Useful Method to Monitor Cerebral Infarction in Atherosclerotic Patients without Atrial Fibrillation by the Combination of Carotid Intima-Media Thickness, Cardio-Ankle Vascular Index, and Plasma D-dimer
Received: 12-Jul-2017 / Accepted Date: 24-Jul-2017 / Published Date: 27-Jul-2017
Abstract
Background and aims: To devise an effective monitoring system for detecting cerebral infarction in severely atherosclerotic patients without atrial fibrillation.
Methods: Atherosclerotic patients (284 cases; men: 97, women: 187; age: 35-96 years old; mean: 71.6 ± 10.7 years old) comprising hypertension, hyperlipidemia, lacunar infarction, ischemic heart disease, and diabetes mellitus, were enrolled and the occurrence of symptomatic cerebral infarction was observed for 40 months. They were stratified into mildly (A) (CAVI<8.0, IMT8.0, IMT>1.1 mm) atherosclerotic groups based on the level of CAVI and IMT and further divided into 2 subgroups: B, D+ (CAVI>8.0, IMT>1.1 mm, Ddimer> 1.0 μg/mL) and B, D- (CAVI>8.0, IMT>1.1 mm, D-dimer
Results: The incidence of cerebral infarction in group A was 0% (0/60), while that in group B was 5.9% (5/84), showing no significance (Chi-square test). Moreover, we examined the incidence of cerebral infarction in the two subgroups, revealing that in subgroup D, B+ it was 31.3% (5/16), while that in subgroup B, D- was 0/73 (Chi-square test, p<0.0001). The rate of aspirin intake in subgroup B, D+ was 43.8% (7/16), while that in subgroup B, D- was 45.2% (33/73), suggesting that the difference in aspirin use was not significant (Chi-square test).
Conclusion: The combination of CAVI, IMT and D-dimer was a useful tool for detecting cerebral infarction in atherosclerotic patients without atrial fibrillation.The incidence of cerebral infarction was increased in severely atherosclerotic patients with a high level of plasma D-dimer.
Keywords: CAVI; IMT; D-dimer; Atherosclerosis; Atrial fibrillation
5969Introduction
Atrial fibrillation is a significant risk factor for cerebral infarction. Atrial fibrillation affects 33 million individuals worldwide, and increases the risk of strokes, heart failure, and death; it also impairs quality of life [1]. Furthermore, non-valvular atrial fibrillation has been reported to be associated with a 5-6-fold increase in the incidence of stroke compared with patients without atrial fibrillation [2,3]. Although much of the evidence associated with cerebral infarction and atrial fibrillation has been collected in recent years, the relationship between the incidence of cerebral infarction and atherosclerosis in patients without atrial fibrillation has yet to be fully elucidated. The cardio-ankle vascular index (CAVI) was found to be related to cardiovascular mortality in the presence of hypertension [4] or the severity of coronary atherosclerosis [5,6]. CAVI provides a noninvasive assessment of atherosclerosis, and an association between CAVI and atherosclerosis [7,8] or cerebral infarction [9,10] has been reported. Furthermore, the carotid intima-media thickness (IMT) using an ultrasound device was related to risk factors for cardiovascular disease [11,12] and was a strong predictor of myocardial infarction [13,14]. IMT was found to be associated with the incidence of stroke [15,16]. A high IMT was predictive of the incidence of stroke [15] . D-dimer, evidence of thrombin generation in vessels, is a reliable and sensitive index of fibrin deposition [17]. The D-dimer level was associated with stroke or thromboembolic events in patients with atrial fibrillation [18,19]. D-dimer is also a predictor of the incidence of thromboembolism in patients with non-valvular atrial fibrillation [20-23].
In the present study, we stratified atherosclerotic patients into mild and severe groups using both CAVI and IMT, and compared the incidence of cerebral infarction in severe atherosclerotic patients with or without a high level of D-dimer.
Methods
Subject
Study patients: Atherosclerotic patients (284; men: 97, women: 187; age: 35-96 years old; mean: 71.6 ± 10.7 years old) who had been treated during the period fromDecember 2006 to September 2008(40 months) in our clinic (established as an annex of the Thrombosis Chemical Institute) were enrolled in this study. Participants with hypertension, hyperlipidemia, ischemic heart disease, or brain lacunar infarction without neurological symptoms were appropriately treated during the observation period. Patients with congestive heart failure, acute myocardial infarction, angina attack, chronic renal failure (serum creatinine>2.1 mg/dL), collagen disease, disseminated intravascular coagulation, acute stroke, varicose veins of the legs, venous thrombosis, acute inflammatory conditions, neoplasm, and recent trauma or surgery were excluded. CAVI and IMT were measured within 2 months after being enrolled. Patients showing symptoms of transient ischemic brain attacks, dizziness, vertigo, and muscle weakness of the extremities were extensively examined by brain MRI (magnetic resonance imaging). Patients showing lacunar infarction or ischemic patterns on MRI received a low dose of aspirin (81 mg/day). After the observation period, the number of patients showing clinical brain infarction was counted. Criteria for clinical brain infarction were as follows: Patients with both MRI spots on T1, T2, or especially diffusion-weighted imaging andconcurrently observed clinical symptoms including muscleweakness of the extremities, speech disturbance, or disturbance of the visual field. Stratification of participants was carried out according to the criteria of CAVI 8.0 and IMT 1.1 mm. That is, patients were divided into 2 groups: group A (mildly atherosclerotic patients: CAVI8.0 and IMT>1.1 mm). Moreover, Group B was divided into subgroups B, D+ and B, Dbased on whether the level of D-dimer exceeded 1.0 μg/mL or not. In group B, D+, the level of D-dimer was counted if it exceeded 1.0 μg/mL once or more during the observation period, whereas it did not exceed 1.0 μg/ml at all in subgroup B, D- during the period. Diseases and medications of patients are summarized in Table 1. This study was approved by the ethics committee of the Thrombosis Chemical Institute. Informed consent was obtained from all patients prior to the study.
| n | % | |
|---|---|---|
| Hypertension | 157 | 67.9 |
| Hyperlipidemia | 76 | 32.9 |
| Cerebral infarction | 77 | 33.3 |
| Ischemic heart disease | 65 | 28.1 |
| Diabetes mellitus | 38 | 16.4 |
| Ca blocker | 154 | 66.7 |
| ARB | 95 | 41.1 |
| ACE inhibitor | 21 | 9 |
| Thyazide | 52 | 22.5 |
| β-Blocker | 27 | 11.6 |
| α-Blocker | 1 | 0.4 |
| Nitrate | 28 | 12.1 |
| Statin | 70 | 30.3 |
| Aspirin | 71 | 30.7 |
Table 1: Diseases and medications of total subjects.
Body measurement: Height and weight were measured, and the body mass index (BMI: kg/m2) was calculated as an index of obesity. The systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded at rest, simultaneously with the measurement of CAVI.
Blood sampling: Blood sampling was carried out in the morning at the clinic. Serum concentrations of total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), creatinine (Cr), and blood urea nitrogen (BUN) were measured by standard laboratory procedures. Serum C-reactive protein (CRP) was measured with the latex immunoturbimetric method. Plasma hemoglobin A1C was measured by standard laboratory procedures. Plasma fibrinogen was measured by the thrombin coagulation time. Plasma D-dimer was measured by the latex immunoturbimetric method (Latecle D-dimer). These laboratory assays were performed at the Special Reference Laboratory Corporation (Tokyo, Japan).
Platelet aggregation: Platelet (Plt) aggregation was measured promptly after blood sampling using a whole-blood impedance aggregometer (Chrono-log, USA) [24] at the clinic.Collagen was added as a stimulator at a final concentration of 5 μg/mL. The degree of aggregation was directly expressed as Ω. The normal range of values was 15 ± 4.3 Ω.
CAVI: CAVI was measured in a supine position with the monitoring of electrocardiograms and phonocardiograms using CAVI-VaSera VS-1000 (Fukuda Denshi, Tokyo, Japan). The principle method and assay procedures were as described previously [25]. Briefly, cuffs were applied to the bilateral upper arms and ankles, with the subject lying supine and the head held in a midline position. After resting for 10 min, the measurement was started. After automatic measurement, the obtained data were analyzed using VSS-10 software (Fukuda Denshi), and the left and right CAVI values were calculated. The average values of the left t and right CAVI were used for analysis. CAVI in patients without signs of atherosclerosis was less than 8.0.
IMT: IMT evaluation was performed with an ultrasound device (Xario SSA-660A, Toshiba Medical Systems Co., Japan) using a 7.5- MHz probe equipped with a Doppler system [26]. IMT was measured on the left and right sides of the carotid artery within 1 cm before the bifurcation and at the bifurcation. The mean IMT was calculated from the four points of IMT.
MRI: Brain MRI (T1-weighted, T2-weighted, T2 star, FLAIR (fluid attenuated inversion recovery), and diffusion-weighted imaging) was performed using Philips gyroscan 1.5 tesla apparatus. All MR images were used for diagnosis by veteran brain surgeons andspecialyzedradiologists.
Statistical analysis: The skewness and kurtosis of data were checked. Mann-Whitney U tests were performed for medians. Student’s t-tests were used for means. The Kruskal-Wallis test was carried out for comparison of D-dimer of the different groups. Chi-square tests with Yates’ correction were carried out. Values are expressed as the mean ± standard deviation (SD) except for medians. A p-value of less than 0.05 was considered significant. All statistical analyses were performed using Excel Analysis.
Results
Stratification of the subjects by IMT and CAVI
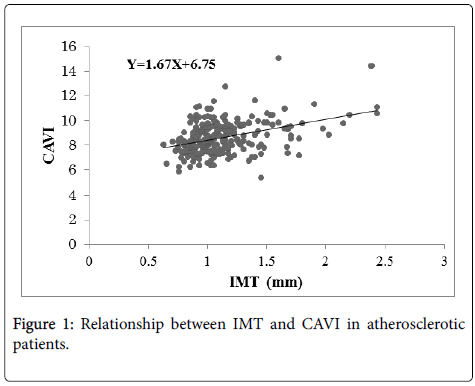
Figure 1 shows the relationship between IMT and CAVI in the atherosclerotic patients (n=284). The regression line is Y=1.67 X+6.75, r=0.39 (p<0.001).
Comparison of clinical parameters between mildly (A) and severely (B) atherosclerotic groups
Table 2 shows that values for age, SBP, pulse pressure, CAVI, and IMT were significantly higher in group B than group A, while the difference of values for male/female ratio, BMI, and DPB were not significant. The difference in age between group A (62.2 years old) and B (76.8 years old) was significant. It was revealed that laboratory data were higher in group B than group A, suggesting that the influence of age was not negligible.
| Group A | Group B | p | |
|---|---|---|---|
| n | 60 | 89 | |
| Age | 62.2 ± 9.3 | 76.8 ± 8.5 | ** |
| Male/Female | 20/40 | 27/62 | NS |
| BMI (kg/mm2) | 23.2 ± 3.3 | 23.1 ± 3.4 | NS |
| SBP (mmHg) | 137.8 ± 14.8 | 147.0 ± 19.0 | ** |
| DBP (mmHg) | 86.9 ± 9.7 | 85.0 ± 9.6 | NS |
| Pulse pressure (mmHg) | 50.9 ± 9.8 | 85.0 ± 9.6 | ** |
| CAVI | 7.3 ± 0.5 | 9.4 ± 1.2 | ** |
| IMT (mm) | 0.9 ± 0.1 | 1.4 ±0.3 | ** |
| Median | 0.9 | 1.3 | ** |
Table 2: Clinical parameters of groups A and B.
Comparison of laboratory data and the incidence of cerebral infarction between groups A and B
Table 3 shows that D-dimer of group B (0.62 ± 0.55 μg/mL) was 2- fold higher than that of group A (0.3 ± 0.12 μg/mL).However, the differences of TC, LDL-C, BUN, Cr, CRP, HbA1C, Alb, Hb, and urine icro alb were not large in the two groups. Moreover, the differences of LDL-C, TG, fibrinogen, Plt aggregation, UA, and TP were not significant. The incidence of cerebral infarction in group A was 0% (0/60), while that in group B was 5.9% (5/84), showing no significance (Chi-square test, Table 4). Therefore, group B was subdivided into two groups based on the value of D-dimer and a comparative study was further conducted of high (D-dimer>1.0 μg/mL) and low (Ddimer< 1.0μg/mL) D-dimer groups.
| Group A | Group B | p | |
|---|---|---|---|
| Total cholesterol (mg/dL) | 210.5 ± 26.8 | 197.2 ± 42.2 | * |
| HDL-cholesterol (mg/dL) | 61.5 ± 14. | 55.5 ± 15.0 | * |
| LDL-cholesterol (mg/dL) | 127.3 ± 36.5 | 117.3 ± 33.8 | NS |
| Triglycerides(mg/dL) | 119.0 ± 88.8 | 118.8 ± 68.9 | NS |
| Median | 94 | 97 | NS |
| BUN (mg/dL) | 15.5 ± 3.7 | 17.5 ± 6.2 | * |
| Creatinine (mg/dL) | 0.7 ± 0.2 | 0.8 ± 0.3 | * |
| Median | 0.65 | 0.7* | * |
| C-reactive protein (mg/dL) | 0.08 ± 0.1 | 0.15 ± 0.2 | * |
| Median | 0.05 | 0.07 | * |
| HbA1C (%) | 5.3 ± 0.4 | 5.8 ± 1.0 | ** |
| Median | 5.3 | 5.5 | ** |
| Fibrinogen (mg/dL) | 279.0 ± 49.2 | 297.1 ± 61.7 | NS |
| Plt aggregation(Ω) | 11.6 ± 5.5 | 11.6 ± 4.6 | NS |
| D-dimer (μg /mL) | 0.3 ± 0.15 | 0.62 ± 0.55 | ** |
| Median | 0.29 | 0.47 | ** |
| Uric acid (mg/dL) | 5.1 ± 1.3 | 5.3 ± 1.2 | NS |
| Total protein (g/dL) | 7.2 ± 0.4 | 7.1 ± 0.5 | NS |
| Albumin (g/dL) | 4.3 ± 0.3 | 4.1 ± 0.3 | ** |
| Hemoglobin (g/dL) | 13.4 ± 1.4 | 12.8 ± 1.2 | ** |
| Median | 13.2 | 12.8 | * |
| Urine micro alb (mg/g Cr) | 14.4 ± 13.8 | 69.8 ± 186.0 | * |
| Median | 11 | 15.3 | * |
Table 3: Laboratory data of groups A and B.
| Group A | Group B | |
|---|---|---|
| Cerebral infarction (+) | 0 | 5 |
| Cerebral infarction (-) | 60 | 84 |
Table 4: Incidence of cerebral infarction in groups A and B.
Comparison of clinical parameters and laboratory data between high and low D-dimer subgroups in severely atherosclerotic group
After the stratification of group B by the level of D-dimer, high (B, D +) and low (B, D-) D-dimer subgroups, between which age, male/ female ratio, BMI, blood pressure, CAVI, and IMT did not significantly differ, were obtained (Table 5). Moreover, Table 6 shows that D-dimer of subgroup B, D+ (1.5 μg/mL) was 3-fold higher than that of subgroup B, D- (0.3 μg/mL), while differences in TG, BUN, Cr, fibrinogen, UA, Alb, and Hb were small and other laboratory data did not differ.
| Group B, D-dimer+ | Group B, D-dimer- | p | |
|---|---|---|---|
| Age (years) | 80.4 ± 8.4 | 76.3 ± 8.0 | NS |
| Male/Female | 2/14 | 25/48 | NS |
| BMI (kg/m2) | 23.2 ± 3.2 | 23.1 ± 3.4 | NS |
| SBP (mmHg) | 142.4 ± 20.5 | 148.2 ± 19.5 | NS |
| DBP (mmHg) | 83.2 ± 9.9 | 85.2 ± 9.9 | NS |
| CAVI | 9.3 ± 0.9 | 9.5 ± 1.3 | NS |
| IMT (mm) | 1.5 ± 0.3, 1.38(Median) |
1.4 ± 0.3, 1.26(Median) |
NS NS |
Table 5: Comparison of clinical data between groups B, D+ and B, D-.
| Group B, D-dimer+ | GroupB, D-dimer- | p | |
|---|---|---|---|
| TC (mg/dL) | 210.1 ± 26.1 | 210.5 ± 36.8 | NS |
| HDL-C (mg/dL) | 56.4 ± 15.8 | 61.5 ± 14.6 | NS |
| LDL-C (mg/dL) | 121.0 ± 25.5 | 127.3 ± 36.5 | NS |
| TG (mg/dL) | 152.8 ± 102.8 | 119.0 ± 88.8 | * |
| Median | 121 | 92.5 | * |
| BUN (mg/dL) | 20.8 ± 9.0 | 15.5 ± 3.7 | * |
| Cr (mg/dL) | 0.9 ± 0.3 | 0.7 ± 0.2 | * |
| Median | 0.85 | 0.685 | * |
| CRP (mg/dL) | 0.12 ± 0.1 | 0.08 ± 0.1 | NS |
| Median | 0.11 | 0.07 | NS |
| A1C (%) | 5.7 ± 0.8 | 5.3 ± 0.4 | NS |
| Median | 5.4 | 5.55 | NS |
| Fibrinogen (mg/dL) | 331.6 ± 68.9 | 279.0 ± 49.2 | * |
| Plt aggregation ( Ω ) | 10.6 ± 4.0 | 11.6 ± 5.5 | NS |
| D-dimer(μg/mL) | 1.5 ± 0.8 | 0.3 ± 0.2 | ** |
| Median | 1.25 | 0.42 | ** |
| UA (mg/dL) | 5.8 ± 1.1 | 5.1 ± 1.3 | * |
| TP (g/dL) | 7.1 ± 0.5 | 7.2 ± 0.4 | NS |
| Alb (g/dL) | 4.1 ± 0.3 | 4.3 ± 0.3 | NS |
| Hb (g/dL) | 11.9 ± 1.1 | 13.4 ± 1.4 | ** |
| Median | 13 | 13.75 | ** |
| Urine microalb (mg/g Cr) | 151.0 ± 318.3 | 14.4 ± 13.8 | NS |
| Median | 12 | 42.5 | NS |
Table 6: Comparison of laboratory data between groups B, D+ and B, D-.
Comparison of the rate of aspirin intake and incidence of cerebral infarction in subgroups B, D+ and B, D-
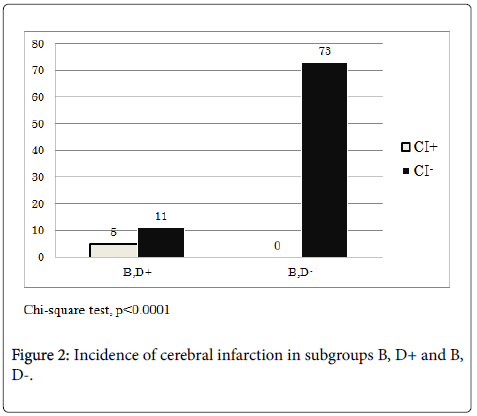
In order to elucidate the difference in the incidence of cerebral infarction between the two subgroups, we examined the rate of aspirin ingestion. The rate of aspirin intake in subgroup B, D+ was 43.8% (7/16), while that in subgroup B, D- was 45.2% (33/73), suggesting that the difference was not significant (Chi-square test). Moreover, we examined the incidence of cerebral infarction in the two subgroups during the observation period, revealing that in subgroup D, B+ it was 31.3% (5/16), while that in subgroup B, D- was 0/73 (Chi-square test, p<0.0001, Figure 2). The incidence of cerebral infarction was high in the high D-dimer subgroup in the severely atherosclerotic group, suggesting that the cerebral infarction monitoring system in combination with CAVI, IMT, and D-dimer was a clinically significant tool to detect cerebral infarction in severely atherosclerotic patients without atrial fibrillation.
Discussion
Atrial fibrillation and a high level of plasma D-dimer were significant risk factors for cerebral infarction [18,19]. The Framingham study revealed that the risk of stroke was increased 5-fold in nonrheumatic chronic atrial fibrillation patientscompared with controls without atrial fibrillation [2,27]. The score of CHADS2 is a good predictor of cerebral infarction in atrial fibrillation, and the incidence of cerebral infarction increased when its score was greater than [2,28]. Moreover, CHADS2 and CHA2DS2-VASc scores were associated with the incidence of cardioembolic stroke in nonvalvular fibrillation [29]. The CHADS2 score was also useful for estimating the risk of stroke or transient ischemic attack in hypertensive patients without atrial fibrillation [30]. However, an effective predictor of cerebral infarction without atrial fibrillation has yet to be fully studied. We reported that thrombosis such as cerebral infarction or myocardial infarction frequently occurred in severely atherosclerotic patients stratified by IMT and CAVI [31].
Recently, the clinical importance of the early detection or effective prevention of stroke has increased. For this purpose, it is significant to identify a higher risk group for cerebral infarction in atherosclerotic patients without atrial fibrillation.
Extraction of the high-risk group of cerebral infarction from atherosclerotic patients by IMT and CAVI is clinically significant, since both methods are associated with cerebral infarction [8-10,15,16]. Furthermore, a high level of plasma D-dimer was also associated with thrombosis [17], cerebral infarction [18,19] or cardioembolic stroke [32]. A comparison of data in groups A (mild) and B (severe) revealed that the incidence of cerebral infarction was not significantly different between the groups. Laboratory findings suggesting thrombosis were also not observed in the groups. Moreover, the age difference (14 years old) between groups A and B did not induce a significant difference in the incidence of cerebral infarction, suggesting that an age difference is not associated with the incidence. Therefore, in our efforts to devise an effective monitoring system for detecting cerebral infarction, we adopted D-dimer, since it has been reported as a sensitive detector of the presence of a thrombus [17] or thrombosis [33].
We could extract the most severely atherosclerotic and highly thrombotic patients using stratification of IMT, CAV, and D-dimer, and finally identified a significant difference in the incidence of cerebral infarction. In the subgroups B, D+ and B, D-, the rate of aspirin intake did not differ, indicating that cerebral infarction occurred without the influence of aspirin.
Regarding the stratification of atherosclerotic patients by CAVI and IMT, the incidences of cerebral infarction in subgroups B, D+ (CAVI>9.0, IMT>1.1 mm, D-dimer>1.0 μg/mL) and B, D- (CAVI<9.0, IMT<1.1 mm, D-dimer<1.0 μg/mL) were 3/7 (42.9%) and 1/47 (2.1%, chi-square test, p<0.01, data not shown in the results), respectively, suggesting that the difference in the level of CAVI (8.0 or 9.0) was not essential. The incidences of cerebral infarction in group B and subgroup B, D+ were 5.6and 31.3%, respectively, while that of apoplexy in the Japanese population aged more than 40 years is about 0.6% [34], suggesting that our methods to extract a high-risk group for cerebral infarction is highly effective. Incidences of stroke in paroxysmal and chronic atrial fibrillation were 2 and 5%, respectively [35]
D-dimer was associated with cerebral infarction with atrial fibrillation [19,20,2] and also a significant indicator to predict cardiovascular events in patients with atrial fibrillation [36]. The level of D-dimer is essentially significant for stratification, as described previously [31]. Although the association of cerebral infarction with CAVI [8,10,37] or IMT [13,16,38] was reported previously, stratification by triple-risk factors such as CAVI, IMT, and D-dimer has not been reported. Consequently, it could be significant for selecting high-risk patients for cerebral infarction and treating them appropriately.
In conclusion, the combination of CAVI, IMT, and D-dimer is a useful tool to detect cerebral infarction in atherosclerotic patients without atrial fibrillation.
References
- Kamel H, Okin PM, Elkind MSV, Ladecola C (2016) Atrial fibrillation and mechanisms of stroke. Stroke 47: 895-900.
- Wolf PA, Dawber TR, Thomas HE Jr, Kannel WB (1978) Epidemiologic assessment of chronic arterial fibrillation and risk of stroke: The Framingham Study. Neurology 28: 973-977.
- Reiffel JA (2014) Atrial fibrillation and stroke: epidemiology. Am J Med 127: 15-16.
- Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, et al. (2006) Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 113: 1213-1225.
- Hirai T, Sasayama S, Kawasaki T, Yagi S (1989) Stiffness of systemic arteries in patients with myocardial infarction. A non-invasive methods to predict severity of coronary atherosclerosis. Circulation 80: 78-86.
- Gatzka CD, Cameron JD, Kingwell BA, Dart AM (1998) Relation between coronary artery disease, aortic stiffness, and left ventricular structure in a population sample. Hypertension 32: 575-578.
- Miyoshi T, Doi M, Hirohara S, Sakane K, Kamikawa S, et al. (2010) Cardio-ankle vascular index is independently associated with the severity of coronary atherosclerosis and left ventricular function in patients with ischemic heart disease. J Atheroscler Thromb 17: 249-258.
- Suzuki J, Sakakibara R, Tomaru T, Tateno F, Kishi M, et al. (2013) Stroke and cardio-ankle vascular stiffness index. J Stroke Carebrovasc Dis 22: 171-175.
- Choi SY, Park HE, Seo H, Kim M, Cho SH, et al. (2013) Arterial stiffness using cardio-ankle vascular index reflects cerebral small vessel disease in healthy young and middle aged subjects. J Atheroscler Thromb 20: 178-185.
- Saji, N, Kimura K, Shimizu H, Kita Y (2012) Silent brain infarct is independently associated with arterial stiffness indicated by cardio-ankle vascular index (CAVI). Hypertens Res 35: 756-760.
- Crouse JR, Toole JF, McKinney WM, Dignan MB, Howard G, et al. (1987) Risk factors for extracranial carotid artery atherosclerosis. Stroke 18: 990-996.
- Poli A, Tremoli E, Colombo A, Pignoli P, Paoletti R (1988) Ultrasonographic measurement of the common carotid artery wall thickness in hypercholesterolemic patients.A new model for the quantitation and follow-up of preclinical atherosclerosis in living human subjects. Atherosclerosis 70: 253-261.
- Robertson CM, Fowkes FGR, Price JF (2012) Carotid intima-media thickness and the prediction of vascular events. Vasc Med 17: 239-248.
- Inlesias dDA, Bots ML, Grobbee DE, Hofman A, Witteman JCM (2002) Carotid intima-media thickness at different sites: relation to incident myocardial infarction; The Rotterdam Study. Eur Heart J 23: 934-940.
- Hollander M, Hak AE, Koudstaal PJ, Bots ML, Grobbee DE, et al. (2003) Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam Study. Stroke 34: 2367-2372.
- Rosvall M, Janzon L, Berglund G, Engstrom G, Hedblad B (2005) Incidence of stroke is related to carotid IMT even in the absence of plaque. Atherosclerosis 179: 325-331.
- Tripodi A (2011) D-Dimer testing in laboratory practice. Clin Chem 57: 1256-1262.
- Nozawa T, Inoue H, Hirai T, Iwasa A, Okumura K, et al. (2006) D-dimer level influences thromboembolic events in patients with arterial fibrillation. Int J Cardiol 109: 59-65.
- Wu N, Chen X, Cai T, Wu L, Xiang Y, et al. (2015) Association of inflammatory and hemostatic markers with stroke and thromboembolic events in atrial fibrillation: a systematic review and meta-analysis. Can J Cardiol 31: 278-286.
- Matsumoto M, Sakaguchi M, Okazaki S, Furukado S, Tagaya M, et al. (2013) Relationship between plasma D-Dimer level and cerebral infarction volume in patients with nonvalvular atrial fibrillation. Cerebrovasc Dis 35: 64-72.
- Gustafsson C, Blomback M, Britton M, Hamsten A, Svensson J (1990) Coagulation?factors?and?the?increased?risk?factors of stroke?in?non-valvular?atrial?fibrillation. Stroke 21: 47-51.
- Inoue H, Nozawa T, Okumura K, Jong-Dae L, Shimizu A, et al. (2004) Prothrombotic activity is increased in patients with nonvalvular atrial fibrillation and risk factors for embolism. Chest 126: 687-692.
- Nozawa T, Inoue H, Hirai T, Iwasa A, Okumura K, et al. (2006) D-Dimer level influences thromboembolic events in patients who have nonvalvular atrial fibrillation. Int J Cardiol 109: 59-65.
- Sathiropas P, Marbet G, Sahaphong S, Duckert F (1988) Detection of small inhibitory effect of acetylsalicylic acid (ASA) by platelet impedance aggregometry in whole blood. Thromb Res 51: 55-62.
- Shirai K, Utino J, Otsuka K, Takata M (2006) A novel blood pressure independent arterial wall stiffness parameter; cardio-ankle vascular index(CAVI). J Atheroscler Thromb 12: 101-107.
- Caplan LR, Ringelstein EB (1999) Color-coded douplex ultrasonography of the cerebral vessels: Atlas and Manual. Stuttgart: Schattauer Verlagesgesellshaft mbH pp: 60-112.
- Petersen P, Madsen EB, Brun B, Pedersen F, Gyldensted C, et al. (1987) Silent cerebral infarction in chronic atrial fibrillation. Stroke 18: 1098-1100.
- Santos C, Pereira T, Conde J (2013) CHADS2 score in predicting cerebrovascular events- A meta-analysis. Arg Bras Cardiol 100: 294-301.
- Duguchi I, Hayashi T, Ohe Y, Kato Y, Fukuoka T, et al. (2014) CHADS2 score/CHA2DS2-VASc score and major artery occlusion in cardioembolic stroke patients with nonvalvular atrial fibrillation. Int J Stroke 9: 576-79.
- Morillas P, Pallares V, Fácila L, Llisterri JL, Sabastián ME, et al. (2015) The CHADS2 score to predict stroke risk in the absence of atrial fibrillation in hypertensive patients aged 65 years or older. Rev Esp Cardiol 68: 485-491.
- Hayashi S (2010) Significance of Plasma D-dimer in relation to the severity of atherosclerosis among patients evaluated by non-invasive indices of cardio-ankle vascular index and carotid intima-media thickness. Int J Haematol 92: 76-82.
- Folsom AR, Gottesman R, Appiah D, Shahar E, Mosley T (2016) Plasma d-Dimer and incident ischemic stroke and coronary heart disease: The Atherosclerosis Risk in Communities Study. Stroke 47: 18-23.
- Carter CJ (1994) The natural history and epidemiology of venous thrombosis. Prog Cardiovasc Dis 36: 423-438.
- Vital statics in Japan (2006) Statics and Information Department, Ministry of Health, Labour and Wellfare, Japan.
- Petersen P, Godtfredsen J (1986) Embolic complications in paroxysmal atrial fibrillation. Stroke 17: 622-626.
- Vene N, Mavri A, Kosmeli K, Stegnar M (2003) High D-dimer levels predict cardiovascular events in patients with chronic atrial fibrillation during oral anticoagulant therapy. Thromb Haemost 90: 1163-1172.
- Shirai K, Hiruta N, Song M, Korosu T, Suzukin J, et al. (2011) Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: Theory, Evidence and Perspectives: J Atheroscler Thromb 18: 924-938.
- Lorenz MW, Markus HS, Bots ML, Rosvall M, Siyzer M (2007) Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 115: 459-467.
Citation: Hayashi S (2017) Useful Method to Monitor Cerebral Infarction in Atherosclerotic Patients without Atrial Fibrillation by the Combination of Carotid Intima-Media Thickness, Cardio-Ankle Vascular Index, and Plasma D-dimer. Atheroscler Open Access 2: 113.
Copyright: © 2016 2017 Hayashi S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 3222
- [From(publication date): 0-2017 - Aug 25, 2025]
- Breakdown by view type
- HTML page views: 2352
- PDF downloads: 870