Research Article Open Access
Validation of a Preoperative Risk Model for Pneumonia in Patients undergoing CABG Surgery
| Hulzebos HJ1*, Buuren SV2,3, Klarenbosch JV4, Gigengack-Baars A5, Brutel de la Riviere6 and Helders PJM1 and Meeteren NLUV2 | |
| 1Child Development and Exercise Center, Wilhelmina Children’s Hospital, University Medical Center Utrecht, The Netherlands | |
| 2TNO Leiden, The Netherlands | |
| 3Department of Methodology and Statistics, FSS, Utrecht University, The Netherlands | |
| 4Department of Anesthesiology, University Medical Center Utrecht, The Netherlands | |
| 5Department of Hospital Hygiene & Infection Prevention, University Medical Center Utrecht, The Netherlands | |
| 6Department of Cardio-thoracic Surgery, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands | |
| Corresponding Author : | Hulzebos HJ Child Development and Exercise Center University Medical Center and Children’s Hospital Room K 02.056, PO Box 85090 3508 AB Utrecht, The Netherlands Tel: 31887554030 E-mail: h.hulzebos@umcutrecht.nl |
| Received July 01, 2014; Accepted August 27, 2014; Published August 29, 2014 | |
| Citation: Hulzebos HJ, Buuren SV, Klarenbosch JV, Gigengack-Baars A, Brutel de la Riviere, et al. (2014) Validation of a Preoperative Risk Model for Pneumonia in Patients undergoing CABG Surgery. J Nov Physiother 4:221. doi: 10.4172/2165-7025.1000221 | |
| Copyright: © 2014 Hulzebos HJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Novel Physiotherapies
Abstract
Background: Postoperative pulmonary complications (PPCs) are among the most frequently reported complications of Coronary Artery Bypass Graft (CABG) surgery. However, the risk to develop a PPC is not the same for all patients. The aim of this study was to validate a previously developed preoperative six-factor pulmonary risk model (age>70 years; productive cough, smoking, diabetes mellitus, inspiratory vital capacity > 75% predicted and maximum expiratory mouth pressure>75% predicted) to predict pneumonia, in patients undergoing CABG surgery.
Methods: Prospectively collected data for 421 adult patients who had undergone elective CABG surgery, in a university medical center in the Netherlands, were used to validate the preoperative risk model for predicting pneumonia. The accuracy of the model was tested by comparing the expected and observed incidence of pneumonia in each patient.
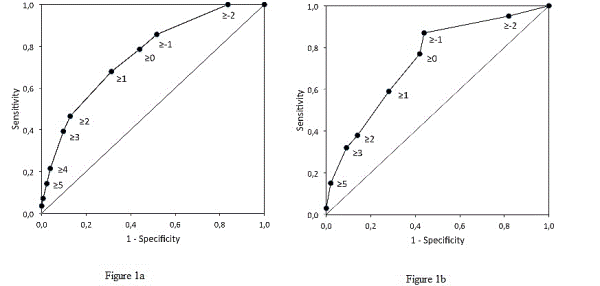
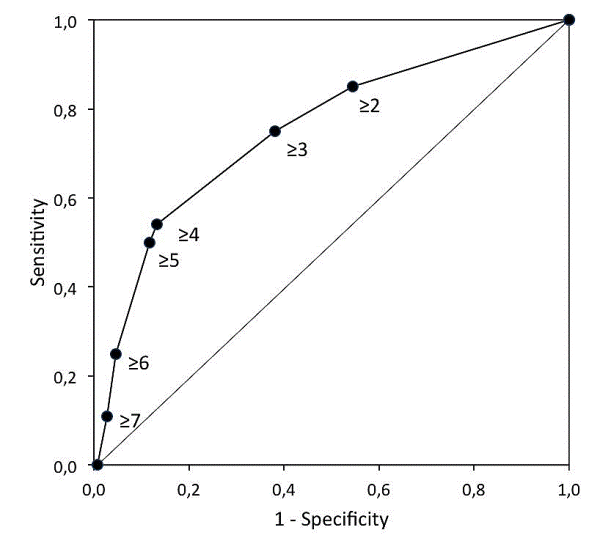
Results: Of the 421 patients, 227 (54%) were classified as being at high pulmonary risk, 24 (11%) of whom developed pneumonia. Only 4 of the 194 (2%) patients classified as being at low pulmonary risk developed pneumonia (OR=5.6; 95%CI, 1.9 to 16.5). The sensitivity (SE) was equal to 0.86, at a specificity (SP) of 0.48, both close to the values calculated for the development sample (SE=0.87, SP=0.56). The negative predictive value (NPV) was 0.98 and the area under curve (AUC) of the receiver-operating characteristics (ROC) curve was 0.76. The model that includes only the four anamnestic risk factors (age≥ 70 year, productive cough, smoking and diabetes mellitus) had an AUC equal to 0.75, with a SE=0.75, SP=0.62, and NPV=0.97.
Conclusion: The study confirms the diagnostic accuracy of the preoperative six-factor pulmonary risk model in an independent sample. Both the six-factor and even the simple anamnestic four-factor models are accurate in identifying preoperative patients at risk of developing pneumonia undergoing CABG surgery.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Electrical stimulation
- High Intensity Exercise
- Muscle Movements
- Musculoskeletal Physical Therapy
- Musculoskeletal Physiotherapy
- Neurophysiotherapy
- Neuroplasticity
- Neuropsychiatric drugs
- Physical Activity
- Physical Fitness
- Physical Medicine
- Physical Therapy
- Precision Rehabilitation
- Scapular Mobilization
- Sleep Disorders
- Sports and Physical Activity
- Sports Physical Therapy
Recommended Journals
Article Tools
Article Usage
- Total views: 13462
- [From(publication date):
September-2014 - Aug 16, 2025] - Breakdown by view type
- HTML page views : 8894
- PDF downloads : 4568
