Using GC-MS to Analyze Bio-Oil Produced from Pyrolysis of Agricultural Wastes - Discarded Soybean Frying Oil, Coffee and Eucalyptus Sawdust in the Presence of 5% Hydrogen and Argon
Received: 26-Jan-2016 / Accepted Date: 17-Feb-2016 / Published Date: 24-Feb-2016 DOI: 10.4172/2155-9872.1000300
Abstract
Pyrolysis is a thermal process for converting various biomasses, wastes and residues to produce high-energydensity fuels (bio-oil). In this paper, we have done some important analysis of bio-oil which is obtained from the pyrolysis of agricultural wastes - discarded soybean frying oil, coffee and eucalyptus sawdust in the presence of 5% Hydrogen and Argon. The bio oil was obtained in one step pyrolysis in which temperature of the system kept 15°C and then increased up to 800°C but in two step condensation processes. 1st condensation step is done on temperature 100°C and 2nd is done on 5°C. So we got two types of fractions, HTPO (Oil condensed at high temperature 100°C after pyrolysis) and LTPO (Oil condensed at low temperature 5°C after pyrolysis). After pyrolysis the thermal cracking is done for both types of oil on the same two temperatures, then we again got two type of fractions HTCO (high temperature 100°C condensed oil after cracking) and LTCO (Low temperature 5°C condensed oil after cracking), these fractions are distillated and analyzed in GC-MS. The resulted compounds are given in the paper and are explained with the help of graphs and tables. The ultimate aim of hydrogenation and Argon is to improve stability and fuel quality by decreasing the contents of organic acids and aldehydes as well as other reactive compounds, as oxygenated and nitrogenated species because they not only lead to high corrosiveness and acidity, but also set up many obstacles to applications.
Keywords: Chromatographic analysis; GC/MS; Biomass pyrolysis; Bio-oil production
303203Abbreviations
CSSB: Bio-oil produced from coffee, sawdust and discarded soybean frying oil; GC/MS: Gas chromatogram/mass spectrometer; HTPO: Oil condensed at 100°C high temperature after pyrolysis; LTPO: Oil condensed at 5°C low temperature after pyrolysis; HTCO: Oil condensed at 100°C high temperature after cracking; LTCO: Oil condensed at 5°C Low temperature after cracking
Introduction
Now-a-days Bio-Oils (Biodiesel or Biofuels) are becoming more famous and attractive for the people in all over the world because of their good aspects for the people and environment around us. Biodiesel is an oxygenated fuel consisting of long chain fatty acid which contain 10-15% oxygen by weight [1,2] and it contains neither sulfur, nor aroma. These facts lead biodiesel to enhance more complete combustion and less emission of particulate matter. The biomass pyrolysis process is an economically feasible option for producing chemicals and/or fuels [3,4]. The bio-oil resulting from the pyrolysis process consists of a mixture of more than 300 organic compounds [5]. In terms of environmental issue biodiesel is more adoptable compare to fossil fuel as it forms low carbon and smoke which are responsible for global warming [6,7]. On the other hand biodiesel has higher molecular weight, density, viscosity and pour point than conventional diesel fuel [8,9]. Higher molecular weight and viscosity of biodiesel causes low volatility and poor fuel atomization, injector coking, piston ring sticking and leading incomplete combustion [10] as well as it has cold flow property which is a barrier to use it in cold or chill weather [11] anyhow the best benefit of Bio-oils is that they are preparing from renewable sources like corpse, plants, trees and residues etc. Approximately 100 years ago, Rudolf Diesel tested Bio oil as the fuel for his engine that was available with him [12,13]. According to scientists and researchers there are 350 oil containing crops and plants identified, among them only soybean, rapeseed, coffee, sunflower, cottonseed, peanut, safflower, and coconut oils are considered that they have the potential and quality of alternative fuels for diesel engines [14,15]. Bio oils have the capacity to substitute for a part or fraction of the petroleum products, distillates and petroleum based petrochemicals in the future. Due to being more expensive than petroleum, bio-oil fuels are nowadays not petroleum competitive fuels. However, due to the misuse, high expenditure and increases in petroleum prices and the uncertainties concerning petroleum availability, there is renewed interest in using Bio-oils in Diesel engines [16]. The emergence of transesterification can be dated back as early as 1846 when Rochieder described glycerol preparation through methanolysis of castor oil and since that time, alcoholysis has been studied in many parts of the world. Scientists, researchers have also investigated the important reaction conditions and parameters on the alcoholysis of triglycerides, such as tallow, fish oils, sunflower, soybean, rapeseed, linseed oils, cottonseed, sunflower, safflower, and peanut [17,18]. Soybean oil was transesterified into ethyl and methylesters, and comparisons of the performances of the fuels with diesel were made [19,20]. Also, methylesters have been prepared from palm oil by transesterification using methanol in the presence of a catalyst (NaOH) or (KOH) in a batch reactor [21]. Ethan oil is a preferred alcohol in the transesterification process compared to methanol because it is derived from natural agricultural products and is renewable and biologically less objectionable in the environment. The success of rapeseed ethylester production would mean that biodiesel`s two main raw materials would be agriculturally produced, renewable and environmentally friend [22].
Methyl, ethyl, 2-propyl and butyl esters were prepared from canola and linseed oils through transesterification using KOH and/or sodium alkoxides as catalysts. In addition, methyl and ethyl esters were prepared from rapeseed and sunflower oils using the same catalysts [23,24].
Experimental
Materials: Discarded soybean frying oil, coffee, eucalyptus sawdust and other reagents
Discarded soybean frying oil, coffee grounds and eucalyptus sawdust were collected from Porto Alegre a Brazilian city. The biooil was obtained by pyrolysis of a mixture (1:1:1 in mass) of discarded soybean frying oil, coffee grounds and eucalyptus sawdust. The frying oil was mixed to the solids after their granulometric reduction (till 0.21 mm). Calcium oxide was added to this mixture (at 20% in mass) and sufficient amount of water to produce a malleable mass that could be fixed and conformed in cylinders (50 mm × 180 mm). After building the cylinders, they were dried at environmental temperature during 3 days. Before the pyrolysis, the system is purged during 20 minutes with Argon with 5% of hydrogen (100 mL/min). The ultimate aim of hydrogenation and Argon is to improve stability and fuel quality by decreasing the contents of organic acids and aldehydes as well as other reactive compounds, as oxygenated and nitrogenated species because if we were not used H and Ar then we found above species which not only lead to high corrosiveness and acidity, but also set up many obstacles to applications.
Production of CSSB
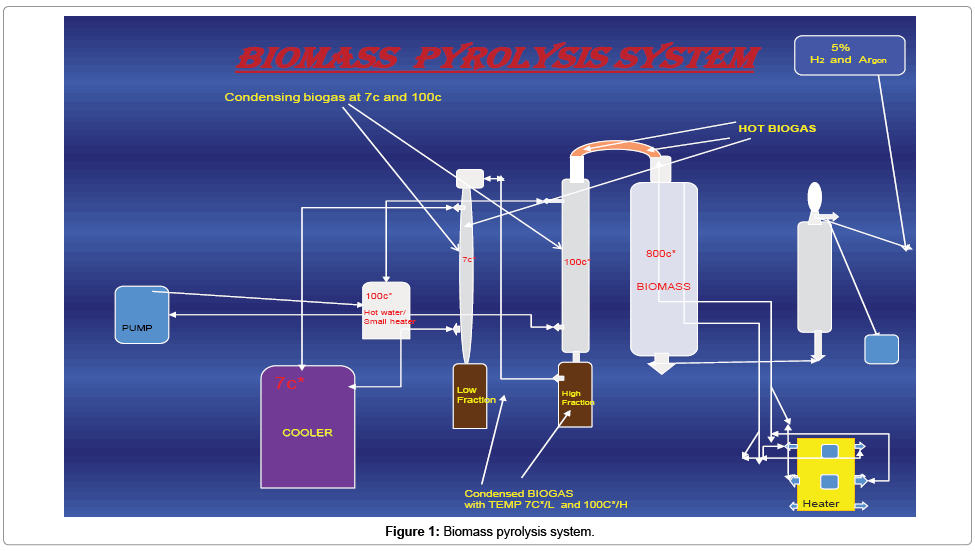
The bio-oil was produced from the pyrolysis of discarded soybean frying oil, coffee grounds and eucalyptus sawdust in the presence of 5% hydrogen and argon. A round block shape structure of sample was made inside the filter paper from (filter paper as side wall of the sample block to keep the biomass tight) biomass, while the weight of this sample is kept 400 g after preparation of this sample block it was kept inside a stainless steel chamber of pyrolysis system which is further connected to two other chambers which are shown in diagram in Figure 1.
The temperature of chamber which has biomass was increased from 15°C to 800°C with help of heater, temperature controller cabinet, and condenser, through which biomass was converted to biogas and then the biogas was condensed in other two chambers which condensed fractions of biogas to bio-oil on temperature 100°C and 5°C respectively, the two condensed fractions from these chambers (HTPO and LTPO) were collected and introduced to further analysis.
GC-MS analysis of HTPO and LTPO (CSSB)
The bio-oil identification and composition determination were performed on a GC Agilent series 6890 with a Agilent mass selective detector of series 5973. A capillary polar wax column, polyethylene glycol (PEG)-coated (length of 30 m, internal diameter of 0.25 mm, and film thickness of 0.25 μm).
Chromatographic conditions were as follows:
Injection volume of 0.2 μL, oven at 40°C (1 min) 6°C min−1 up to 300°C (10/Min) split mode with a ratio of 100:1 and injection temperature of 290°C. Time taken was 54.3 minutes, He (helium) as carrier gas with a flow rate of 2.9 mL min−1.
GC-MS chromatograms of CSSB
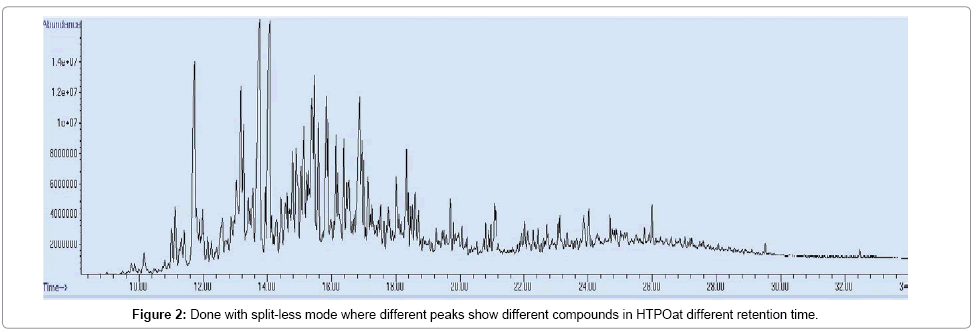
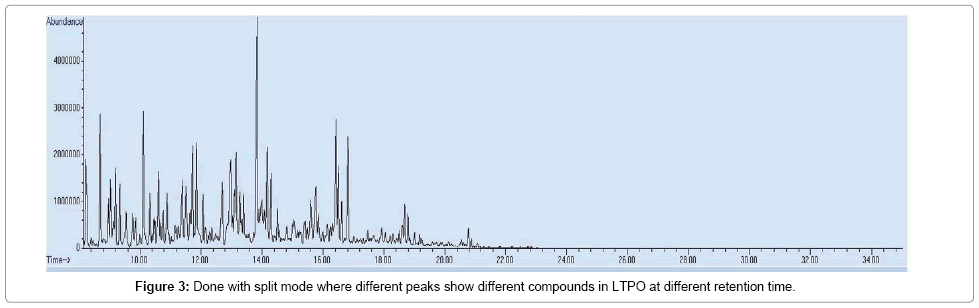
Below Figures 2 and 3 are different chromatograms of HTPO and LTPO respectively which show different peaks which show different compounds in both cases.
Results and Discussion
Chemical composition of CSSB
CSSB is a dark and sticky liquid the compounds detected in CSSB can be classified into hydrocarbons, alcohols, phenol, ethers, aldehydes, ketones, carboxylic acids, and other esters. But large peaks of GC/MS mostly shows aromatic, aliphatic and cyclic hydrocarbons while small peaks show other groups. Library match used for identification of compounds based on probability score and each compound was detected very clearly and with high probability value. According to GC/MS analysis summarized in Tables 1 and 2 mostly aromatics and aliphatic groups were enriched in the sample. After GC/MS analysis each peak of chromatogram was matched with library one by one, where different peaks showed different Aliphatic and Aromatic compounds.
| No | Name | Formula | Retention time |
|---|---|---|---|
| 1 | Benzene, 1-ethyl-3-methyl- | C9H12 | 8.18 |
| 2 | Benzene, 1,2,3-trimethyl- | C9H12 | 9 |
| 3 | Decane | C10H22 | 9.15 |
| 4 | 2-Decene, (Z)- | C10H20 | 9.32 |
| 5 | cis-3-Decene | C10H20 | 9.52 |
| 6 | Benzene, 1,2,3-trimethyl- | C9H12 | 9.72 |
| 7 | Benzene, 2-propenyl- | C9H10 | 9.82 |
| 8 | Indane | C9H10 | 10.17 |
| 9 | Indene | C9H8 | 10.3 |
| 10 | Benzene, butyl- | C10H14 | 10.52 |
| 11 | Benzene, 1,2-diethyl- | C10H14 | 10.72 |
| 12 | 2,4-Dimethylstyrene | C10H12 | 11.3 |
| 13 | Undecane | C11H24 | 11.7 |
| 14 | 3-Undecene, (Z)- | C11H22 | 11.82 |
| 15 | 3-Undecene, (Z)- | C11H22 | 12.05 |
| 16 | Benzene, 4-ethenyl-1,2-dimethyl- | C10H12 | 12.67 |
| 17 | 1H-Indene, 2,3-dihydro-4-methyl- | C10H12 | 12.95 |
| 18 | Benzene, pentyl- | C11H16 | 13.15 |
| 19 | Naphthalene, 1,2,3,4-tetrahydro- | C10H12 | 13.25 |
| 20 | Benzene, (1-methylbutyl)- | C11H16 | 13.35 |
| 21 | Azulene | C10H8 | 13.75 |
| 22 | Dodecane | C12H26 | 14.15 |
| 23 | 6-Dodecene, (Z)- | C12H24 | 14.25 |
| 24 | 3-Dodecene, (Z)- | C12H24 | 14.5 |
| 25 | Benzene, hexyl- | C12H18 | 15.55 |
| 26 | Benzene, (1-methylpentyl)- | C12H18 | 15.7 |
| 27 | Naphthalene, 1-methyl- | C11H10 | 16.35 |
| 28 | Tridecane | C13H28 | 16.47 |
| 29 | 3-Tridecene, (E)- | C13H26 | 16.57 |
| 30 | 1H-Indene, 1-ethylidene- | C11H10 | 16.75 |
| 31 | Naphthalene, 2-methyl- | C11H10 | 16.85 |
| 32 | 1-Isopropenylnaphthalene | C13H12 | 16.95 |
| 33 | Tetradecane | C14H30 | 18.65 |
| 34 | 3-Tetradecene, (E)- | C14H28 | 18.75 |
| 35 | Pentadecane | C15H32 | 20.72 |
Table 1: Aliphatic and Aromatic Hydrocarbons detected in HTPO with their retention time.
| No | Name | Formula | Retention time |
|---|---|---|---|
| 1 | 3-Undecene, (Z)- | C11H22 | 11.45 |
| 2 | Undecane | C11H24 | 11.65 |
| 3 | 5-Undecene, (Z)- | C11H22 | 11.8 |
| 4 | 3-Dodecene, (Z)- | C12H24 | 13.9 |
| 5 | 3-Dodecene, (Z)- | C12H24 | 14.05 |
| 6 | Dodecane | C12H26 | 14.15 |
| 7 | 3-Dodecene, (E)- | C12H24 | 14.25 |
| 8 | 3-Dodecene, (Z)- | C12H24 | 14.5 |
| 9 | 2-Tridecene, (Z)- | C13H26 | 16.3 |
| 10 | Tridecane | C13H28 | 16.45 |
| 11 | 3-Tridecene, (E)- | C13H26 | 16.55 |
| 12 | 3-Tetradecene, (E)- | C14H28 | 18.5 |
| 13 | Tetradecane | C14H30 | 18.65 |
| 14 | 3-Tetradecene, (E)- | C14H28 | 19 |
| 15 | Pentadecane | C15H32 | 20.72 |
| 16 | Hexadecane | C16H34 | 22.7 |
| 17 | Heptadecane | C17H36 | 24.58 |
Table 2: Aliphatic hydrocarbons in CSSB and their retention time.
Enrichment of chemicals in CSSB sample
According to GC/MS analysis summarized in Tables 1 and 3, C11-C17 alkanes, alkene, cyclic hydrocarbons and aromatic hydrocarbons were enriched in the CSSB sample.
| No | Name of compound | Formula | Retention time |
|---|---|---|---|
| 1 | Benzene, 1-methyl-4-(1-methylethyl)- | C10H14 | 11.3 |
| 2 | 3-Undecene, (Z)- | C11H22 | 11.45 |
| 3 | Undecane | C11H24 | 11.65 |
| 4 | 5-Undecene, (Z)- | C11H22 | 11.8 |
| 5 | Benzene, 4-ethenyl-1,2-dimethyl- | C10H12 | 12.65 |
| 6 | 1H-Indene, 2,3-dihydro-5-methyl- | C10H12 | 12.9 |
| 7 | 7-Methyl-1,2,3,5,8,8a-hexahydronaphthalene | C11H16 | 13 |
| 8 | 2-Methylindene | C10H10 | 13.1 |
| 9 | Benzene, pentyl- | C11H16 | 13.13 |
| 10 | Naphthalene, 1,2,3,4-tetrahydro- | C10H12 | 13.25 |
| 11 | Benzene, (1-methylbutyl)- | C11H16 | 13.38 |
| 12 | Naphthalene | C10H8 | 13.75 |
| 13 | 3-Dodecene, (Z)- | C12H24 | 13.9 |
| 14 | 3-Dodecene, (Z)- | C12H24 | 14.05 |
| 15 | Dodecane | C12H26 | 14.15 |
| 16 | 3-Dodecene, (E)- | C12H24 | 14.25 |
| 17 | 3-Dodecene, (Z)- | C12H24 | 14.5 |
| 18 | 2-Ethyl-2,3-dihydro-1H-indene | C11H14 | 14.95 |
| 19 | Benzene, hexyl- | C12H18 | 15.55 |
| 20 | Benzene, (1-methylpentyl)- | C12H18 | 15.7 |
| 21 | 2-Tridecene, (Z)- | C13H26 | 16.3 |
| 22 | Naphthalene, 1-meth yl- | C11H10 | 16.35 |
| 23 | Tridecane | C13H28 | 16.45 |
| 24 | 3-Tridecene, (E)- | C13H26 | 16.55 |
| 25 | 1H-Indene, 1-ethylidene- | C11H10 | 16.75 |
| 26 | Benzene, heptyl- | C13H20 | 17.9 |
| 27 | 1-Methyl-2-n-hexylbenzene | C13H20 | 18 |
| 28 | Naphthalene, 2-ethyl- | C12H12 | 18.6 |
| 29 | 3-Tetradecene, (E)- | C14H28 | 18.5 |
| 30 | Tetradecane | C14H30 | 18.65 |
| 31 | 3-Tetradecene, (E)- | C14H28 | 19 |
| 32 | Naphthalene, 1,7-dimethyl- | C12H12 | 19.15 |
| 33 | Pentadecane | C15H32 | 20.72 |
| 34 | n-Nonylcyclohexane | C15H30 | 21.8 |
| 35 | Hexadecane | C16H34 | 22.7 |
| 36 | Cyclopentane, undecyl- | C16H32 | 22.8 |
| 37 | Heptadecane | C17H36 | 24.58 |
Table 3: Aliphatic and Aromatic Hydrocarbons detected in LTPO with their retention time.
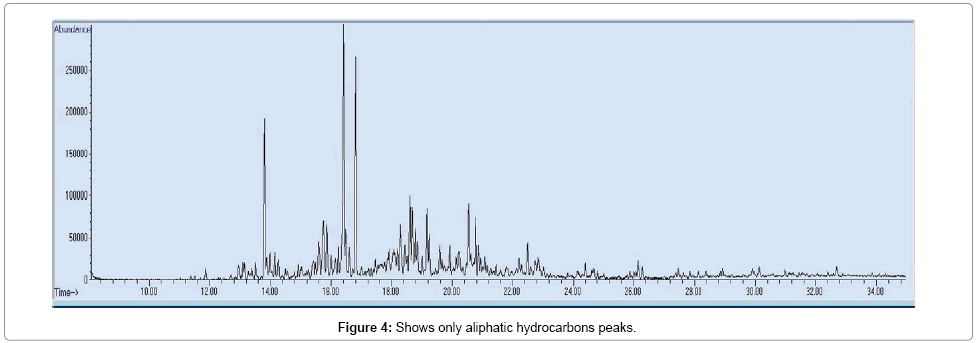
Enrichment of C11-C17 aliphatic hydrocarbons (alkane, alkene, cyclic): As Tables 1 and 4 shows, aliphatic hydrocarbons with C11–C17 are predominant in the sample with a % area of 51.255, 34.843, and 31.204 in LTCO, LTPO and HTCO respectively. Figure 4 and Table 2 show only aliphatic hydrocarbons in CSSB.
| No | Name | Formula | Retention time |
|---|---|---|---|
| 1 | Benzene, 1-methyl-4-(1-methylethyl)- | C10H14 | 11.3 |
| 2 | Benzene, 4-ethenyl-1,2-dimethyl- | C10H12 | 12.65 |
| 3 | 1H-Indene, 2,3-dihydro-5-methyl- | C10H12 | 12.9 |
| 4 | 7-Methyl-1,2,3,5,8,8a-hexahydronaphthalene | C11H16 | 13 |
| 5 | 2-Methylindene | C10H10 | 13.1 |
| 6 | Benzene, pentyl- | C11H16 | 13.13 |
| 7 | Naphthalene, 1,2,3,4-tetrahydro- | C10H12 | 13.25 |
| 8 | Benzene, (1-methylbutyl)- | C11H16 | 13.38 |
| 9 | Naphthalene | C10H8 | 13.75 |
| 10 | 2-Ethyl-2,3-dihydro-1H-indene | C11H14 | 14.95 |
| 11 | Benzene, hexyl- | C12H18 | 15.55 |
| 12 | Benzene, (1-methylpentyl)- | C12H18 | 15.7 |
| 13 | Naphthalene, 1-methyl- | C11H10 | 16.35 |
| 14 | 1H-Indene, 1-ethylidene- | C11H10 | 16.75 |
| 15 | Benzene, heptyl- | C13H20 | 17.9 |
| 16 | 1-Methyl-2-n-hexylbenzene | C13H20 | 18 |
| 17 | Naphthalene, 2-ethyl- | C12H12 | 18.6 |
| 18 | Naphthalene, 1,7-dimethyl- | C12H12 | 19.15 |
| 19 | n-Nonylcyclohexane | C15H30 | 21.8 |
| 20 | Cyclopentane, undecyl- | C16H32 | 22.8 |
Table 4: Main Aromatic compounds detected in CSSB and their retention time.
Enrichment of aromatic hydrocarbons: As Table 4 and Figure 5 show only aromatic hydrocarbons detected in CSSB and also occupied large part of the CSSB with in the sample with a % area of 0.246, 30.008, 32.241, 24.892 in HTPO, LTCO, LTPO and HTCO respectively.
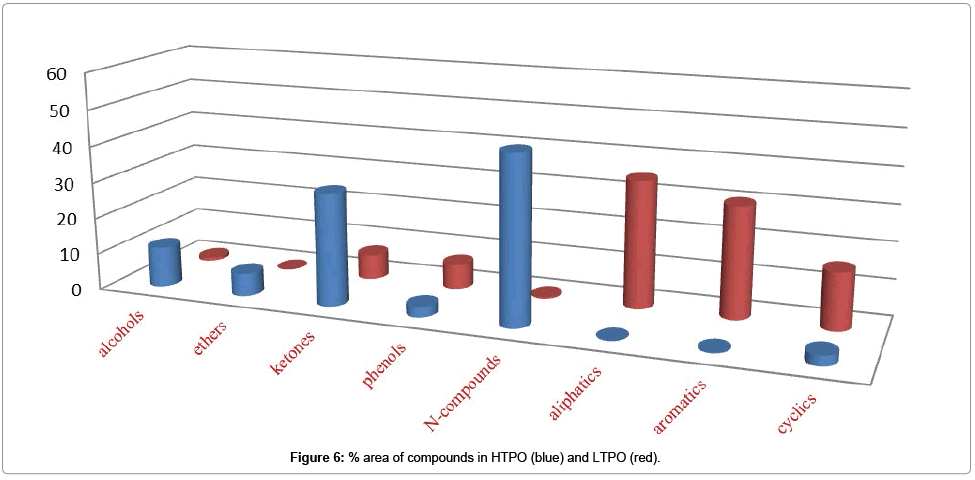
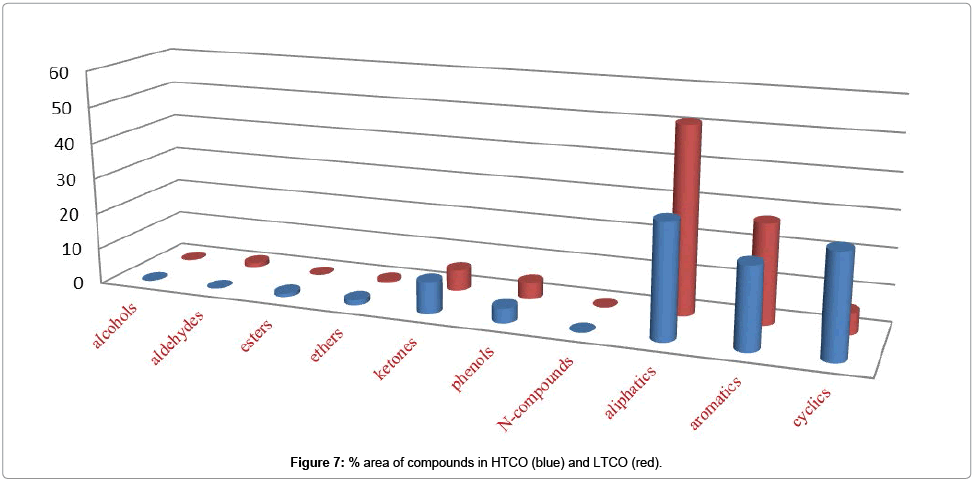
Enrichment of other compounds: Alcohols, Aldehydes, Ketones, Ethers, Esters, Phenols, and Nitrogenous also contained some part of the CSSB while in these classes Phenols and ketones were occupied more space as compared to others as shown in Table 5. Which mean that in these we also have Phenols and ketones, which need to be separated.
Figure 6 shows % area of alcohols, ethers, ketones, phenols, N-compounds, aliphatic, aromatic and cyclic compounds in HTPO (blue) and LTPO (red) while Figure 7. Shows % area of alcohols, ethers, ketones, phenols, N-compounds, aliphatic, aromatic and cyclic compounds in HTCO (blue) and LTCO (red) and their detail is also given in Table 5.
| Different Classes of compounds | % Area | |||
|---|---|---|---|---|
| HTCO | LTPO | LTCO | HTPO | |
| Alcohols | n.d | 0.894 | 0.168 | 10.930 |
| Aldehydes | n.d. | 0.416 | 1.289 | n.d. |
| Ketones | 9.088 | 7.92 | 6.526 | 30.827 |
| Ethers | 1.459 | n.d. | 0.468 | 6.263 |
| Esters | 0.921 | n.d. | n.d. | n.d. |
| Phenols | 4.018 | 6.809 | 4.458 | 2.858 |
| Nitrogenous | n.d. | 0.354 | n.d. | 45.779 |
| Aromatics hydrocarbons | 24.892 | 32.241 | 30.008 | 0.246 |
| Cyclic hydrocarbons | 28.319 | 16.420 | 5.829 | 3.096 |
| Aliphatic hydrocarbons | 31.304 | 34.843 | 51,255 | n.d. |
Table 5: % area of different compounds detected in CSSB and its Graphical representation.
Conclusion
More than 120 compounds were detected in the CSSB. Among them, aromatic, aliphatic and cyclic hydrocarbons, especially alkanes, alkenes and benzene containing compounds were dominant. A laboratory scale effort is made in this work however to improve efficiency and process thus, this process can be successfully applied in large-scale operations because the demand for liquid transportation fuels is increasing day by day, and biofuels might be one of the best solutions for this problem. Technologies for converting biomass to biodiesel also are at various stages of development, which include the pretreatment of biomass. Although cost of biomass may be high or the costs of processing it can be high but for the time being it may be an alternative for fossil fuels, Future work is going to improve the recovery of phenols, ketones and other chemicals from the CSSB.
Acknowledgements
The authors want to thank CNPq, Brazil.
References
- Shahabuddin M, Kalam M, Masjuki H, Bhuiya M, Mofijur M (2012) An experimental investigation into biodiesel stability by means of oxidation and property determination. Energy 44: 616-622.
- Puhan S, Vedaraman N, Sankaranarayanan G, Ram BVB (2005) Performance and emission study of Mahua oil (madhuca indica oil) ethyl ester in a 4-stroke natural aspirated direct injection diesel engine. Renew Energy 30: 1269-1278.
- Vispute TP, Zhang H, Sanna A, Xiao R, Huber GW (2010) Renewable chemical commodity feedstocks from integrated catalytic processing of oils. Science 330: 1222-1227.
- Wright MM, Daugaard DE, Satrio JA, Brown RC (2010) Techno-economic analysis of biomass fast pyrolysis to transportation fuels. Fuel 89: S2-S10.
- Mythili R, Venkatachalam P, Subramanain P, Uma D (2013) Characterization of bioresidues for bioil production through pyrolysis. Bioresour Technol 138: 71-78.
- Murayama T, Oh YT, Miyamoto N, Chikahisa T, Takagi N, et al. (1984) Low carbon, lower buildup, low smoke, and efficient diesel operation with vegetable oils by conversion to mono-esters and blending with diesel oil or alcohols. SAE 841161: 292-302.
- Abbaszaadeh A, Ghobadian B, Omidkhah MR, Najafi G (2012) Current biodiesel production technologies: a comparative review. Energy Convers Manage 63: 138-148.
- Ashraful AM, Masjuki HH, Kalam MA, Rizwanul Fattah IM, Imtenan S, et al. (2014) Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: a review. Energy Convers Manage 80: 202-228.
- Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition Renew Sustain Energy Rev 16: 143-169.
- Bagby (1987) Vegetable oils for diesel fuel: opportunities for development. ASAE, pp: 87-1588.
- Sivalakshmi S, Balusamy T (2013) Effect of biodiesel and its blends with diethyl ether on the combustion, performance and emissions from a diesel engine. Fuel 106: 106-110.
- Shay EG (1993) Diesel fuel from vegetable oils: status and opportunities. Biomass Bioenergy 4: 227-242.
- Özbay N, Uzun BB, Varol EA, Pütün AE (2006) Comparative analysis of pyrolysis oils and its subfractions under different atmospheric conditions. Fuel Processing Technology 87: 1013-1019.
- Goering CE, Schwab AW, Daugherty MJ, Pryde EH, Heakin AJ (1982) Fuel properties of eleven vegetable oils. Trans ASAE 25: 1472-1477.
- Pryor RW, Hanna MA, Schinstock JL, Bashford LL (1983) Soybean oil fuel in a small Diesel engine. Trans ASAE 26: 0333-0337.
- Giannelos PN, Zannikos F, Stournas S, Lois E, Anastopoulos G (2002) Tobacco seed oil as an alternative Diesel fuel: physical and chemical properties. Ind Crop Prod 16: 1-9.
- Formo MW (1979) Physical properties of fats and fatty acids. In: Bailey’s industrial oil and fat products. John Wiley and Sons, New York, USA.
- Acaroglu M, Oguz H, Ogut H (2001) An investigation of the use of rapeseed oil in agricultural tractors as engine oil. Energy Sources 23: 823-830.
- Diasakou M, Louloudi A, Papayannakos N (1998) Kinetics of the non-catalytic transesterification of soybean oil. Fuel 77: 1297-1302.
- Adams C, Peters JF, Rand MC, Schorer BJ, Ziemke MC (1983) Investigation of soybean oil as a Diesel fuel extender: endurance tests. JAOCS 60: 1574-1579.
- Darnoko D, Cheryan M (2000) Kinetics of palm oil transesterification in a batch reactor. JAOCS 77: 1263-1267.
- Isi Gigur A, Karaosmonoglu F, Aksoy HA (1994) Methyl ester from safflower seed oil of Turkish origin as a biofuel for Diesel engines. Appl Biochem Biotechnol 45: 103-112.
- Freedman B, Pryde EH (1982) Fatty esters from vegetable oils for use as a Diesel fuel. In: Proceedings of the International Conference on Plant and Vegetable Oils as Fuels, pp: 17-122.
- Fuls J, Hugo FJC (1981) On farm preparation of sunflower oil esters for fuel. In: Third International Conference on Energy Use Management, pp: 1595-1602.
Citation: Shah Z, Veses RC, Silva R (2016) Using GC-MS to Analyze Bio-Oil Produced from Pyrolysis of Agricultural Wastes - Discarded Soybean Frying Oil, Coffee and Eucalyptus Sawdust in the Presence of 5% Hydrogen and Argon. J Anal Bioanal Tech 7:300. DOI: 10.4172/2155-9872.1000300
Copyright: © 2016 Shah Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 14374
- [From(publication date): 4-2016 - Aug 29, 2025]
- Breakdown by view type
- HTML page views: 13181
- PDF downloads: 1193