Ultrastructural Changes of Endosymbiotic Symbiodinium of Galaxea astreata under Thermal Stress and after Short Time Recovery Process
Received: 19-Jul-2018 / Accepted Date: 17-Oct-2018 / Published Date: 25-Oct-2018 DOI: 10.4172/2155-9910.1000262
Abstract
This study aimed to elucidate the mechanism underlying coral bleaching. Galaxea astreata corals were maintained in aquaria either under non-thermal stress (28°C) or under thermal stress (32°C) for 5 days followed by non-thermal stress for 7 days. Changes in the morphology and ultrastructure of the zooxanthellae of these corals were observed using transmission electron microscopy. Results showed that zooxanthella cells under 28°C and 30°C for 24 h displayed normal morphological features. Zooxanthella cells were damaged and their membranes began to fold after exposure to thermal stress for 72 h. These cells became severely damaged and underwent apoptosis upon prolonged exposure (168 h) to thermal stress. When thermal stress was further prolonged to 218 h, necrosis characterized by ruptured cell wall was observed. Recovery process was initiated by gradually reducing the temperature from 32°C to 28°C. The external morphology of Symbiodinium cells recovered at 28°C for 216 h. However, the cytoplasmic organelles needed more time to repair. These results implied that symbiont cells can survive and potentially be reabsorbed for symbiosis after coral bleaching.
Keywords: Symbiodinium; Ultrastructural; Thermal stress; Recovery
Introduction
Coral reefs, which accounts for only 0.2% of the world sea area, are the most diverse biological marine ecosystem [1]. Tropical corals usually live in symbiosis with yellow-brown single-celled algae or zooxanthellae, which comprise approximately 2000 species [2,3]. Freudenthal [4] first proposed that these zooxanthophyta belonged to the symphysis of analga. Zooxanthellae are located in the vacuole of the endodermal cells of the coral host. Under normal conditions, the symbiotic relationship between zooxanthellae and the host is stable, and the number of algae in corals is constant [5]. The mutualism between coral and zooxanthella is the basis for the survival of the symbiosis in extremely poor tropical seawater environments. Ecologists have observed that even small changes in the physical parameters of the marine environment could cause dramatic effects on the stability of the coral-zooxanthella symbiosis system [6]. The most common effect is coral bleaching, a phenomenon where corals lose their symbiosis or symbiotic algae lose their pigments [7]. Temperature is a major factor that causes coral bleaching, and the symbiotic system is highly sensitive to an increase in seawater temperature. Coral bleaching occurs even if the water temperature is higher than the optimum growth temperature by only 1-2°C [8]. Algal density decreases at the start of bleaching, and approximately 70%-90% of algae in corals are lost during visible bleaching. Coral hosts exhibit stronger tolerance to high temperatures than zooxanthellae [9-11], which may be associated with the high sensitivity of the photosynthetic system of the latter. Different from the apoptosis of zooxanthella cells, bleaching is observed in coral hosts only when the experimental temperature reaches 34°C [12]. Chromosome polymerization and DNA fragmentation occur during the necrosis of stony corals [13]. Endosymbiotic algae in corals showing visible bleaching usually undergo apoptosis or necrosis, but some algae remain normal [14]. This finding indicates that the symbiont has a chance to survive and recover [15]. Bleaching corals die if they fail to grow new algae or if the stress condition lasts too long [16]. In addition, zooxanthellae and corals exhibit species-specific differences in thermal sensitivity [17-19]. Thus, the injury and recovery of symbiotic algae also differ.
Galaxea astreata is a Chinese endemic species, and molecular identification studies have found that its symbiotic algae belong to the Clade C.
In the present study, we observed the changes in the morphology and ultrastructure of zooxanthella of G. astreata under thermal stress and at the early recovery by transmission electron microscopy (TEM) to understand the damage and recovery of the symbiosis of the G. astreata from the cell level and to provide a basis mechanism for the anti-albinism of coral-zooxanthella symbiosis.
Material and Methods
Collection of materials
G. astreata corals were collected from the coast near the Xuwen, Guangdong Province, China (109° 54′ 45’’ E, 20° 16′ 20’’ N). Samples were maintained in seawater with 12:12 h light and dark phase and temperature between 27 ± 0.5°C for 2 weeks before the experiment.
Methods
Temperature treatment: After transient raising, the samples were divided into 90, 5cm × 5cm nubbins and were randomly divided into two groups: control and stressed. The control group was kept at 28°C, while the stressed group was exposed to an increase in temperature of 1°C for 5 days, maintained at 32°C for 7 additional days, exposed to a decrease in temperature of 1°C every 24 h until 28°C, and then maintained for 7 days. All samples were obtained 24 h after the stress treatment (each group with 3 tips, 100 mg each tip). In accordance with the method described by Sammarco et al. [20], the samples were washed with PBS and then fixed in 2.5% glutrardehyde and 2% paraformaldehyde in 0.1 M sodium cacodylate buffer at pH 7.4 and 4°C for 12 h.
TEM: After the fixative was removed, the samples were washed with PBS and then post-fixed in 0.1% osmium tetroxide (OsO4) in the same cacodylate buffer. After 0.1% OsO4 was discarded, the samples were gradient dehydrated with different concentrations of acetone (50%, 70%, 80%, 90%, 95%, and 100%) and embedded in Epon 812 (Sigma, St. Louis, Mo, USA). Ultrathin sections were stained in uranyl acetate and lead citrate, and the samples were viewed under a JEM-1400 transmission electron microscope (JEOL, Tokyo, Japan) operated at 80 kV.
Results
Ultrastructure of Symbiodinium under normal temperature
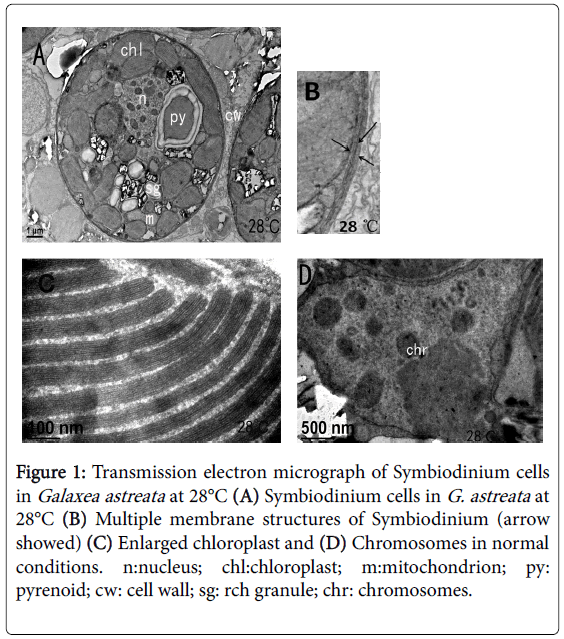
As shown in Figure 1, under TEM, the normal Symbiodinium cells were oval shaped with approximately 8-13 μm diameters. The cell wall, including three non-uniform thickness membranes, was approximately 50-150 nm. Several chloroplasts with different shapes were found alongside the inner membrane of the cell wall. After amplification, the lamellar structure of chloroplasts can be observed clearly (Figure 1C).
Figure 1: Transmission electron micrograph of Symbiodinium cells in Galaxea astreata at 28°C (A) Symbiodinium cells in G. astreata at 28°C (B) Multiple membrane structures of Symbiodinium (arrow showed) (C) Enlarged chloroplast and (D) Chromosomes in normal conditions. n:nucleus; chl:chloroplast; m:mitochondrion; py: pyrenoid; cw: cell wall; sg: rch granule; chr: chromosomes.
Starch and several mitochondria were found near the chloroplasts, indicating that the cells were performing active photosynthesis. “Horseshoe”-shaped pyrenoid was found embedded in the inner side of the chloroplasts with starch sheath package. No thylakoid was found in the pyrenoid. Obvious rounded accumulation body can be found near the central cell. The nucleus was nearly elliptical in shape in the cell side and partly surrounded by chloroplasts. A set of Golgi apparatus was found close to the lateral side of the nuclear membrane, and multiple structural integrity and dying dark oval chromosomes were found. All organelles within the cytoplasm had distinct boundaries.
Ultrastructure of Symbiodinium under thermal stress
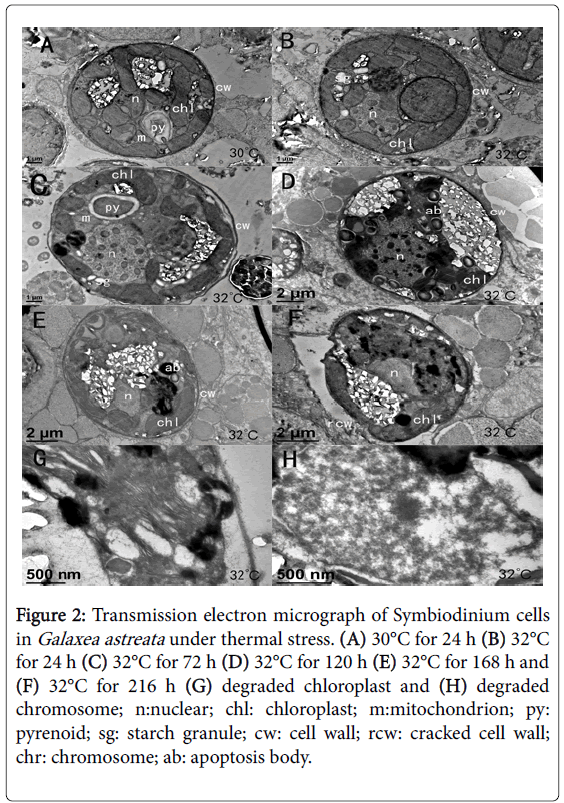
After the temperature was slowly increased from 28°C to 32°C and kept for 24 h, the morphology and ultrastructure of the Symbiodinium cells remained normal without obvious changes (Figures 2A and 2B), indicating that the short-term high temperature stress did not exert obvious stress effects on the zooxanthella. When the stress was continued until 32°C and kept for 72 h, the membrane of the zooxanthella started to fold and layer (Figure 2C). Over time, the damage of thermal stress on zooxanthella cells became more obvious.
After exposure to 32°C for 120 h, numerous white crystalline carboxylations occurred in the cells, and intracellular organelle positions changed (Figure 2D). At 168 h of exposure to 32°C, the zooxanthella cell damage became more severe; spaces began to form between the cells and the host tissue, cell size shrank, cell membrane sagged, the nuclear shape became abnormal, and apoptotic bodies appeared, which all indicated that the zooxanthella cells began to undergo apoptosis (Figure 2E). After 216 h of exposure at 32°C, a few zooxanthella cells were observed to undergo necrosis, cells suffered serious injury, internal organelles began to degrade and melt, the cell wall began to burst, and the cell content began to overflow (Figure 2F). Upon magnification, the granum lamella of the chloroplast became distorted (Figure 2G) and irregular in shape, and the chromosome appeared intensely degraded (Figure 2H).
Figure 2: Transmission electron micrograph of Symbiodinium cells in Galaxea astreata under thermal stress. (A) 30°C for 24 h (B) 32°C for 24 h (C) 32°C for 72 h (D) 32°C for 120 h (E) 32°C for 168 h and (F) 32°C for 216 h (G) degraded chloroplast and (H) degraded chromosome; n:nuclear; chl: chloroplast; m:mitochondrion; py: pyrenoid; sg: starch granule; cw: cell wall; rcw: cracked cell wall; chr: chromosome; ab: apoptosis body.
Ultrastructure of Symbiodinium under recovery
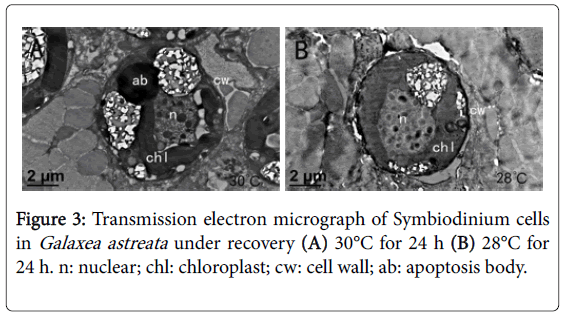
When the temperature was decreased from 32°C to 28°C and was kept for 24 h, the external morphology and internal structure of the zooxanthella cells showed slight recovery, but the cell membrane continued to shrink, was fuzzy, and the cells were still in a state of obvious damage (Figures 3A and 3B).
Ultrastructure of Symbiodinium after short-term recovery
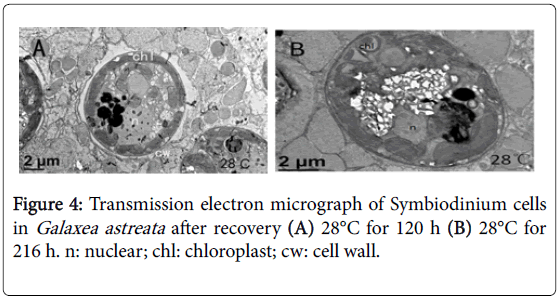
Until 28°C for 120 h, the external morphology of the zooxanthella cells looked normal, the cell membrane was intact and clear, and the intracellular organelles exhibited obvious recovery (Figure 4A). However, the entire cell did not fully recover to normal condition. A few cell cavities can be observed in the chloroplast of some cells until 28°C for 216 h (Figure 4B).
Discussion
Effect of thermal stress on the morphology and structure of the zooxanthella cells
Corals inhibited in the Persian Gulf have a growth temperature of 13°C-38°C. Most corals that are symbiotic with Symbiodinium lived at 18°C-33°C, with the most appropriate temperature at 25°C-29°C [21]. Corals living in water whose temperature is higher than the appropriate level undergo bleaching. In this study, we observed the structures of Symbiodinium under high temperature stress. When the temperature was increased from 28°C to 32°C and lasted for 24 h, the cell morphology and structures of the Symbiodinium did not show any change, indicating that G. astreata and Symbiodinium show certain tolerance to temperature stress. Short-term stress did not cause any damage to the symbiosis system. After 72 h of stress at 32°C, obvious changes in the Symbiodinium cell morphology were observed, and the cell membrane was wrinkled. After 120 h of stress, the number of carboxylated cells in the cells increased rapidly, indicating that the cell was affected by heat stress, and the photorespiration of chloroplasts increased dramatically. Dramatic changes in cells started from 120 h to 168 h, and even a few zooxanthella cells underwent apoptosis at 32°C for 168 h. Meanwhile, the surface color of the coral became clear, indicating that the coral began to show faint bleaching. Studies on coral bleaching caused by high temperature have found that the host cells can release the injured algae to adapt to heat stress [14]. Given the continuous high-temperature stress, part of the algal cells began to dissolve and released lysozyme, which might cause further damage to the healthy algal cells. This process amplified the cell damage effect and accelerated the rate of index or logarithm of cell necrosis and apoptosis in the late stage of the stress, eventually leading to the emergence of coral bleaching. However, several controversies remained about the bleaching process from coral symbiotic algae released from the host. Some studies suggested that part of the expelled zooxanthellae still have normal morphological structure and can exist in the environment as non-symbiotic zooxanthellae [22]. Other studies considered that the expelled zooxanthellae incur some physiological damage and can only survive for a short time in the environment [15]. In the present study, continuous heat stress on the Galaxea astreata caused the apoptosis of the symbiotic algal cells, and the loss of algal cells resulted in coral bleaching.
Thermal sensitivity of zooxanthella cells
Different types of Symbiodinium cells show different degrees of thermal tolerance. D-type confers higher thermal tolerance than the others because the former cells contain more polyunsaturated fatty acids, which could reduce the sensitivity of the cell membrane to oxygen free radicals [17]. Symbiodinium cells are generally used to increase antioxidant properties [23], antibacterial properties [24], and anti-ultraviolent function [25] to protect host bleaching. However, even the same Symbiodinium cells that are endosymbiotic with different corals would show different responses to thermal stress. In the present study, the structure of the Symbiodinium cells changed when the temperature was increased to 32°C and kept for 72 h. However, Sammarco [20] found that Symbiodinium that hosts at soft corals has stronger resistance to bleaching than stone corals; for example, meat soft corals that host Symbiodinium can survive at 36°C. Although both of them are clade C Symbiodinium, they show different levels of heat sensitivity. The different protective effects on the endosymbiotic algae can be attributed to the different types and proportions of the various sublines of Clade and the different structures of corals.
Other studies showed that the coral host has stronger temperature tolerance than Symbiodinium. Apoptosis of Symbiodinium cells under temperature stress occurs before the cells leaves the host [12,14,26]. Vivekanandan [27] also found that the Symbiodinium in the Indian Ocean has more sensitivity to stress than the host coral, which may be attributed to the temperature sensitivity of the photosynthetic mechanism of Symbiodinium. A temperature sensitivity difference was found between coral hosts, such as staghorn coral, honeycomb coral, and Porites nigrescens, and the algae [13]. The explanation for this phenomenon, according to Cervino [28], was that the host synthesized more heat shock proteins than the endosymbionts to resist the heat stress. Hoegh-Guldberg [29] found that the algae also display greater sensitivity to nutrients than the coral host. In the present study, the damage of zooxanthella cells under thermal stress (32°C for 72 h) occurred earlier than coral bleaching (32°C for 168 h), which might also prove that algae are more sensitive to heat stress than corals.
Morphological and structural changes of Symbiodinium in recovery
After slowly cooling the temperature to 30°C for 24 h and 28°C for 24 h, the external morphology and ultrastructure of the cells recovered slightly. The cell memberane structure of the cells was still fuzzy, and the whole algal cells were still in the state of injury. Until the temperature of 28°C for 120 h, the whole cell structure basically returned to normal, the cell membrane was intact, and the intracellular organelles showed obvious recovery. However, the recovery was incomplete until 216 h, at which some organelle structures exhibited cell abnormalities, such as part of the chloroplast was empty. This result indicates that the internal organelles of the Symbiodinium cells may take longer time to completely recover. During the whole heat stress process, the vast majority of symbiotic algal cells did not experience serious damage or apoptosis. Thus, after the stress, most of the cells showed good recovery. Strychar [12] thought that even after coral bleaching, the corals still hosted a small number of normal Symbiodinium cells. Rodrigues [15] proposed that bleaching corals exposed to appropriate recovery temperature can still survive or even rebuild symbiosis with a small number of normal endosymbionts.
Conclusion
In normal cells, chloroplast thylakoids, starch, and condensed chromosomes were obvious in the nucleus, indicating that the cells were performing active photosynthesis. A thick vacuolated coral cell surrounded the cell undergoing degradation, and the subcellular organelles in the degrading cell became indistinct. Finally, the organelles of the degraded cells nearly deteriorated, the cell membrane disappeared, the accumulation body became enlarged, and the cell size shrank to approximately half the normal size.
Acknowledgement
This research was supported by the Guangdong Ocean University Scientific Research Start-up Fund for the Doctoral Program.
References
- Dishon G, Fisch J, Horn I, Kaczmarek K, Bijma J, et al. (2015) A novel paleo-bleaching proxy using boron isotopes and high-resolution laser ablation to reconstruct coral bleaching events. Biogeosci 12: 5677-5687.
- Zhang Z, Green BR, Cavalier-Smith T (2000) Phylogeny of Ultra-Rapidly Evolving Dinoflagellate Chloroplast Genes: A Possible Common Origin for Sporozoan and Dinoflagellate Plastids. J Mol Evol 51: 26-40.
- Rogers K (2010) Fungi, Algae and Protists. Britannica Educational Pub in association with Rosen Educational Services, New York, pp 25-30.
- Freudenthal HD (1962) Symbiodiniumgen nov. and Symbiodinium microadriaticumsp. nov., a Zooxanthella: Taxonomy, Life Cycle, and Morphology. The J Protozoology 9: 45-52.
- Drew EA (1972) The biology and physiology of alga-invertebrates symbioses. II. The density of symbiotic algal cells in a number of hermatypic hard corals and alcyonarians from various depths. J Exp Marine Biol Ecol 9: 71-75.
- Glynn PW (1990) Coral Mortality and Disturbances to Coral Reefs in the Tropical Eastern Pacific. Elsevier Oceanography Series 55-126.
- Vidal-Dupiol J, Adjeroud M, Roger E, Foure L, Duval D, et al. (2009) Coral bleaching under thermal stress: putative involvement of host/symbiont recognition mechanisms. BMC Physiology 9: 14.
- Glynn PW, Maté JL, Baker AC, Calderón MO (2001) Coral Bleaching and Mortality in Panama and Ecuador During The 1997-1998 El Niño-Southern Oscillation Event: Spatial/Temporal Patterns and Comparisons With The 1982-1983 Event. Bulletin of Marine Sci 69: 79-109.
- Hill R, Ulstrup KE, Ralph PJ (2009) Temperature Induced Changes in Thylakoid Membrane Thermostability of Cultured, Freshly Isolated, and Expelled Zooxanthellae From Scleractinian Corals. Bulletin of Marine Sci 85: 223-244.
- Strychar KB, Sammarco PW (2011) Effects of Heat Stress on Phytopigments of Zooxanthellae (Symbiodinium spp.) Symbiotic with the Corals Acropora hyacinthus, Porites solida, and Favites complanata. Int J Biol 4: 3-19.
- Roth MS, Goericke R, Deheyn DD (2012) Cold induces acute stress but heat is ultimately more deleterious for the reef-building coral Acropora yongei. Scient Reports 2: 240.
- Strychar KB, Sammarco PW (2009) Exaptation in corals to high seawater temperatures: Low concentrations of apoptotic and necrotic cells in host coral tissue under bleaching conditions. J Exp Marine Biol Ecol 369: 31-42.
- Sammarco PW, Strychar KB (2009) Effects of Climate Change/Global Warming on Coral Reefs: Adaptation/Exaptation in Corals, Evolution in Zooxanthellae, and Biogeographic Shifts. Environ Bioindicators 4: 9-45.
- Strychar KB, Coates M, Sammarco PW (2004) Loss of Symbiodinium from bleached Australian scleractinian corals (Acroporahyacinthus,Favites complanata and Porites solida). Marine Freshwater Res 55: 135.
- Rodrigues LJ, Grottoli AG (2007) Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnol Oceanograph 52: 1874-1882.
- Diaz-Pulido G, McCook L (2002) The fate of bleached corals: patterns and dynamics of algal recruitment. Marine Ecol Prog Series 232: 115-128.
- Abrego D, Ulstrup KE, Willis BL, Van Oppen MJ (2008) Species-specific interactions between algal endosymbionts and coral hosts define their bleaching response to heat and light stress. Proc Royal Soc B: Biol Sci 275: 2273-2278.
- Oliver TA, Palumbi SR (2011) Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30: 429-440.
- Krueger T, Gates RD (2012) Cultivating endosymbionts- Host environmental mimics support the survival of Symbiodinium C15 ex hospite. J Exp Marine Biol Ecol 413: 169-176.
- Sammarco PW, Strychar KB (2013) Responses to High Seawater Temperatures in Zooxanthellate Octocorals. PLoS ONE 8: e5498.
- Achituv Y, Dubinsky Z (1990) Evolution and zoogeography of coral reefs. In: Dubinsky Z (editor) Coral reefs. Ecosystems of the world. Elsevier, Amsterdam pp 1-9.
- ladriere O, Compere P, Decloux N, Vandewalle P, Oulicek Mp (2008) Morphological alterations of zooxanthellae in bleached cnidarian hosts. Cah Biol Mar 49: 215-227.
- Lesser MP, Stochaj WR, Tapley DW, Shick JM (1990) Bleaching in coral reef anthozoans: effects of irradiance, ultraviolet radiation, and temperature on the activities of protective enzymes against active oxygen. Coral Reefs 8: 225-232.
- Dunlap WC, Shick JM (1998) Review-Ultraviolet Radiation-Absorbing Mycosporine-Like Amino Acids in Coral Reef Organisms: A Biochemical and Environmental Perspective. J Phycol 34: 418-430.
- Salih A, Larkum A, Cox G, Kühl M, Hoegh-Guldberg O (2000) Fluorescent pigments in corals are photoprotective. Nature 408: 850-853.
- Stambler N (2010) Coral Symbiosis Under Stress. Symbioses and Stress, Springer Netherlands, pp 197-224.
- Vivekanandan E (2008) Vulnerability of corals to seawater warming-Winter School on Impact of Climate Change on Indian Marine Fisheries held at CMFRI, Cochin, India.
- Cervino Jm, Hayes R, Goreau Tj, Smith Gw (2004) Zooxanthellae Regulation in Yellow Blotch/Band and Other Coral Diseases Contrasted with Temperature Related Bleaching: In Situ Destruction vs Expulsion. Symbiosis 37: 63-85.
- Hoegh-Guldberg O (1997) Effect of nutrient enrichment in the field on the biomass, growth and calcification of the giant clam Tridacna maxima. Marine Biol 129: 635-42.
Citation: Zhang YP, Shen C, Li Feng, Shen YC, Liu L (2018) Ultrastructural Changes of Endosymbiotic Symbiodinium of Galaxea astreata under Thermal Stress and after Short Time Recovery Process. J Marine Sci Res Dev 8: 262. DOI: 10.4172/2155-9910.1000262
Copyright: © 2018 Zhang Yan-Ping, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5385
- [From(publication date): 0-2018 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 4319
- PDF downloads: 1066