Uncoupling of the Electron Transport Chain Compromises Mitochondrial Oxidative Phosphorylation and Exacerbates Stroke Outcomes
Received: 29-Nov-2018 / Accepted Date: 21-Dec-2018 / Published Date: 31-Dec-2018 DOI: 10.4172/2314-7326.1000283
Abstract
Objective: Mitochondrial dysfunction is known to be implicated in stroke, but the complex mechanisms of stroke have led to few stroke therapies. The present study to disrupted mitochondrial oxidative phosphorylation through a known electron transport chain (ETC) uncoupler, Carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP). Analyzing the resulting neurological deficits as well as infarct volume could help determine the role of mitochondria in stroke outcome and determine whether uncoupling the ETC could potentially be a strategy for new stroke therapies. The objective of this study was to determine the effects of uncoupling electron flow on mitochondrial oxidative phosphorylation and stroke infarction.
Methods: Cerebral endovascular cells (CECs) were treated with various concentrations of FCCP, and bioenergetics were measured. For the stroke mouse model, FCCP (1 mg/kg, i.p) or vehicle was administered followed by 1-hour transient middle cerebral artery occlusion (tMCAO). Infarct volume was measured after a 23-hour reperfusion, and triphenyl tetrazolium chloride (TTC) staining was used to assess infarct volume.
Results: FCCP significantly decreased basal respiration, ATP turnover, maximal respiration, and spare capacity when the concentration of FCCP was greater than 1000 nM. The mice pretreated with FCCP had a significantly increased infarct volume within the cortex, striatum, and total hemisphere. Mice receiving FCCP had a significantly increased neurological deficit score compared to the vehicle.
Conclusions: FCCP compromised mitochondrial oxidative phosphorylation in CECs in a dose-dependent manner. Uncoupling the electron transport chain with FCCP prior to tMCAO exacerbated stroke infarction in mice.
Keywords: Blood-Brain Barrier (BBB); Cerebral Endovascular Cells (CECs); Carbonyl Cyanide-4 (trifluoromethoxy) Phenylhydrazone (FCCP); Electron Transport Chain (ETC); Ischemia; Transient Middle Cerebral Artery Occlusion (tMCAO); Triphenyl Tetrazolium Chloride (TTC)
Introduction
Stroke remains the third leading cause of death worldwide and is considered to be a leading cause of disability in adults [1]. Annually, 15 million people are affected by stroke, leaving 5 million dead and another 5 million disabled [2]. Stroke therapies are limited for patients due to the complicated mechanisms of stroke that have yet to be investigated.
Mitochondrial dysfunction is not only implicated in stroke, but other studies indicate that disruption of the electron transport chain also leads to mitochondrial associated metabolic diseases, such as obesity, neurodegenerative diseases, and cancer [3]. Mitochondrial oxidative phosphorylation is imperative to the proper functioning of aerobic cells, as i1t produces most of the energy by coupling respiration to ATP production [4]. Previous studies have shown five levels of mitochondrial oxidative phosphorylation regulation. These include direct modulation of the electron transport chain kinetic parameters, regulation of intrinsic efficiency, mitochondrial network dynamics, mitochondrial biogenesis and degradation, and the cellular and mitochondrial environment [5]. Mitochondrial dysfunction is known to affect stroke due to its incorporation in multiple cellular pathways. These pathways include ATP and reactive oxygen species generation, energy metabolism, cell cycle, calcium homeostasis, and apoptosis [6]. It controls the fate of neuronal cells while also influencing blood-brain barrier (BBB) permeability after the occurrence of ischemic stroke. Mitochondria heavily regulate the ETC since it is the site of redox reactions that facilitate phosphorylation of ADP to ATP. Defects in mitochondria and subsequently the ETC are known to have fatal consequences for cells.
Carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP), a mobile ion carrier, is a known uncoupling agent of the mitochondrial electron transport chain [7]. FCCP is able to disrupt ATP synthesis through uncoupling the proton gradient generated by the mitochondrial membrane. FCCP has been associated with mitochondrial inhibition and has been shown to activate ionic currents and depolarize the plasma membrane potential in a dose-dependent manner [8,9]. Other in vivo studies found that FCCP disrupts mitochondrial H+ gradients, disrupts the microtubular network, and decreases the rate of protein synthesis. Stroke studies have used FCCP to reduce the mitochondrial membrane potential and increase K+ conductance to shunt action potentials [10]. However, the role of FCCP on stroke outcomes were not reported.
In this study, we investigated the relationship between the uncoupling of the electron transport chain and infarct volume in stroke. Using an in vitro cell culture model, we demonstrated that FCCP compromised mitochondrial phosphorylation in a dose-dependent manner. Using a transient middle cerebral artery occlusion stroke model, we demonstrated that uncoupling the electron transport chain exacerbates stroke infarction in mice. This novel study determined that the role of uncoupling the electron transport chain is detrimental to stroke brain and targeting of the electron transport chain could potentially be a strategy for stroke therapy.
Materials and Methods
Animals
C57BL/6J male mice (3-6-month-old, 25~30 g) from the Jackson laboratory were used for experiments. All procedures were approved prior to experimentation by the West Virginia University Animal Care and Use Committee.
Cell culture
The cerebrovascular endothelial cells (CECs, b. End3 cell line from ATCC) are originally derived from mouse brain endothelial cells. Passages 25~30 were used for in vitro experiments. The cells (16,000 cells/ well) were seeded into Seahorse Bioscience XFe96 cell culture plates overnight then treated with FCCP in varying concentrations (10 nM, 100 nM, 1000 nM, 10000 nM) or the vehicle control for 24 hours in a 37°C humidified incubator with 5% CO2.
Measuring oxygen consumption in CECs
Oxygen consumption rate (OCR) was measured at 37°C using a Seahorse XFe96 Analyzer (Agilent, Santa Clara, CA) according to our protocol published [11]. Briefly, the cell culture media was changed to Mito-Stress Test Assay Media, which is DMEM that contains the following: 2 mM GlutaMax, 1 mM sodium pyruvate, and 25 mM glucose (media is un-buffered, pH 7.4). The cells were incubated at 37°C without CO2 1 hr prior to the start of the extracellular flux assay. Oligomycin, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), rotenone and antimycin A (all from Sigma, St. Louis, MO ) of 10 × compound dilutions were prepared for the assay and loaded into the assigned ports. A sensor cartridge was calibrated 24 hr prior to the assay. Following calibration, the cell plate was placed in the Bioanalyzer, and the Mito-Stress Test assay protocol was completed on the samples. This protocol allowed determination of the basal level of oxygen consumption, the amount of oxygen consumption linked to ATP turnover, the maximal respiration capacity, and the spare capacity.
Drug administration
For the in vivo study, mice were pretreated with FCCP (Sigma, 1 mg/kg n=8) or vehicle (10% DMSO in saline) (n=8) via intraperitoneal injection 30 min prior to stroke model.
Murine ischemic stroke model
Transient middle cerebral artery occlusion (tMCAO) was performed after induction of anesthesia with 4~5% isoflurane and mice did not respond to toe pinch. Isoflurane (1-2%) via face mask in 30%O2: 70%N2 mixture was used to maintain the unresponsive state, and a rectal body temperature of 37 ± 0.5 ˚C was maintained throughout surgery. Right middle cerebral artery was occluded for 60 minutes with MCAO suture (diameter with coating 0.22 ± 0.01 mm from Doccol Corp., MA). Blood flow and success of occlusion was determined using Laser Speckle Imager (Moor Instruments, United Kingdom).
Brain histology
Mice were euthanized under deep anesthesia with 4~5% isoflurane; brains were retrieved and cut in 2 mm coronal matrix. Sliced brain sections underwent three different staining procedures: Triphenyl tetrazolium chloride (TTC, Sigma, Saint Louis, MO), Cresyl Violet, and H&E staining. Brain sections (2 mm) were stained for 30 minutes with 2% TTC in phosphate buffered solution (PBS) at 37°C staining to analyze infarct volume within the cortex, striatum, and total hemisphere. Resulting brain sections were imaged with photo scanner (CanonScan, 9000 F, Japan). Brain sections were fixed in 10% formalin for one week then embedded in paraffin. Coronal slices (10 μm) were cut through the brain hippocampus area. Cresyl violet [12] and H&E [13] staining were performed using published protocols. One FCCP treated mouse died before 23-hour end-point was not included for stroke infraction.
Neurological deficits
After being subjected to tMCAO, a score was given to each mouse to evaluate neurological deficits. Neurological functioning was measured on a 0-5 scale, where 0=no neurological dysfunction; 1=failure to extend contralateral forelimb when lifted by tail; 2=circling to the contralateral side: 3=falling to the contralateral side; 4=nonspontaneous walk or in a comatose state; 5=death.
Statistical analysis
A one-way ANOVA and post hoc Tukey’s test were used to analyze the graph and calculate basal respiration, ATP turnover, maximal respiration, and spare capacity, which are all derivatives of mitochondrial oxidative phosphorylation. Student’s t-test was used to analyze the significance of infarction and neurological deficits between groups. p<0.05 was considered statistical significance.
Results
FCCP compromises oxidative phosphorylation in CECs
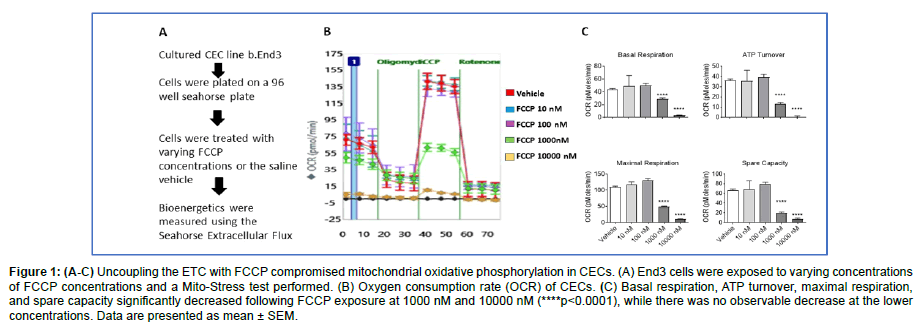
To assess the effects of uncoupling the electron transport chain in CECs, Seahorse XFe96 Bioanalyzer with the Mito-Stress Test was utilized. Briefly, the injection of different compounds, sequentially, disrupts the ETC. Measurements taken after each injection allow for different mitochondrial parameters to be calculated, which include basal respiration, ATP turnover, maximal respiration, and spare capacity. After culturing CECs and exposing them to various concentrations of FCCP, basal respiration, ATP production, maximal respiration, and spare capacity were determined. Results indicate all four mitochondrial parameters were significantly decreased at doses of both 1000 nM and 10000 nM (p<0.0001), while there was no difference observed at the 10 nM and 100 nM exposures (Figure 1). These data suggest that uncoupling of the ETC decreases mitochondrial phosphorylation in CECs at 1000 nM or greater concentrations of FCCP.
Figure 1: (A-C) Uncoupling the ETC with FCCP compromised mitochondrial oxidative phosphorylation in CECs. (A) End3 cells were exposed to varying concentrations of FCCP concentrations and a Mito-Stress test performed. (B) Oxygen consumption rate (OCR) of CECs. (C) Basal respiration, ATP turnover, maximal respiration, and spare capacity significantly decreased following FCCP exposure at 1000 nM and 10000 nM (****p<0.0001), while there was no observable decrease at the lower concentrations. Data are presented as mean ± SEM.
FCCP worsens stroke outcomes in mice
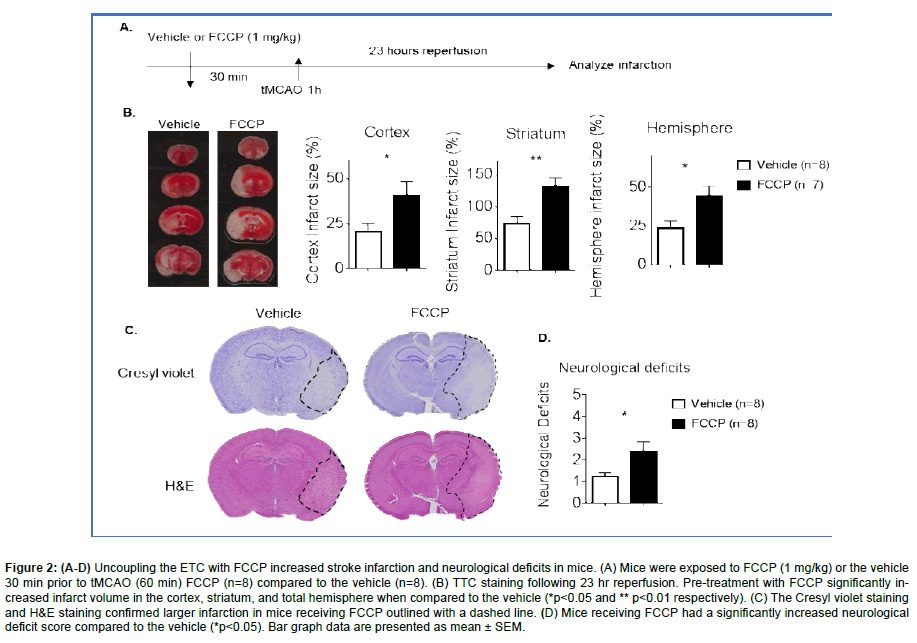
In order to understand the impact of the uncoupling of the ETC might have during a stroke, animals were treated with either vehicle control or FCCP (1 mg/kg) at 30 min prior to tMCAO (Figure 2A). Following a 23 hr reperfusion, mice were sacrificed and TTC staining was completed. Results demonstrate that pretreatment of mice with FCCP prior to tMCAO significantly increased infarct volume when compared to the vehicle control. Increases in infarct size were observed in the cortex, striatum, and total hemisphere of FCCP treated mice (Figure 2B).
Figure 2: (A-D) Uncoupling the ETC with FCCP increased stroke infarction and neurological deficits in mice. (A) Mice were exposed to FCCP (1 mg/kg) or the vehicle 30 min prior to tMCAO (60 min) FCCP (n=8) compared to the vehicle (n=8). (B) TTC staining following 23 hr reperfusion. Pre-treatment with FCCP significantly increased infarct volume in the cortex, striatum, and total hemisphere when compared to the vehicle (*p<0.05 and ** p<0.01 respectively). (C) The Cresyl violet staining and H&E staining confirmed larger infarction in mice receiving FCCP outlined with a dashed line. (D) Mice receiving FCCP had a significantly increased neurological deficit score compared to the vehicle (*p<0.05). Bar graph data are presented as mean ± SEM.
Cresyl violet staining and H&E staining confirmed there was a larger infarction in mice receiving FCCP (Figure 2C). Mice receiving FCCP demonstrated increased severity on the neurological deficit scale (Figure 2D). The data suggests that the uncoupling of the ETC prior to a stroke may lead to exacerbated stroke infarction and neurological dysfunction.
Discussion
The importance of maintaining mitochondrial function following an ischemic event in the brain has been well-demonstrated. Previous work revealed that loss of the mitochondrial uncoupling protein-2, which leads to inhibition of the ETC, led to an increased infarct volume of 61% per hemisphere compared to 18% in wildtype [14]. Other
studies have found that low mitochondrial DNA content is associated with heightened effects of ischemic stroke [15]. In two other studies, the effects of LPS and miR-34a were shown to affect the integrity of the BBB, and in both of these studies, there was measurable mitochondrial dysfunction. Along with miR-34a and LPS effects, NAD-Dependent Protein Deacetylase Sirtuin-1 (SIRT1) may be another main player in mitochondrial dysfunction [16]. SIRT1 is known to play a crucial role in cancers, obesity, stroke, and dementia along with regulation of metabolism and the modulation of metabolic diseases [17-19]. Our previous study demonstrated that using rotenone, a Complex I inhibitor, compromised mitochondrial activity in a murine model, and this dysfunction, along with altered body temperature, led to poor outcome following a stroke [20]. In a study by Feng et al. [21], treatment with rotenone reduced SIRT1 levels and AMPK phosphorylation. Furthermore, miR-34a regulates SIRT1 [17], and miR-34a is related to mitochondrial dysfunction. However, it is not known whether FCCP in involved in the regulation of SIRT1- this could be an interesting topic to be addressed further. These studies provide evidence for the importance of maintaining mitochondrial function following an injury to the brain [22,23]. Our previous study demonstrated that the mitochondria inhibitor FCCP disrupts the integrity of the BBB and increases BBB permeability in vitro [17]. The data from our studies provides additional support that compromising the integrity of the ETC by using an uncoupling agent like FCCP leads to mitochondrial dysfunction in a dosedependent manner. Pre-treatment of animals with FCCP 30 min prior to a tMCAO (60 min) led to a significantly larger infarct volume when compared to vehicle control.
Some limitations in our studies are derived from the fact that overall infarct size can be dependent on other factors such as ischemia duration and severity, sufficient blood pressure, age, comorbidities, genetic background, etc [24], and it will be important to consider these factors in order to determine the full effects that mitochondrial dysfunction may have on stroke outcome.
Conclusion
In conclusion, our results demonstrate that the uncoupling of the electron transport chain compromises mitochondrial oxidative phosphorylation in CECs, observed by a decrease in spare capacity, ATP turnover, basal respiration, and maximal respiration. Chemically uncoupling the ETC exacerbated stroke infarction in a murine tMCAO model, with measurable increases in infarct volume within the cortex, striatum, and total hemisphere. Given the data from our studies, as well as previously reported work, it is increasingly apparent that maintaining and/or restoring mitochondrial function following an ischemic stroke is critical to minimize infarct damage. Future studies should address how to restore mitochondrial function and focus on development of therapeutics that could be given to patients following an ischemic stroke, with the idea that restoration of mitochondrial function and decreased infarct size will lead to better outcomes for stroke patients.
Acknowledgements
This study is supported by AHA SDG (16SDG31170008 to XR), WVCTSI to XR (NIH/NIGMS U54GM104942), and NIH WV stroke-CoBRE to JWS (P20 GM109098).
References
- Yuan MZ, Li F, Fang Q, Wang W, Peng JJ, et al. (2018) Research on the cause of death for severe stroke patients. J Clin Nurs 27: 450-460.
- Shrivastava SR, Shrivastava PS, Ramasamy JD (2013) Reduction in global burden of stroke in underserved areas. Journal of Neurosciences in Rural Practice 4: 475-476.
- Sreedhar A, Zhao Y (2017) Uncoupling protein 2 and metabolic diseases. Mitochondrion 34: 135-140.
- Figarola JL, Singhal J, Tompkins JD, Rogers GW, Warden C, et al. (2015) SR4 Uncouples mitochondrial oxidative phosphorylation, modulates AMP-dependent Kinase (AMPK)-mammalian target of rapamycin (mTOR) Signaling, and inhibits proliferation of HepG2 hepatocarcinoma cells. J Biol Chem 290: 30321-30341.
- Hroudova J, Fisar Z (2013) Control mechanisms in mitochondrial oxidative phosphorylation. Neural Regeneration Research 8: 363-375.
- Yang JL, Mukda S, Chen SD (2018) Diverse roles of mitochondria in ischemic stroke. Redox biology 16: 263-275.
- Terada H (1990) Uncouplers of oxidative phosphorylation. Environmental Health Perspectives 87: 213-218.
- Park KS, Jo I, Pak K, Bae SW, Rhim H, et al. (2002) FCCP depolarizes plasma membrane potential by activating proton and Na+ currents in bovine aortic endothelial cells. Pflugers Archiv: European Journal of Physiology 443: 344-352.
- Kenwood BM, Weaver JL, Bajwa A, Poon IK, Byrne FL, et al. (2014) Identification of a novel mitochondrial uncoupler that does not depolarize the plasma membrane. Molecular Metabolism 3: 114-123.
- Duchen MR (1990) Effects of metabolic inhibition on the membrane properties of isolated mouse primary sensory neurones. The Journal of Physiology 424: 387-409.
- Rellick SL, Hu H, Simpkins JW, Ren X (2016) Evaluation of bioenergetic function in cerebral vascular endothelial cells. JoVE p: 117.
- Rousselet E, Kriz J, Seidah NG (2012) Mouse model of intraluminal MCAO: Cerebral infarct evaluation by cresyl violet staining. JoVE p: 69.
- Blazquez M (2008) Phenotypic analysis of Arabidopsis mutants: Gibberellin/abscisic acid/paclobutrazol hormone response. CSH Protocols: pdb. prot p: 4964.
- Haines BA, Mehta SL, Pratt SM, Warden CH, Li PA (2010) Deletion of mitochondrial uncoupling protein-2 increases ischemic brain damage after transient focal ischemia by altering gene expression patterns and enhancing inflammatory cytokines. Journal of Cerebral Blood flow and Metabolism: Official Journal of the International Society Of Cerebral Blood Flow and Metabolism 30: 1825-1833.
- Lien LM, Chiou HY, Yeh HL, Chiu SY, Jeng JS, et al. (2017) Significant association between low mitochondrial DNA content in peripheral blood leukocytes and ischemic stroke. Journal of the American Heart Association 6: e006157.
- Martins I (2017) The future of genomic medicine involves the maintenance of Sirtuin 1 in global populations. Int J Mol Biol 2: 13.
- Yamakuchi M (2012) MicroRNA regulation of SIRT1. Frontiers in Physiology 3: 68.
- Martins I (2015) Unhealthy nutrigenomic diets accelerate NAFLD and adiposity in global communities. Journal of Molecular and Genetic Medicine 9: 11.
- Martins I (2014) The global obesity epidemic is related to stroke, dementia and Alzheimer's disease. JSM Alzheimer's Dis Related Dementia 1: 1010.
- Hu H, Doll DN, Sun J, Lewis SE, Wimsatt JH, et al. (2016) Mitochondrial Impairment in Cerebrovascular endothelial cells is involved in the correlation between body temperature and stroke severity. Aging and Disease 7: 14-27.
- Feng Y, Liu T, Dong SY, Guo YJ, Jankovic J, et al. (2015) Rotenone affects p53 transcriptional activity and apoptosis via targeting SIRT1 and H3K9 acetylation in SH-SY5Y cells. J Neurochem 134: 668-676.
- Doll DN, Hu H, Sun J, Lewis SE, Simpkins JW, et al. (2015) Mitochondrial crisis in cerebrovascular endothelial cells opens the blood-brain barrier. Stroke: A Journal of Cerebral Circulation 46: 1681-1689.
- Bukeirat M, Sarkar SN, Hu H, Quintana DD, Simpkins JW, et al. (2016) MiR-34a regulates blood-brain barrier permeability and mitochondrial function by targeting cytochrome c. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism 36: 387-392.
- Sommer CJ (2017) Ischemic stroke: Experimental models and reality. Acta Neuropathologica 133: 245-261.
Citation: Grasmick KA, Heng Hu, Hone EA , Farooqi I, Rellick SL, et al. (2018) Uncoupling of the Electron Transport Chain Compromises Mitochondrial Oxidative Phosphorylation and Exacerbates Stroke Outcomes. J Neuroinfect Dis 9: 283. DOI: 10.4172/2314-7326.1000283
Copyright: © 2018 Grasmick KA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 11011
- [From(publication date): 0-2018 - Oct 08, 2025]
- Breakdown by view type
- HTML page views: 10131
- PDF downloads: 880