatigue, fever, myalgias, and headache. Initially intermittent, headaches eventually worsen and become continuous. Headaches observed in cases 1 and 3 were reported by 86% of authors. Fever is also absent in up to 20% of patients with tuberculous meningitis [7-9]. Cranial nerve palsies observed in cases 2 and 3 are seen on admission in 30% to 50% of adults with NTB. The sixth cranial nerve is most commonly affected followed in descending order of frequency by the third, fourth, seventh, second, eighth, tenth, eleventh, and twelfth [10]. Causes of Cranial nerve palsies other than tuberculous meningitis including arachnoiditis and tuberculomas compressing of cranial nerves, may explain symptoms [3,11-14]. Among focal neurologic signs observed in our patients are hemiparesis hemiplegia and cerebellar ataxia. These abnormalities are dependent on mass lesions from tuberculomas (shown at MRI as typical features of contrast enhancing ring lesions with surrounding oedema) but maybe the consequence of infarction due to tuberculous vasculitis [12- 14]. With identical pathophysiology to tuberculous meningitis, spinal meningitis occurs in between less than 1%; TB can affect any part of the spinal cord, including the nerve roots and therefore can present with upper or lower motor neuron involvement, or a mixed clinical picture [2]. Approximately 12% of cases may manifest as radiculomyelopathy, transverse myelitis or anterior spinal artery syndromes. Tuberculous radiculomyelitis observed in case 4, is characterized by sub-acute paraparesis, radicular pain and bladder disturbance that typically develops after the onset of tuberculous meningitis [15], involving more than one spinal region in >80%, with the thoracic and cervical region being most commonly affected. MRI contrast enhance mentmay be seen in the meninges (80%), cord (20%), and nerve roots (30%) [16]. As seen in ours cases (1 and 4), MR imaging studies of the spine often show features of adhesive arachnoiditis, intramedullary spinal tuberculoma and clumping of the cauda equine nerve roots [17]. Given the clinical polymorphism of NTB, establishing the diagnosis is still challenging. Routine analysis of CSF is also pivotal in diagnosing in tuberculous meningitis usually showing clear appearance, pleocytosis (with lymphocyte predominance), raised protein concentration and a low glucose concentration [8,9,11,13]. The hallmark of diagnosis is the demonstration of acid-fast bacilli (AFB) in the CSF what is less commonly found in the CSF of patients without meningitis. With lack of a “gold standard” for diagnosing mycobacterial infection, the best diagnostic technique is polymerase chain reaction (PCR) in CSF with a higher specificity of 98%. Nevertheless, its sensitivity may not exceed 50% in our study none patient was positive as global rate of positivity is known to be less than 25% [18]. The contribution of cerebral and medullar imaging to the diagnosis of CNS involvement is well established. MRI was the preferred investigation because of its high sensitivity for the detection of abnormalities such as meningeal enhancement and tuberculomas, especially for lesions involving the brainstem, although it cannot accurately distinguish NTB from differential diagnosis and tissue examination is usually required to confirm the diagnosis [12,16]. Thus, in some cases of CNS involvement, there are currently no real diagnostic tools to distinguish NTB from a variety of infectious, inflammatory, neoplastic and vascular conditions that need to be explored. The main differential diagnosis that should be considered is Neurosarcoidosis emphasizing the value of brain biopsy in establishing the diagnosis in some patients [19]. However, it is not always obvious as seen in our case 2 where infiltration of the cavernous sinus is not accessible to biopsy. In default, as noted in our four cases, NTB diagnosis could be based on strong clinical and radiological evidence followed by an adequate response to anti tubercular treatment [9,11]. Chemotherapy for CNS TB follows the model of short course chemotherapy for pulmonary TB: an intensive phase of treatment, followed by a continuation phase. But unlike pulmonary TB, the optimal drug regimen and duration of each phase are not clearly established. However, most authorities recommend 12 months treatment. Adjunctive cortico-steroids are also recommended (dexamethasone 0.4 mg/kg/24 h with a reducing course over 6-8 weeks) [2,9]. In view of the absence of standardised diagnostic criteria for NTB, an international workshop (in Cape Town (South Africa) May 2009) have proposed a consensus case definition for tuberculous meningitis form and a diagnosis score (with categories are definite, probable, possible, and not tuberculous meningitis) depending on the strength of clinical, laboratory or radiological findings (Figure 5) [18,19]. Because of the limitations of available diagnostic techniques and the urgent need of treatment of tuberculous meningitis this consensus even if not yet validated could be an alternative for early diagnosis and treatment.
Figure 5: Categories for patients with suspected tuberculosis meningitis.
Definite tuberculous meningitis: microbiological identification or evidence from commercial nucleic acid amplification tests of CNS Mycobacterium tuberculosis infection. Probable tuberculous meningitis: when imaging is available a diagnostic score of 12 or above is required, and when imaging is not available, a diagnostic score of 10 or above is required. Possible tuberculous meningitis: when imaging is available a diagnostic score of 6-11 is required and when imaging is not available, a score of 6-9 is required.
Conclusion
In Tunisia we are still suffering from serious infectious disease that should be kept in mind because of the challenging early treatment. The large spectrum of the clinical manifestations of NTB has received considerable research attention. It includes three clinical categories: tuberculous meningitis, tuberculoma, and spinal tuberculous features. As Tunisia considered an endemic area, the diagnosis of NTB must not be forgotten and should be done following the national TB guidelines based on bacteriological and/or histological proof. In default, as noted in our four cases, NTB diagnosis could consists on strong clinical and radiological evidence followed by an adequate response to anti tubercular treatment. Standardized diagnostic criteria need to be established for the different clinical categories in order to be uniformly applicable progress in NTB diagnosis and management.
References
- Hosoglu S, Geyik MF, Balik I, Aygen B, Erol S, et al. (2002) Predictors of outcome in patients withtuberculousmeningitis. Int J Tuberc Lung Dis 6: 64-70.
- Thwaites G, Fisher M, Hemingway C, Scott G, Solomon T, et al. (2009) British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect 59: 167-87.
- Ben Ayed H, Koubaa M, Gargouri L, Ben Jemaa M, Trigui M, et al. (2019) Epidemiology and disease burden of tuberculosis in south of Tunisia over a 22-year period: Current trends and future projections. PLoS ONE 14: e0212853.
- Zunt JR (2018) Tuberculosis of the Central Nervous System. J Continuum (MinneapMinn) 24: 1422-38.
- Aher A, Paithankar M, Bhurke B (2018) Study of Central Nervous System Tuberculosis. J Assoc Physicians India 66: 41-4.
- Williamson EML, Berger JR (2015) Central Nervous System Infections with Immunomodulatory Therapies. CONTINUUM: Lifelong Learning in Neur 21: 1577.
- Davis AG, Rohlwink UK, Proust A, Figaji AA, Wilkinson RJ (2019) The pathogenesis of tuberculousmeningitis. J LeukocBiol 105: 267-80.
- Kent SJ, Crowe SM, Yung A, Lucas CR, Mijch AM (1993) Tuberculousmeningitis: a 30-year review. Clin Infect Dis 17: 987-94.
- Cecchini D, Ambrosioni J, Brezzo C, Corti M, Rybko A, et al. (2009) Tuberculousmeningitis in HIV-infected and non-infected patients: comparison of cerebrospinalfluidfindings. Int J Tuberc Lung Dis 13: 269-71.
- Schaller MA, Wicke F, Foerch C, Weidauer S (2019) Central Nervous System Tuberculosis : Etiology, Clinical Manifestations and Neuroradiological Features. Clin Neuroradiol 29: 3-18.
- Traub M, Colchester AC, Kingsley DP, Swash M (1984) Tuberculosis of the central nervous system. Q J Med 53: 81-100.
- Leonard JM (2017) Central Nervous SystemTuberculosis. MicrobiolSpectr 5: 2.
- Chaudhary V, Bano S, Garga UC (2017) Central Nervous System Tuberculosis: An Imaging Perspective. Can Assoc Radiol J 68: 161-70.
- Li H, Liu W, You C (2012) Central nervous system tuberculoma. J Clin Neurosci 19: 691-5.
- DeLance AR, Safaee M, Oh MC, Clark AJ, Kaur G, et al. (2013) Tuberculoma of the central nervous system. J Clin Neurosci 20 : 1333-41.
- Hernández-Albújar S, Arribas JR, RoyoA, González-GarcÃa JJ, Peña JM, et al. (2000) Tuberculous radiculomyelitis complicating tuberculous meningitis: case report and review. Clin Infect Dis 30: 915-21.
- Wasay M, Arif H, Khealani B, Ahsan H (2006) Neuroimaging of tuberculousmyelitis: analysis of ten cases and review of literature. J Neuroimaging 16: 197-205.
- Moghtaderi A, AlaviNaini R (2003) Tuberculousradiculomyelitis: review and presentation of five patients. Int J Tuberc Lung Dis 7: 1186-90.
- Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, et al. (2010)Tuberculousmeningitis: a uniform case definition for use in clinicalresearch. Lancet Infect Dis 10: 803-12.
Citation: Mahmoud MB, Ali NB, Fray S, jamoussi H, Chebbi S, et al. (2020) Tuberculosis of Central Nervous System: Report of Tunisian Cases and Literature Review. J Neuroinfect Dis 11: 302. DOI: 10.4172/2314-7326.1000302
Copyright: © 2020 Mahmoud MB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2893
- [From(publication date): 0-2020 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 2075
- PDF downloads: 818