Review Article Open Access
Polyamines and Gut Mucosal Homeostasis
| Jennifer Timmons1, Elizabeth T. Chang1, Jian-Ying Wang1,2,3 and Jaladanki N. Rao1,3* | |
| 1Department of Surgery, University of Maryland School of Medicine, USA | |
| 2Department of Pathology, University of Maryland School of Medicine, USA | |
| 3Baltimore Veterans Affairs Medical Center, Baltimore, Maryland 21201, USA | |
| Corresponding Author : | Jaladanki N. Rao Ph.D Department of Surgery University of Maryland School of Medicine and Baltimore Veterans Affairs Medical Center 10 North Greene Street, Baltimore MD 21201, USA Tel: 410-605-7808 Fax: 410-605-7949 E-mail: jrao@umaryland.edu |
| Received January 25, 2012; Accepted February 20, 2012; Published February 22, 2012 | |
| Citation: Timmons J, Chang ET, Wang JY, Rao JN (2012) Polyamines and Gut Mucosal Homeostasis. J Gastroint Dig Syst S7:001. doi: 10.4172/2161-069X.S7-001 | |
| Copyright: © 2012 Timmons J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
The epithelium of gastrointestinal (GI) mucosa has the most rapid turnover rate of any tissue in the body
and its integrity is preserved through a dynamic balance between cell migration, proliferation, growth arrest and apoptosis. To maintain tissue homeostasis of the GI mucosa, the rates of epithelial cell division and apoptosis must be highly regulated by various extracellular and intracellular factors including cellular polyamines. Natural polyamines spermidine, spermine and their precursor putrescine, are organic cations in eukaryotic cells and are implicated in the control of multiple signaling pathways and distinct cellular functions. Normal intestinal epithelial growth depends on the available supply of polyamines to the dividing cells in the crypts, and polyamines also regulate intestinal epithelial
cell (IEC) apoptosis. Although the specific molecular processes controlled by polyamines remains to be fully defined, increasing evidence indicates that polyamines regulate intestinal epithelial integrity by modulating the expression of various growth-related genes. In this review, we will extrapolate the current state of scientific knowledge regarding the roles of polyamines in gut mucosal homeostasis and highlight progress in cellular and molecular mechanisms of polyamines and their potential clinical applications.
| Keywords |
| Ornithine decarboxylase; Mucosal injury; Restitution; Apoptosis; Cell proliferation; RNA-binding proteins; MicroRNAs |
| Introduction |
| The gut is an important organ responsible for digestion, absorption, and metabolism of dietary nutrients. The mucosa of the gastrointestinal (GI) tract is lined with epithelium that has the shortest turnover rate of any tissue in the body [1-3]. Maintenance of GI epithelial homeostasis depends on a complex interplay between processes involving intestinal epithelial cell (IEC) proliferation, differentiation, migration, and apoptosis [3-6]. Under normal physiological situations, undifferentiated epithelial cells continuously replicate in the proliferative zone within the crypts and differentiate as they migrate up towards the luminal surface of the colon and villous tips in the small intestine [7,8]. To maintain a stable number of enterocytes, cell division must be counterbalanced by the process of apoptotic cell death, a fundamental biological process involving selective cell deletion to regulate tissue homeostasis [3,9,10]. Apoptosis occurs in the crypt area, where it maintains a critical balance in cell number between newly divided and surviving cells, and at the luminal surface of the colon and villous tips in the small intestine, where differentiated cells are lost. This rapid dynamic turnover rate of intestinal epithelial cells is highly regulated and critically controlled by numerous factors, including cellular polyamines [11-13]. |
| Natural polyamines, spermidine, spermine, and their precursor putrescine, are ubiquitous biogenic amines of low molecular weight found in abundance intracellularly in nearly all eukaryotic cells. Polyamines are intimately involved in many distinct cellular functions [11,14,15], but their exact roles at the molecular level remains largely unknown. A series of observations from our previous studies [16-19] and others [20-22] have shown that normal intestinal mucosal growth depends on polyamine availability to dividing cells within crypts, and that polyamines, either synthesized endogenously or supplied luminally, are absolutely required for epithelial cell division. Cellular polyamine content increases rapidly when cells are stimulated to grow and divide, whereas inhibition of ornithine decarboxylase (ODC), the rate-limiting enzyme in polyamine biosynthesis, decreases cellular polyamines and represses IEC proliferation both in vivo and in vitro [13,23,24]. Because of the absolute requirement of cellular polyamines for GI mucosal cell growth and proliferation, the polyamine metabolic pathway has been an attractive therapeutic target for antineoplastic intervention [25]. It has also been shown that the excessive expression of ODC and the resulting heightened levels of polyamine in human colon and other cancers [11,15,26] suggests that this sustained overexpression of ODC may lead to malignant transformation [27,28]. Since there are already several excellent reviews which focus on polyamines and GI cancers [11,15,29,32] we decided to highlight primarily on the role of polyamines during normal GI mucosal integrity. In this review we discuss what has been defined thus far in regards to the role of polyamines and its clinical significance in gut mucosal homeostasis including mucosal repair, barrier function, growth, and apoptosis. |
| Polyamine Biosynthesis |
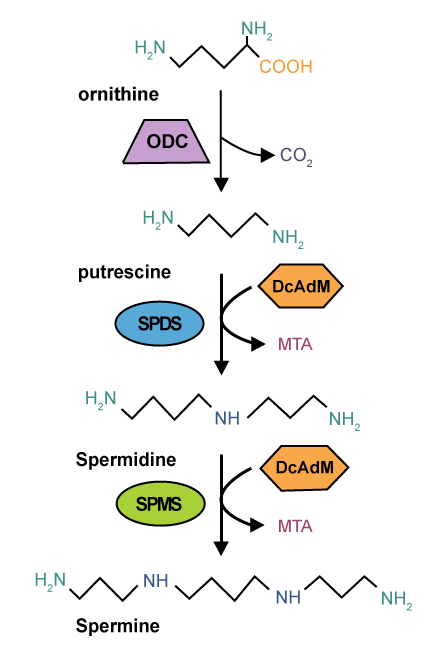
| Biosynthesis and catabolism of polyamines are carefully controlled processes in all types of eukaryotic cells. Intracellular polyamine levels is maintained and highly regulated by endogenous biosynthesis, exogenous transport, and degradation [11,14,33,34]. Natural polyamines found in the intestinal lumen arise from diet (such as red meat and cheeses), bacteria, and villous extrusion from sloughed epithelial cells. Polyamine metabolism involves both the forward and reverse component pathways, although cellular control of regulatory enzymes and the polyamine transporter act in concert with each other in order to maintain appropriate levels of individual polyamines. Polyamines derive from two amino acids, ornithine and methionine [14,23]. During polyamine synthesis in mammals, ODC, the catalyst for the first rate-limiting step of polyamine synthesis, decarboxylates the amino acid ornithine to form putrescine; propylamine groups are then added to one or both amino groups of putrescine to form spermidine and spermine [12,35,36]. Methionine is the precursor for S-adenosylmethionine (AdoMet). The decarboxylation product of AdoMet is the precursor of the aminopropyl moieties of spermidine and spermine (Figure 1). |
| In contrast, polyamines are degraded by diamine oxidase and spermidine/ spermine-Nacetyltransferase (SSAT). Putrescine, spermidine and spermine are interconverted according to cellular physiological needs [36,37]. Polyamine homeostasis is regulated by a feedback mechanism controlled primarily by polyamines themselves via regulation of de novo synthesis, release, uptake and catabolism. In mammalian cells, the degradation of ODC is facilitated by a specific ODC-antizyme [38,39], a protein that also appears to downregulate polyamine transport. DL-α-difluoromethylornithine (DFMO) is an irreversible inactivator of the ODC enzyme that specifically inhibits ODC activity. The discovery of DFMO has provided an enormous stimulus to the field of mammalian polyamine biology leading to the unraveling of various polyamine-related mechanisms over the two decades. |
| Polyamines and Gut Mucosal Repair: Epithelial Restitution and Chronic Healing |
| Cellular decisions which regulate signaling pathways and control gene expression involved in migration, proliferation, and apoptosis, are required for the successful repair of mucosal damage and wounds [40-42]. The repair of damaged mucosa occurs through two distinct mechanisms: restitution and chronic healing. Mucosal restitution is an important and primary repair modality in the GI tract, and its dysregulation underlies various critical pathological states such as mucosal bleeding and ulcers, disruption of epithelial integrity, and barrier dysfunction [8,43,44]. Epithelial restitution occurs as a consequence of IEC migration to reseal superficial wounds, a process independent of cell proliferation [13,45-48]. In contrast, chronic healing is the much slower mechanism of replacing lost cells through DNA synthesis and cell division, which takes a much longer time compared with epithelial restitution [42,49]. |
| Studies from our own [13,46,50-53] and others [43,44,54,55] have demonstrated a strong relationship between cellular polyamines and mucosal repair processes both in vivo and in vitro. In a rat stress ulcer model, results show that ODC activity levels were significantly elevated in GI tissues after stress [8,13], and are associated with increased levels of mucosal putrescine, spermidine, and spermine content [8,13]. In contrast, administration of DFMO prevented this normal GI mucosal healing process from occurring [13]. Gastric administration of polyamines immediately following the period of stress prevented the inhibition of repair caused by DFMO and instead increased the normal rate of healing. In order to study the involvement of polyamines in mucosal restitution, an in vitro model of healing was developed to demonstrate cell migration after wounding [45,56-59]. In this model, a confluent monolayer of IEC cells were wounded by scraping with a razor blade and cell migration was assessed on short time (~6h after wounding) by counting the number of cells that crossed the wounded edge. Cell migration in this model was independent of DNA synthesis but dependent on cytoskeletal reorganization. Several studies showed that cells grown in the presence of DFMO inhibited migration by ~80%, and that exogenous polyamines prevented the decreased migration [45,47,57]. We have demonstrated that polyamines regulate IEC migration by altering K+ channel activity, membrane potential (Em), and cytosolic free Ca2+ concentration [Ca2+]cyt , and that the induced changes in [Ca2+]cyt exerts its regulatory effects on cell motility through interacting with specific targets during restitution [47,51-53,60,61]. Decreased [Ca2+]cyt by depolarization of Em inhibited normal cell migration and prevented the restoration of cell migration by exogenous spermidine in polyamine-deficient cells. In contrast, increased [Ca2+]cyt by Ca2+ ionophore ionomycin stimulated cell migration in the absence of cellular polyamines [53,57], indicating that polyamine-mediated IEC migration is due partially to increased levels of Kv channel activity. |
| In another set of experiments we have demonstrated that differentiated IEC-Cdx2L1 cells migrate over the wounded edge much faster than undifferentiated parental IEC-6 cells [45,47,62]. Differentiated IEC-Cdx2L1 cells express higher basal levels of Kv1.1 and Kv1.5 mRNAs and proteins, and depletion of intracellular polyamines decreases the expression of both Kv1.1 and Kv1.5 channel genes, resulting in an inhibition of whole cell K+ currents, Em, and reduced resting [Ca2+]cyt. The migration rates in differentiated IECCdx2L1 cells are ~4 times that of parental IEC-6 cells [45]. Inhibition of Kv channel expression by depletion of cellular polyamines reduced [Ca2+]cyt, resulting in cellular reorganization of cytoskeletal proteins, along with a marked reduction in actomyosin stress fiber formation and inhibited epithelial cell migration. To determine the mechanism by which polyamine-modulated Ca2+ induces cell migration during restitution, we have further showed that migration of IECs after wounding is associated with a significant increase in β-catenin tyrosine phosphorylation [56]. Decreased levels of cellular polyamines by DFMO prevented the induction of β-catenin phosphorylation and consequently decreased cell migration after wounding [56], while elevation of [Ca2+] cyt restored β-catenin phosphorylation and stimulated migration in polyamine-deficient cells. These data indicate that β-catenin tyrosine phosphorylation plays a critical role in polyamine-dependent cell migration and that polyamines induce β-catenin phosphorylation at least partially through [Ca2+]cyt. |
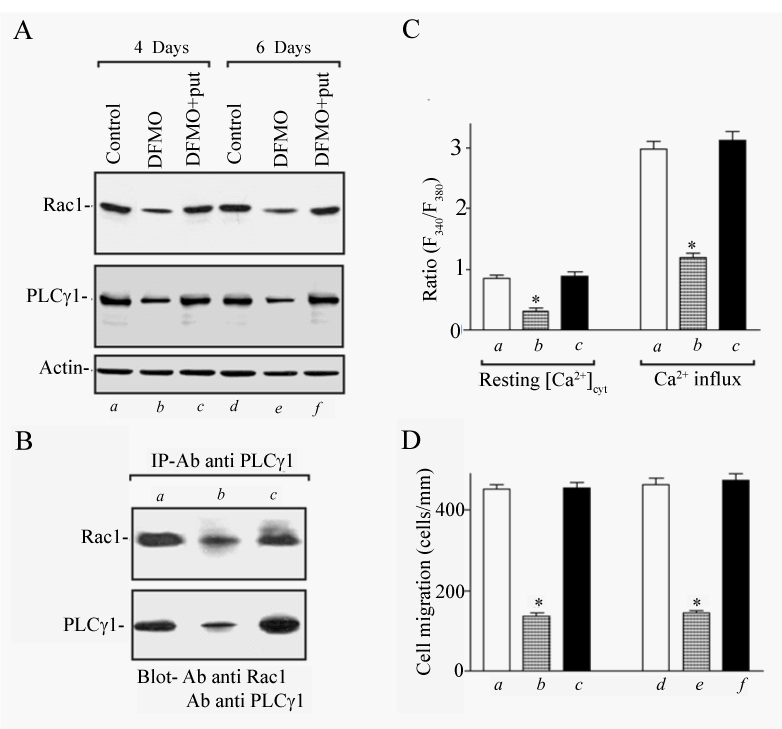
| During restitution, intestinal columnar cells must undergo rearrangement-flattening, stretching, migrating, and the eventual repolarizing of their cytosol and membrane [44,45,63,64]. Major stimuli and modulators of epithelial cell migration comes from local growth factors produced at the site of injury and elsewhere (e.g. platelets and macrophages) as well as from metabolic substrates, the extracellular matrix, regulatory peptides, integrins, cytokines, and polyamines. These growth factors stimulate cytoplasmic and cytoskeletal rearrangement in order for migration to occur [65,66]. More specifically, genes affecting the components that make up these structures, including microfilaments and stress fibers composed of actin and/or myosin, are key to successful migration and restitution. Several studies have indicated that small GTP binding proteins such as RhoA, Rac1, and Cdc42 play an important role in polyamine-dependent IEC migration after wounding. We have shown that polyamines are required for expression of Rac1 protein in differentiated IEC-Cdx2L1 cells and are implicated in modulating the interaction between Rac1 and phospholipase C-γ1 (PLC-γ1) after wounding, partially through [Ca2+]cyt. Figure 2 shows that the depletion of cellular polyamines decreased the formation of Rac1/ PLC-γ1 complexes, attenuated store depletion-induced Ca2+ influx, and repressed cell migration after wounding. We also reported that PLC-γ1 expression requires polyamines and that polyamine-induced PLC-γ1 is involved in the control of [Ca2+]cyt during epithelial restitution [47]. These results prove the possibility that Rac1/PLC-γ1 complexes are necessary for the stimulatory effect of polyamines on Ca2+ influx after wounding. Studies from these experiments suggest that Rac1 functions as an upstream regulator of PLC-γ1-induced Ca2+ signaling and that induced interaction between Rac1 and PLC-γ1 enhances polyaminedependent cell migration after wounding by increasing [Ca2+]cyt. Given the fact that Rac1/PLC-γ1-induced Ca2+ signaling is highly regulated by cellular polyamines and that levels of tissue polyamines in the damaged intestinal mucosa are dramatically increased [51,67], activation of this signaling pathway is crucial for polyamine-dependent IEC migration after injury and contributes to the maintenance of intestinal epithelial homeostasis. Our recent studies further revealed that the major storeoperated Ca2+ (SOC) channels such as Transient Receptor Potential Channel 1 (TRPC1), TRPC5, and Stromal Interaction Molecule 1 (STIM1) are upstream of Rac1/PLC-γ1 signaling and plays a critical role in IEC migration after wounding [52,68]. |
| Deeper mucosal defects such as ulcers, often a result of tissue necrosis and penetration of the muscularis mucosa, and chronic injury, necessitate a somewhat different repair process. As opposed to the rapid mucosal restitution that occurs in the acute phase of GI mucosal injury, chronic healing process requires much more in the way of cellular replication, protein synthesis and de novo DNA and mRNA synthesis [49]. Because several studies indicate that cellular polyamines are required for the growth of all eukaryotic cells, it is not surprising that the cell replacement stage of mucosal wound healing is dependent on polyamine levels. It has been shown that there was almost no replacement of gastric mucosal cells 24 h after stress in rats treated with DFMO [43]. Polyamine depletion blocked the increases in protein, RNA and DNA synthesis, and content that normally follow damage [69]. One of the earliest events that are triggered in the stress ulcer model is a significant transient increase in the expression of the c-fos and c-myc protein levels that were preceded by increases in ODC activity and putrescine levels [46,70,71]. Blocking ODC with DFMO totally prevented the increased expression of protooncogenes. In vitro wound healing model results also show that the expression of c-fos, cmyc, and c-jun, protooncogenes is required for healing, which was inhibited by polyamine depletion. These cellular protooncogenes are responsible for the regulation of the cell cycle and are involved in healing as well as normal growth and development [72]. Thus polyamines are essential for healing process by enhancing expression of these protooncogenes [71,72]. Several studies demonstrated that cellular polyamines plays a critical role during chronic healing by altering signaling mechanisms involving Wnt, TGF-β, MAPK, ERK1/2, EGF, and EGF-R [49,73-75]. |
| Polyamines and Gut Epithelial Barrier Function |
| Epithelial cells line the intestinal mucosa and form an important barrier that protects the subepithelial tissue against a wide array of noxious substances, allergens, and luminal microbial pathogens [2,3]. The effectiveness and stability of this epithelial barrier depends on the activity of junctional complexes that include tight junctions (TJs), adherens junctions, desmosomes, and gap junctions, all of which seals epithelial cells together in a way that prevents even small molecules from leaking between cells [76-78]. An increasing body of evidence indicates that formation of adherens junctions is essential for the assembly of TJs between epithelial cells and that alteration in levels of the cadherin-dependent adherens junctions regulates the stability of TJ complexes and affects intestinal epithelial paracellular permeability [79-81]. We [82-84] have demonstrated that polyamines regulate the intestinal epithelial barrier function and that polyamine depletion increases epithelial paracellular permeability partially by repressing expression of Zona Occludens-1 (ZO-1), occludin and E-cadherin. Our studies have further shown that polyamines modulate expression of various intercellular junction proteins through distinct cellular signaling pathways. In this regard, polyamines promote E-cadherin mRNA translation and increases its protein stability [85], whereas polyamines modulate ZO-1 transcription by altering the interaction of the ZO-1 gene promoter [86]. We also provide further evidence showing that polyamines are necessary for E-cadherin transcription by activating c-myc interaction with the E-Pal box in the proximal region of the E-cadherin promoter [85]. |
| Occludin is a transmembrane TJ protein that plays an important role in TJ assembly and regulation of the epithelial barrier function. Our most recent studies indicated that decreasing cellular polyamines inhibited occludin expression and also decreased the levels of phosphorylated Human Antigen R (HuR) [84]. The RNA-binding protein HuR modulates the stability and translation of many target mRNAs leading us to investigate whether polyamines regulate occludin expression via HuR and subsequently the intestinal epithelial barrier function. Although the mechanisms by which polyamines regulate occludin expression remains to be fully elucidated, our studies have shown that polyamine depletion by DFMO decreased HuR/occludin mRNA complexes and repressed occludin mRNA translation. Exogenous polyamine putrescine, when given together with DFMO, prevented Chk2 kinase inhibition and restored HuR phosphorylation and its binding affinity to occludin mRNA, thereby promoting occludin translation. Consistent with our current findings, polyamines are also shown to increase HuR’s binding to c-Myc mRNA through enhancement of Chk2-dependent HuR phosphorylation in IECs [84,87]. In vivo studies from our laboratory further revealed that Chk2dependent HuR phosphorylation is implicated in the maintenance and re-establishment of gut barrier integrity under critical surgical stress. CLP stress decreased Chk2 levels, reduced HuR/occludin mRNA complexes, and inhibited occludin abundance, thus contributing to the pathogenesis of gut barrier dysfunction [84]. In contrast, induced Chk2-dependent HuR phosphorylation appears to be crucial for the recovery of occludin expression and gut barrier function. These results indicate that polyamines are implicated in the Chk2-dependent HuR phosphorylation that regulates occludin mRNA translation and helps to maintain and re-establish epithelial barrier function in the intestinal tract when responding to septic stress [84]. |
| Polyamines and Mucosal Growth |
| Mucosal cell turnover is extremely fast; in rodent gut, cells are replaced approximately every three days and in humans about every four days [3,88,89]. Several studies show that cellular polyamine content increases rapidly in cells stimulated to grow and divide, while reducing cellular polyamines by inhibiting ODC [11,90-94] represses IEC proliferation in vivo and in vitro [13,72,95]. Since the recognition that polyamines are absolutely required for mammalian cell growth, the targeting of their function and metabolism has been an attractive strategy for anti proliferative therapy [26,96,97]. Polyamines are shown to positively regulate the transcription of growth-promoting genes such as c-fos, c-jun, and c-myc [70-72] and negatively affect growth inhibiting genes including p53, NDRG1, NPM, JunD, and TGFβ/TGFβ receptor at the posttranscriptional level [94,98-106]. |
| Our recent studies revealed that depletion of cellular polyamines increases the nuclear abundance of ATF-2 by stabilizing its mRNA, which is associated with a decrease in the levels of cyclin-dependent kinase 4 (CDK4) and cell proliferation [107-109]. We have shown a novel function of ATF-2 in the modulation of CDK4 expression and demonstrate that induction of ATF-2 represses CDK4 gene transcription, thus contributing to the inhibition of IEC proliferation following polyamine depletion [107]. Studies aimed at characterizing the molecular aspects of this process indicate that induced ATF-2 in polyamine-deficient cells physically interacts with JunD and forms ATF-2/JunD heterodimers that directly bind to the CDK4-promoter. |
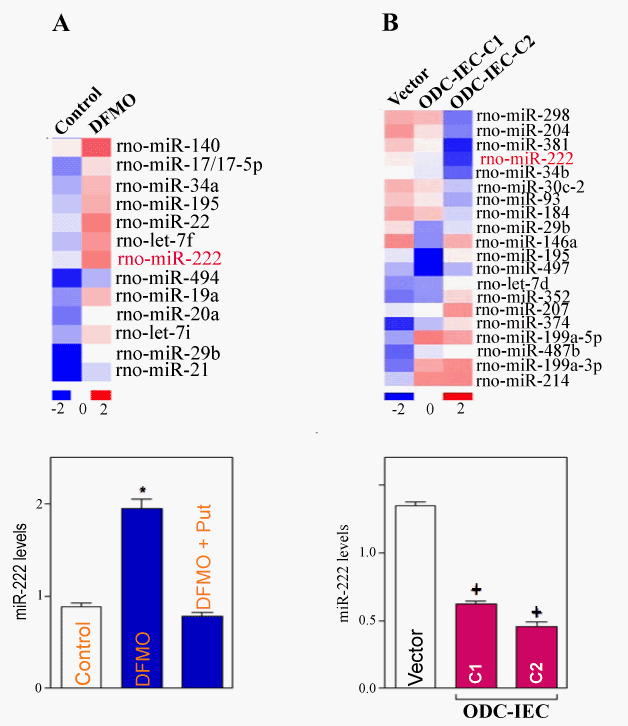
| Most recently, we went further to provide evidence that polyamines promote the translation of CDK4 by repressing CUG-binding protein 1 (CUGBP1) and microRNA-222 (miR-222) in IECs. Specific inhibition of CDK4 activity delays cell-cycle progression and results in growth arrest in the G1 phase, similar to the phenotype observed in polyamine-deficient IECs [110]. We reported that CUGBP1 and miR-222 jointly bind the CDK4 mRNA and repress CDK4 translation synergistically. First, we determined the effect of cellular polyamines on global miRNA expression by miRNA array analysis. A comparison of the miRNA expression profiles in untreated relative to polyaminedeficient cells revealed several increased miRNAs after polyamine depletion, including miR-222, miR-195, miR-140, and miR-29b (Figure 3A). In contrast, increasing cellular polyamines by the overexpression of ODC gene in IEC cells (ODC-IEC) decreased the levels of some miRNAs, such as miR-222 and miR-29b (Figure 3B). Although a sizable subset of miRNAs showed altered abundance in IECs after modulating polyamines, we focused on miR-222, based on its strong dependence on polyamine abundance and its predicted interaction with the CDK4 mRNA. Real-time quantitative PCR (Q-PCR) analysis confirmed changes in the levels of miR-222 after altering the levels of cellular polyamines and revealed that miR-222 levels increased by polyamine depletion (Figure 3A) but decreased in ODC-IECs, which contained high polyamine levels (Figure 3B). Taken together, these findings show that polyamines negatively regulate CUGBP1 and miR-222 expression in normal IECs. |
| In a second set of experiments, we found that CUGBP1 directly interacts with both the 3’-UTR and coding region (CR) of CDK4 mRNA, but miR-222 only binds to the CDK4 CR. Moreover, polyamine depletion inhibited CDK4 translation by inducing cytoplasmic CUGBP1 and miR-222 levels, whereas increased levels of cellular polyamines stimulated CDK4 expression by decreasing CDK4 mRNA associations with CUGBP1 and miR-222 [110]. Because intracellular polyamines are tightly regulated by stress stimulation and the status of cell growth, this suggests that polyamine-mediated activation of CDK4 expression by targeting CUGBP1 and miR-222 directly regulates the growth of the intestinal mucosa and thereby contributes to maintaining the integrity of the intestinal epithelium. |
| Polyamines and Apoptosis |
| It has been shown that apoptosis, rather than simple exfoliation of enterocytes, accounts for the majority of cell loss at the luminal surface of the colon and villous tips in the small intestine [3]. Apoptosis also occurs in the crypt area. Previous work from our lab [19,71,101,104] and others [10,44,55] have demonstrated that polyamines are crucial for the maintenance of epithelial homoeostasis and that depletion of cellular polyamines promotes the resistance of IECs to apoptosis through multiple signaling pathways. Although the exact roles of polyamines in apoptotic pathways has been rather controversial, depending on the cell type and death stimulus, polyamine depletion by inhibition of ODC with DFMO promotes resistance to tumor necrosis factor-α (TNF-α)/cycloheximide (CHX)-induced apoptosis in normal IEC-6 cells. We have also shown that polyamines downregulate NFκB activity and that depletion of cellular polyamines increases NFκB transcriptional activity, thus stimulating the expression of c-IAPs [106,111]. Polyamines are also needed for the inhibition of focal [106,111]. Polyamines are also needed for the inhibition of focal adhesion kinase (FAK) [112] and Akt [113], as polyamine depletion induces the phosphorylation of FAK and Akt and increases their kinase activities. Recently, polyamines are shown to inhibit the expression of ATF-2 and XIAP genes at the post-transcriptional level, as decreasing the levels of cellular polyamines increases the steady-state levels of ATF-2 [107,109] and XIAP [111] through stabilization of their mRNAs. |
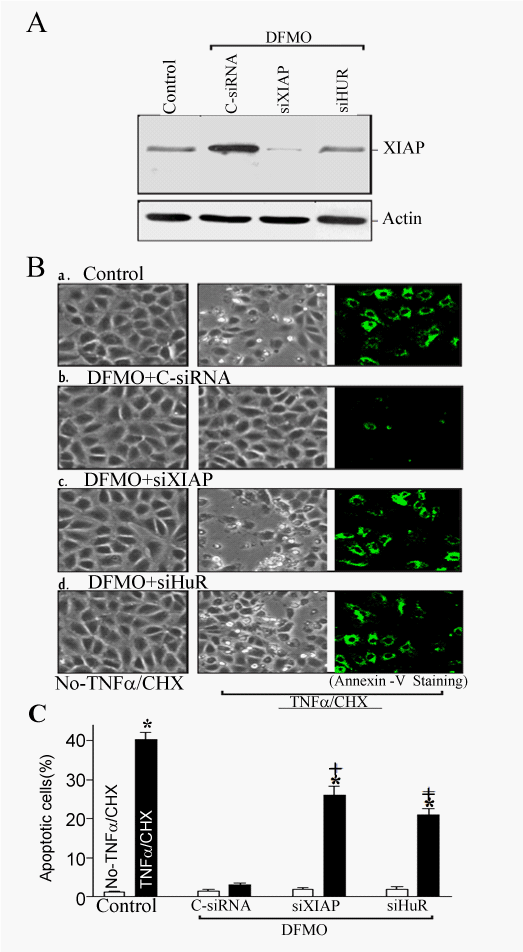
| To investigate the physiological consequences of inducing endogenous XIAP by HuR following polyamine depletion, we studied the possible involvement of this process in regulating IEC apoptosis. We first examined spontaneous apoptotic cell death without any challenge of apoptotic stimulators after inhibition of XIAP expression by using siRNA targeting XIAP mRNA (siXIAP) or siHuR in the absence of cellular polyamines. Transient transfection with the siXIAP or siHuR prevented the increased expression of XIAP in polyaminedeficient cells (Figure 4A) but failed to directly induce apoptosis in polyamine-deficient cells (Figure 4B). Second, we determined whether XIAP silencing altered the polyamine depletion-mediated resistance to apoptosis elicited by treatment with TNF-α/CHX. As shown in (Figure 4Ba), when control cells were exposed to TNF-α/ CHX for 4 h, morphological features characteristic of programmed cell death were observed and annexin V staining showed significant phosphatidylserine presence in the cell membrane, a classic indicator of apoptotic cells (Figure 4Ba). This increased resistance to TNF-α/ CHX-induced apoptosis was not altered when polyamine-deficient cells were transfected with C-siRNA (Figure 4Bb), but was lost when XIAP expression was silenced by siXIAP (Figure 4Bc) or siHuR (Figure 4Bd). The percentages of apoptotic cells (Figure 4C) in DFMO-treated cells transfected with siXIAP or siHuR were significantly increased compared with those observed in DFMO-treated cells transfected with C-siRNA after exposure to TNF-α/CHX. These results indicate that the HuR-mediated increase in XIAP expression contributes to an increase in resistance to apoptosis following polyamine depletion. |
| Given our long-standing interest in understanding polyamine function in gut mucosal homeostasis, we also analyzed the association of HuR with p53, NPM, AUF-1, ATF-2 and MEK1 [105,107,109,114,116]. Increased levels of cytoplasmic HuR following polyamine depletion is associated with the abundance and steady state levels of p53, ATF-2, and MEK1 mRNA complexes. Taken together, our studies revealed that HuR-mediated protein expression plays an important role in the regulation of IEC apoptosis and thus is implicated in the maintenance of gut mucosal homeostasis. |
| Summary and Clinical Significance |
| Among mammalian cells the link between polyamine metabolism, synthesis, and neoplastic growth has been well established [26,117,121]. The role of polyamines is extensive and differs among various cell types. Several studies have showed that increased polyamine synthesis is linked to colon carcinogenesis in preclinical models and in humans. Dietary polyamines are an important factor in adenoma prevention, whereas controlling exogenous polyamines is an adjunctive strategy to chemoprevention with polyamine-inhibitory agents [11,15,12,23]. DFMO has been in use as a chemotherapeutic agent for many years and polyamines are thought to be potentially useful for enhancing drug absorption [122-125]. In addition, polyamine metabolism is affected by a variety of non-steroidal anti-inflammatory drugs (NSAIDs) [126,127]. Polyamines are strongly reactive within various cell types of the digestive tract including pancreatic acinar cells, gastric chief cells, insulin cells and cells of the submandibular gland [128-130]. Polyamines also function as “important growth factors in breast milk” which have important implications in neonatal gut maturity and potential formula supplementation [123,131,132]. With the wide array of cell types and roles of cellular polyamines, the possibilities for clinical application seem limitless. |
| Acknowledgements |
| The authors sincerely apologize to all colleagues whose work has been omitted due to space limitations. Authors’ work was supported by a Merit Review Grants from the Department of Veterans Affairs (to JNR & J-YW) and by National Institutes of Health Grants DK-57819, DK-61972, and DK-68491 (to J-YW). J-Y Wang is a Senior Research Career Scientist, Medical Research Service, U.S. Department of Veterans Affairs. The authors have no conflicting financial interest. |
References
- Jankowski JA, Goodlad RA, Wright NA (1994) Maintenance of normal intestinal mucosa: function, structure, and adaptation. Gut Supplement 1: 1-4.
- Antonioli DA, Madara JL (1998) Functional anatomy of the gastrointestinal tract. Ming SC, Goldman H, In: Pathology of Gastrointestinal Tract, Williams & Wilkins, 2: 13-33.
- Rao JN, Wang JY (2011) Regulation of gastrointestinal mucosal growth. Granger ND, Granger J, Morgan and Claypool Publishers 1-114.
- Wong WM, Wright NA (1999) Cell proliferation in gastrointestinal mucosa. J Clin Pathol 52: 321-333.
- McCole DF, Barrett KE (2007) Varied role of the gut epithelium in mucosal homeostasis. Curr Opin Gastroenterology 23: 647-654.
- Marasa BS, Xiao L, Rao JN, Zou T, Liu L, et al. (2008) Induced TRPC1 expression increases protein phosphatase 2A sensitizing intestinal epithelial cells to apoptosis through inhibition of NF-?B activation. Am J Physiol Cell Physiol 294: 1277-1287.
- Silen W, Ito S (1985) Mechanism for rapid-epithelialization of the gastric mucosal surface. Annu Rev Physiol 47: 217-229.
- Wang JY, Johnson LR (1990) Luminal polyamines stimulate repair of gastric mucosal stress ulcers. Am J Physiol 259: 584-592.
- Hauck AL, Swanson KS, Kenis PJ, Leckband DE, Gaskins HR, et al. (2005) Twists and turns in the development and maintenance of the mammalian small intestine epithelium. Birth Defects Res C Embryo Today 75: 58-71.
- Bhattacharya S, Ray RM, Johnson LR (2009) Role of polyamines in p53-dependent apoptosis of intestinal epithelial cells. Cell Signal 21: 509-522.
- Seiler N, Raul F (2007) Polyamines and the intestinal tract. Crit Rev Clin Lab Sci 44: 365-411.
- Seiler N, Raul F (2005) Polyamines and apoptosis. J Cell Mol Med 9: 623-642.
- Wang JY, Johnson LR (1991) Polyamines and ornithine decarboxylase during repair of duodenal mucosa after stress in rats. Gastroenterology 100: 333-343.
- Tabor CW, Tabor H (1984) Polyamines. Annu Rev Biochem 53: 749-790.
- Gerner EW, Meyskens FL (2004) Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer 4: 781-792.
- Liu L, Santora R, Rao JN, Guo X, Zou T, et al. (2003) Activation of TGF-ß-Smad signaling pathway following polyamine depletion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 285: 1056-1067.
- Rao JN, Li L, Bass BL, Wang JY (2000) Expression of the TGF-ß receptor gene and sensitivity to growth inhibition following polyamine depletion. Am J Physiol Cell Physiol 279: 1034-1044.
- Patel AR, Li J, Bass BL, Wang JY (1998) Expression of the transforming growth factor-ß gene during growth inhibition following polyamine depletion. Am J Physiol 275: 590-598.
- Liu L, Li L, Rao JN, Zou T, Zhang HM, et al. (2005) Polyamine-modulated expression of c-myc plays a critical role in stimulation of normal intestinal epithelial cell proliferation. Am J Physiol Cell Physiol 288: 89-99.
- Johnson LR (1988) Regulation of gastrointestinal mucosal growth. Physiol Rev 68: 456-502.
- Ray RM, Bavaria MN, Bhattacharya S, Johnson LR (2011) Activation of Dbl restores migration in polyamine-depleted intestinal epithelial cells via Rho-GTPases. Am J Physiol Gastrointest Liver Physiol 300: 988-997.
- Murphy GM (2001) Polyamines in the human gut. Eur J Gastroenterol Hepatol 13: 1011-1014.
- Thomas T, Thomas TJ (2003) Polyamine metabolism and cancer. J Cell Mol Med 7: 113-126.
- Seidel ER, Ginty DD (1990) Apparent post-transcriptional modification of ornithine decarboxylase accounts for its induction in IEC-6 cells in culture. Digestion 2: 383-389.
- Casero RA, Marton LJ (2007) Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov 6: 373-390.
- Linsalata M, Russo F (2008) Nutritional factors and polyamine metabolism in colorectal cancer. Nutrition 24: 382-389.
- Laukaitis CM, Gerner EW (2011) DFMO: targeted risk reduction therapy for colorectal neoplasia. Best Pract Res Clin Gastroenterol 25: 495-506.
- Goodwin AC, Destefano Shields CE, Wu S, Huso DL, Wu X, et al. (2011) Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci U S A 108: 15354-15359.
- Babbar N, Gerner EW (2011) Targeting polyamines and inflammation for cancer prevention. Recent Results Cancer Res 188: 49-64.
- Ignatenko NA, Gerner EW, Besselsen DG (2011) Defining the role of polyamines in colon carcinogenesis using mouse models. J Carcinog 10:10.
- Rial NS, Meyskens FL, Gerner EW (2009) Polyamines as mediators of APPC-dependent intestinal carcinogenesis and cancer chemoprevention. Essays Biochem 46: 111-124.
- Fu S, Xiao C, Zhao W, Yu X (2012) Polyamines analysis by HPLC and their application as tumor markers. Front Biosci (Elite Ed) 4: 1795-1801.
- Shantz LM, Pegg AE (1999) Translational regulation of ornithine decarboxylase and other enzymes of the polyamine pathway. Int J Biochem Cell Biol 31: 107-122.
- Uemura T, Gerner EW (2011) Polyamine transport systems in mammalian cells and tissues. Methods Mol Biol 720: 339-348.
- Parveen N, Cornell KA (2011) Methylthioadenosine/S-adenosylhomocysteine nucleosidase, a critical enzyme for bacterial metabolism. Mol Microbiol 79: 7-20.
- Shantz LM, Holm I, Janne OA, Pegg AE (1992) Regulation of S-adenoxylmethionine deccarboxylase activity by alterations in the intracellular polyamine content. Biochem J 288: 511-518.
- Hobbs CA, Gilmour SK (2006) Role of Polyamines in the regulation of chromatin acetylation. Wang JY, Casero RA, Polyamine cell signaling, Humana Press, Totowa, New Jersey, 75-89.
- Tang H, Ariki K, Ohkido M, Murakami Y, Matsufuji S, et al. (2009) Role of ornithine decarboxylase antizyme inhibitor in vivo. Genes Cells 14: 79-87.
- Wallace HM, Fraser AV, Hughes A (2003) A perspective of polyamine metabolism. Biochem J 376: 1-14.
- McCormack SA, Johnson LR (1991) Role of polyamines in gastrointestinal mucosal growth. Am J Physiol Gastrointest Liver Physiol 260: 795-806.
- Nusrat A, Delp C, Madara JL (1992) Intestinal epithelial restitution: characterization of cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Invest 89: 1501-1511.
- Tarnawski AS (2005) Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci 50: 24-33.
- Johnson LR, McCormack SA (1999) Healing of Gastrointestinal Mucosa: Involvement of Polyamines. News Physiol Sci 14: 12-17.
- Santos MF, McCormack SA, Guo Z, Okolicany J, Zheng Y, et al. (1997) Rho proteins play a critical role in cell migration during the early phases of mucosal restitution. J Clin Invest 100: 216-225.
- Rao JN, Li J, Li L, Bass BL, Wang JY (1999) Differentiated intestinal epithelial cells exhibit increased migration through polyamines and myosin II. Am J Physiol Gastrointest Liver Physiol 277: 1149-1158.
- Wang JY, Johnson LR (1994) Expression of protooncogenes c-fos and c-myc in healing of gastric mucosal stress ulcers. Am J Physiol 266: 878-886.
- Rao JN, Liu L, Zou T, Marasa BS, Boneva D, et al. (2007) Polyamines are required for phospholipase C-?1 expression promoting intestinal epithelial cell restitution after wounding. Am J Physiol Gastrointest Liver Physiol 292: 335-343.
- Rao JN, Wang JY (2002) Ca2+ signaling in epithelial restitution. In: Gastrointestinal Mucosal Repair and Experimental Therapeutics, edited by Cho CH, Wang JY Switzerland: S KARGER AG, 29-42.
- Liu L, Rao JN, Zou T, Xiao L, Smith A, et al. (2012) Activation of Wnt3a signaling stimulates intestinal epithelial repair by promoting c-Myc-regulated gene expression. Am J Physiol Cell Physiol 302: 277-285.
- Wang JY, McCormack SA, Viar MJ, Johnson LR (1991) Stimulation of proximal small intestinal mucosal growth by luminal polyamines. Am J Physiol Gastrointest Liver Physiol 261: 504-511.
- Rao JN, Liu SV, Zou T, Liu L, Xiao L, et al. (2008) Rac1 promotes intestinal epithelial restitution by increasing Ca2+ influx through interaction with phospholipase C-?1 after wounding. Am J Physiol Cell Physiol 295: 1499-1509.
- Rao JN, Platoshyn O, Golovina VA, Liu L, Zou T, et al. (2006) TRPC1 functions as a store-operated Ca2+ channel in intestinal epithelial cells and regulates early mucosal restitution after wounding. Am J Physiol Gastrointest Liver Physiol 290: 782-792.
- Wang JY, Wang J, Golovina VA, Li L, Platoshyn O, et al. (2000) Role of K+ channel expression in polyamine-dependent intestinal epithelial cell migration. Am J Physiol Cell Physiol 278: 303-314.
- Bavaria MN, Ray RM, Johnson LR (2011) The phosphorylation state of MRLC is polyamine dependent in intestinal epithelial cells. Am J Physiol Cell Physiol 300: 164-175.
- McCormack SA, Viar MJ, Johnson LR (1993) Polyamines are necessary for cell migration by a small intestinal crypt cell line. Am J Physiol 264: 367-374.
- Guo X, Rao JN, Liu L, Rizvi M, Turner DJ, et al. (2002) Polyamines regulate ß-catenin tyrosine phosphorylation via Ca2+ during intestinal epithelial cell migration. Am J Physiol Cell Physiol 283: 722-734.
- Rao JN, Li L, Golovina VA, Platoshyn O, Strauch ED, et al. (2001) Ca2+-RhoA signaling pathway required for polyamine-dependent intestinal epithelial cell migration. Am J Physiol Cell Physiol 280: 993-1007.
- Rao JN, Guo X, Liu L, Zou T, Murthy KS, et al. (2003) Polyamines regulate Rho-kinase and myosin phosphorylation during intestinal epithelial restitution. Am J Physiol Cell Physiol 284: 848-859.
- Ray RM, Guo H, Patel M, Jin S, Bhattacharya S, et al. (2007) Role of myosin regulatory light chain and Rac1 in the migration of polyamine-depleted intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 292: 983-995.
- Rao JN, Platoshyn O, Li L, Guo X, Golovina VA, et al. (2002) Activation of K+ channels and increased migration of differentiated intestinal epithelial cells after wounding. Am J Physiol Cell Physiol 282: 885-898.
- Rao JN, Wang JY (2006) Regulation of Kv channel activity and intracellular junctions by polyamines in intestinal epithelial cells. Wang JY, Casero RA, Totowa, In: Polyamine Cell Signaling: Physiology, Pharmacology and Cancer Research, NJ: Humana, 363-381.
- Suh E, Traber PG (1996) An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol 16: 619-625.
- Cho CH, Wang JY (2002) Expression of Early Primary Response Genes in Healing of Gastrointestinal Mucosal Injury (eds): Gastrointestinal Mucosal Repair and Experimental Therapeutics. Front Gastrointest Res, Besel, Karger, 25: 82-100.
- Gu S, Papadopoulou N, Nasir O, Föller M, Alevizopoulos K, et al. (2011) Activation of membrane androgen receptors in colon cancer inhibits the prosurvival signals Akt/bad in vitro and in vivo and blocks migration via vinculin/actin signaling. Mol Med 17: 48-58.
- Solanas G, Batlle E (2011) Control of cell adhesion and compartmentalization in the intestinal epithelium. Exp Cell Res 317: 2695-2701.
- Agle KA, Vongsa RA, Dwinell MB (2010) Calcium mobilization triggered by the chemokine CXCL12 regulates migration in wounded intestinal epithelial monolayers. J Biol Chem 285: 16066-16075.
- Ray RM, McCormack SA, Covington C, Viar MJ, Zheng Y, et al. (2003) The requirement for polyamines for intestinal epithelial cell migration is mediated through Rac1. J Biol Chem 278: 13039-13046.
- Rao JN, Rathor N, Zou T, Liu L, Xiao L, et al. (2010) STIM1 translocation to the plasma membrane enhances intestinal epithelial restitution by inducing TRPC1-medidated Ca2+ signaling after wounding. Am J Physiol Cell Physiol 299: 579-588.
- McCormack SA, Viar MJ, Johnson LR (1993) Polyamines are necessary for cell migration by a small intestinal crypt cell line. Am J Physiol 264: 367-374.
- Pfeffer LM, Yang CH, Pfeffer SR, Murti A, McCormack SA, et al. (2000) Inhibition of ornithine decarboxylase induces STAT3 tyrosine phosphorylation and DNA binding in IEC-6 cells. Am J Physiol Cell Physiol 278: 331-335.
- Patel AR, Wang JY (1997) Polyamines modulate transcription but not posttranscription of c-myc and c-jun in IEC-6 cells. Am J Physiol 273: 1020-1029.
- Wang JY, McCormack SA, Viar MJ, Wang HL, Tzen CY, et al. (1993) Decreased expression of protooncogenes c-fos, c-myc, and c-jun following polyamine depletion in IEC-6 cells. Am J Physiol 265: 331-338.
- Yamaoka T, Frey MR, Dise RS, Bernard JK, Polk DB (2011) Specific epidermal growth factor receptor autophosphorylation sites promote mouse colon epithelial cell chemotaxis and restitution. Am J Physiol Gastrointest Liver Physiol 301: 368-376.
- Wilson AJ, Gibson PR (1999) Role of epidermal growth factor receptor in basal and stimulated colonic epithelial cell migration in vitro. Exp Cell Res 250: 187-196.
- Frey MR, Golovin A, Polk DB (2004) Epidermal growth factor-stimulated intestinal epithelial cell migration requires Src family kinase-dependent p38 MAPK signaling. J Biol Chem 279: 44513-44521.
- Ey B, Eyking A, Gerken G, Podolsky DK, Cario E (2009) TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury. J Biol Chem 284: 22332-22343.
- Greenspon J, Li R, Xiao L, Rao JN, Sun R, et al. (2011) Sphingosine-1-phosphate regulates the expression of adherens junction protein E cadherin and enhances intestinal epithelial cell barrier function. Dig Dis Sci 56: 1342-1353.
- Smalley-Freed WG, Efimov A, Burnett PE, Short SP, Davis MA, et al. (2010) p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J Clin Invest 120: 1824-1835.
- Mao X, Zeng X, Qiao S, Wu G, Li D (2011) Specific roles of threonine in intestinal mucosal integrity and barrier function. Front Biosci (Elite Ed) 3: 1192-1200.
- Tanaka-Okamoto M, Hori K, Ishizaki H, Itoh Y, Onishi S, et al. (2011) Involvement of afadin in barrier function and homeostasis of mouse intestinal epithelia. J Cell Sci 124: 2231-2240.
- Catalioto RM, Maggi CA, Giuliani S (2011) Intestinal epithelial barrier dysfunction in disease and possible therapeutical interventions. Curr Med Chem 18: 398-426.
- Guo X, Rao JN, Liu L, Zou T, Turner DJ, et al. (2003) Regulation of adherens junctions and epithelial paracellular permeability: a novel function for polyamines. Am J Physiol Cell Physiol 285: 1174-1187.
- Chen J, Rao JN, Zou T, Liu L, Marasa BS, et al. (2007) Polyamines are Required for Expression of Toll-like Receptor 2 Modulating Intestinal Epithelial Barrier Integrity. Am J Physiol Gastrointest Liver Physiol 293: 568-576.
- Yu T, Wang PY, Rao JN, Zou T, Liu L, et al. (2011) ChK2-dependent HuR phosphorylation regulates occludin mRNA translation and epithelial barrier function. Nucleic Acids Res 39: 8472-8487.
- Liu L, Guo X, Rao JN, Zou T, Xiao L, et al. (2009) Polyamines regulate E-cadherin transcription through c-myc modulating intestinal epithelial barrier function. Am J Physiol Cell Physiol 296: 801-810.
- Chen J, Xiao L, Rao JN, Zou T, Liu L, et al. (2008) JunD represses transcription and translation of the tight junction protein Zona Occludens-1 modulating intestinal epithelial barrier function. Mol Biol Cell 19: 3701-3712.
- Liu L, Rao JN, Zou T, Xiao L, Wang PY, et al. (2009) Polyamines regulate c-Myc translation through Chk2-dependent HuR phosphorylation. Mol Biol Cell 20: 4885-4898.
- Silberg DG, Wu GD (2003) Development of the alimentary tract, liver and pancreas. Rustgi AK, Saunders, Gastrointestinal cancers 7: 105-119.
- Drozdowski LA, Clandinin T, Thomson AB (2010) Ontogeny, growth and development of the small intestine: Understanding pediatric gastroenterology. World J Gastroenterol 16: 787-799.
- Milovic V (2001) Polyamines in the gut lumen: bioavailability and biodistribution. Eur J Gastroenterol Hepatol 13: 1021-1025.
- Wang JY (2007) Polyamines and mRNA stability in regulation of intestinal mucosal growth. Amino Acids 33: 241-252.
- Celano P, Baylin SB, Casero RA (1989) Polyamines differentially modulate the transcription of growth-associated genes in human colon carcinoma cells. J Biol Chem 264: 8922-8927.
- Pegg AE, Shantz LM, Coleman CS (1995) Ornithine decarboxylase as a target for chemoprevention. J Cell Biochem Suppl 22: 132-138.
- Rathor N, Wang SR, Chang ET, and Rao JN (2011) Differentiated intestinal epithelial cells express high levels of TGF-ß receptors and exhibit increased sensitivity to growth inhibition. Int J Clin Exp Med 4: 299-308.
- Yatin M (2002) Polyamines in Living Organisms. Journal of Cell and Molecular Biology 1: 57-67.
- Gao Y, He L, Katsumi H, Sakane T, Fujita T, et al. (2008) Improvement of intestinal absorption of water-soluble macromolecules by various polyamines: Intestinal mucosal toxicity and absorption-enhancing mechanism of spermine. Int J Pharm 354: 126-134.
- Tome ME, Fiser SM, Payne CM, Gerner EW (1997) Excess putrescine accumulation inhibits the formation of modified eukaryotic initiation factor 5A (eIF-5A) and induces apoptosis. Biochem J 328: 847-854.
- Zhang AH, Rao JN, Zou T, Liu L, Marasa BS, et al. (2007) P53-dependent NDRG1 expression induces inhibition of intestinal epithelial cell proliferation but not apoptosis after polyamine depletion. Am J Physiol Cell Physiol 293: 379-389.
- Li L, Li J, Rao JN, Li M, Bass BL, et al. (1999) Inhibition of polyamine synthesis induces p53 gene expression but not apoptosis. Am J Physiol 276: 946-954.
- Rao JN, Li L, Bass BL, Wang JY (2000) Expression of the TGF-ß receptor gene and sensitivity to growth inhibition following polyamine depletion. Am J Physiol Cell Physiol 279: 1034-1044.
- Li L, Rao JN, Guo X, Liu L, Santora R, et al. (2001) Polyamine depletion stabilizes p53 resulting in inhibition of normal intestinal epithelial cell proliferation. Am J Physiol Cell Physiol 281: 941-953.
- Zou T, Rao JN, Liu L, Marasa BS, Keledjian KM, et al. (2005) Polyamine depletion induces nucleophosmin modulating stability and transcriptional activity of p53 in intestinal epithelial cells. Am J Physiol Cell Physiol 289: 686-696.
- Patel AR, Wang JY (1999) Polyamine depletion is associated with an increase in JunD/AP-1 activity in small intestinal crypt cells. Am J Physiol 276: 441-450.
- Li L, Liu L, Rao JN, Esmaili A, Strauch ED, et al. (2002) JunD stabilization results in inhibition of normal intestinal epithelial cell growth through p21 after polyamine depletion. Gastroenterology 123: 764-779.
- Zou T, Mazan-Mamczarz K, Rao JN, Liu L, Marasa BS, et al. (2006) Polyamine depletion increases cytoplasmic levels of RNA-binding protein HuR leading to stabilization of nucleophosmin and p53 mRNAs. J Biol Chem 281: 19387-19394.
- Marasa BS, Rao JN, Zou T, Liu L, Keledjian KM, et al. (2006) Induced TRPC1 expression sensitizes intestinal epithelial cells to apoptosis by inhibiting NF-?B activation through Ca2+ influx. Biochem J 397: 77-87.
- Xiao L, Rao JN, Zou T, Liu L, Marasa BS, et al. (2007) Polyamines regulate the stability of activating transcription factor-2 mRNA through RNA-binding protein HuR in intestinal epithelial cells. Mol Biol Cell 18: 4579-4590.
- Xiao L, Rao JN, Zou T, Liu L, Marasa BS, et al. (2007) Induced JunD in intestinal epithelial cells represses CDK4 transcription through its proximal promoter region following polyamine depletion. Biochem J 403: 573-581.
- Xiao L, Rao JN, Zou T, Liu L, Yu TX, et al. (2010) Induced ATF-2 represses CDK4 transcription through dimerization with JunD inhibiting intestinal epithelial cell growth after polyamine depletion. Am J Physiol Cell Physiol 298: 1226-1234.
- Xiao L, Cui YH, Rao JN, Zou T, Liu L, et al. (2011) Regulation of Cyclin-Dependent Kinase 4 Translation through CUG-Binding Protein 1 and microRNA-222 by Polyamines. Mol Biol Cell 22: 3055-3069.
- Zhang X, Zou T, Rao JN, Liu L, Xiao L, et al. (2009) Stabilization of XIAP mRNA through the RNA binding protein HuR regulated by cellular polyamines. Nucleic Acids Res 37: 7623-7637.
- Zhang HM, Keledjian KM, Rao JN, Zou T, Liu L, et al. (2006) Induced focal adhesion kinase expression suppresses apoptosis by activating NF-kB signaling in intestinal epithelial cells. Am J Physiol Cell Physiol 290: 1310-1320.
- Zhang HM, Rao JN, Guo X, Liu L, Zou T, et al. (2004) Akt kinase activation blocks apoptosis in intestinal epithelial cells by inhibiting caspase-3 after polyamine depletion. J Biol Chem 279: 22539-22547.
- Wang PY, Rao JN, Zou T, Liu L, Xiao L, et al. (2010) Post-transcriptional regulation of MEK-1 by polyamines through the RNA-binding protein HuR modulating intestinal epithelial apoptosis. Biochem J 426: 293-306.
- Zou T, Liu L, Rao JN, Marasa BS, Chen J, et al. (2008) Polyamines modulate the subcellular localization of RNA-binding protein HuR through AMP-activated protein kinase-regulated phosphorylation and acetylation of importin a1. Biochem J 409: 389-398.
- Zou T, Rao JN, Liu L, Xiao L, Yu TX, et al. (2010) Polyamines regulate the stability of JunD mRNA by modulating the competitive binding of its 3' untranslated region to HuR and AUF1. Mol Cell Biol 30: 5021-5032.
- Gawel-Thompson KJ, Greene RM (1989) Epidermal growth factor: modulator of murine embryonic palate mesenchymal cell proliferation, polyamine biosynthesis, and polyamine transport. J Cell Physiol 140: 359-370.
- Cui YH, Xiao L, Rao JN, Zou T, Liu L, et al. (2012) miR 503 represses CUG binding protein 1 translation by recruiting CUGBP1 mRNA to processing bodies. Mol Biol Cell 23: 151-162.
- Jänne J, Alhonen L, Pietilä M, Keinänen TA (2004) Genetic approaches to the cellular functions of polyamines in mammals. Eur J Biochem 271: 877-894.
- Wang C, Delcros JG, Cannon L, Konate F, Carias H, et al. (2003) Defining the molecular requirements for the selective delivery of polyamine conjugates into cells containing active polyamine transporters. J Med Chem 46: 5129-5138.
- Milovic V, Turchanowa L (2003) Polyamines and colon cancer. Biochem Soc Trans 31: 381-383.
- Scemama JL, Grabié V, Seidel ER (1993) Characterization of univectorial polyamine transport in duodenal crypt cell line. Am J Physiol 265: 851-856.
- Larqué E, Sabater-Molina M, Zamora S (2007) Biological significance of dietary polyamines. Nutrition 23: 87-95.
- Ignatenko NA, Besselsen DG, Roy UK, Stringer DE, Blohm-Mangone KA, et al. (2006) Dietary putrescine reduces the intestinal anticarcinogenic activity of sulindac in a murine model of familial adenomatous polyposis. Nutr Cancer 56: 172-181.
- Woster PM (2006) Polyamine structure and synthetic analogs. From: polyamine cell signaling: physiology, pharmacology, and cancer research. Wang JY, Casero RA 271: 877-894.
- Mackenzie GG, Ouyang N, Xie G, Vrankova K, Huang L, et al. (2011) Phospho-sulindac (OXT-328) combined with difluoromethylornithine prevents colon cancer in mice. Cancer Prev Res 4: 1052-1060.
- Motawi TK, Abd Elgawad HM, Shahin NN (2007) Modulation of indomethacin-induced gastric injury by spermine and taurine in rats. J Biochem Mol Toxicol 21: 280-288.
- Biczó G, Hegyi P, Sinervirta R, Berczi S, Dósa S, et al. (2010) Characterization of polyamine homeostasis in l-ornithine-induced acute pancreatitis in rats. Pancreas 39: 1047-1056.
- Tirupathi Pichiah PB, Suriyakalaa U, Kamalakkannan S, Kokilavani P, Kalaiselvi S, et al. (2011) Spermidine may decrease ER stress in pancreatic beta cells and may reduce apoptosis via activating AMPK dependent autophagy pathway. Med Hypotheses 77: 677-679.
- Blume GB, Koenig H, Goldstone AD (1985) Association of increased polyamine levels with isoproterenol-stimulated mucin secretion in the rat submandibular gland. Biochem Biophys Res Commun 132: 118-125.
- Gómez Gallego C, Ros Berruezo G, Bernal Cava MJ, Pérez Conesa D, Periago Castón MJ (2008) Role of polyamines in diet. Importance of polyamines in infant nutrition. Arch Latinoam Nutr 58: 117-125.
- Matsumoto M, Kurihara S, Kibe R, Ashida H, Benno Y (2011) Longevity in mice is promoted by probiotic-induced suppression of colonic senescence dependent on upregulation of gut bacterial polyamine production. PLoS One 6: e23652.
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15128
- [From(publication date):
specialissue-2013 - Dec 20, 2025] - Breakdown by view type
- HTML page views : 10444
- PDF downloads : 4684
