Review Article Open Access
Raman Endoscopy for Objective Diagnosis of Early Cancer in the Gastrointestinal System
Mads Sylvest Bergholt1, Wei Zheng1, Khek Yu Ho2, Ming Teh3, Khay Guan Yeoh2 and Zhiwei Huang1*
1Optical Bioimaging Laboratory, Department of Biomedical Engineering, Faculty of Engineering, National University of Singapore, Singapore
2Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore
3Department of Pathology, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore
- Corresponding Author:
- Zhiwei Huang
Optical Bioimaging Laboratory
Department of Bioengineering Faculty of Engineering
National University of Singapore 9
Engineering Drive 1, Singapore 117576
Tel: +65- 6516-8856
Fax: +65- 6872-3069
E-mail: biehzw@nus.edu.sg
Received Date: June 15, 2013; Accepted Date: August 16, 2013; Published Date: August 19, 2013
Citation: Bergholt MS, Zheng W, Ho KY, Yeoh KG, Huang Z (2013) Raman Endoscopy for Objective Diagnosis of Early Cancer in the Gastrointestinal System. J Gastroint Dig Syst S1:008. doi: 10.4172/2161-069X.S1-008
Copyright: © 2013 Bergholt MS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
In recent years, there has been an increasing interest in the use of optical spectroscopic technologies for early cancer detection in the gastrointestinal system. Raman endoscopy is a unique fiber-optic spectroscopic technology for in vivo histopathological assessment of tissue based on endogenous biomolecules. Recent technical advances have enabled in vivo tissue Raman measurements in real-time during clinical endoscopic examination. The endoscopist can apply Raman endoscope to analyze the biomolecular signature of cells and tissues in vivo, thereby enabling noninvasive optical biopsies of suspicious lesions during ongoing endoscopy. In this article, we review the applications of Raman endoscopy in the gastrointestinal system and also discuss its potential function and role in gastrointestinal endoscopy and therapeutics. The results of the latest Raman clinical trials are summarized, illustrating the great potential of Raman endoscope technology for non-invasive, in vivo diagnosis and detection of pre-cancer and early cancer in the gastrointestinal system.
Keywords
Early gastrointestinal cancer; Optical biopsy; in vivo diagnosis; Endoscopy
Abbreviations
WLR: White-Light Reflectance; NBI: Narrow-Band Imaging; AFI: Auto Fluorescence Imaging; NIR: Near-Infrared; CCD: Charge-Coupled Device; PCA: Principal Component Analysis; PLSDA: Partial Least Squares-Discriminant Analysis
Introduction
Gastrointestinal malignancies are the major health burdens and one of the leading causes of death in humans world-wide [1,2]. For instance, a total of 989,600 new stomach cancer cases and 738,000 deaths are estimated to occur annually in the United States, accounting for 8% of the total cancer cases and 10% of total deaths [1]. Early identification and adequate intervention are critical measures to reducing the cancer-related mortality rates. Flexible endoscopy in patients with suspicious symptoms is the primary choice for the clinician. However, dysplastic lesions or grossly inconspicuous cancers are endoscopically indistinguishable from the surrounding benign tissue using conventional white-light reflectance (WLR) endoscopy, narrowband imaging (NBI) or autofluorescence imaging (AFI) [3]. This is because these modalities largely rely on visual assessment of structural and morphological details and provide little or no biomolecular information [3]. Endoscopic inspections are therefore associated with a vast number of unnecessary biopsies, which are clinically labour intensive and also financial burden to the patients. Moreover, histopathological characterization of minute specimens can also be highly subjective. Inter-observer disagreements of up to >50% have been reported for low-grade dysplasia (LGD) [4]. Hence, it is highly desirable to develop new advanced endoscopic modalities for improving early diagnosis, surveillance and management of patients with gastrointestinal malignancies [3]. The targeted biopsies with a minimally invasive objective technique based on endogenous biomolecules of tissue for identifying high-risk tissue regions in situ could greatly reduce biopsy sampling errors as well as health care expenses and burden on the individual patient.
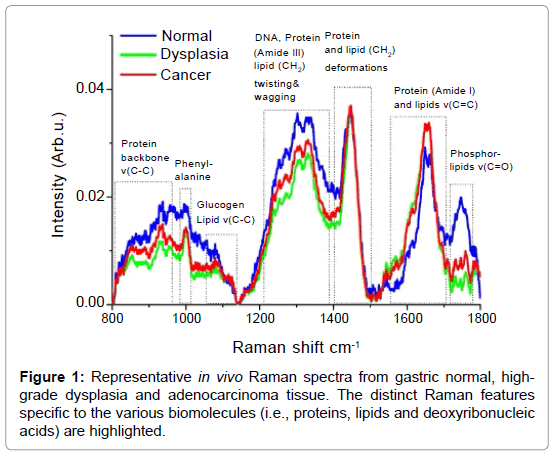
Raman spectroscopy is a unique optical spectroscopic technique capable of probing fundamental vibrations of biomolecules in tissue and cells [5]. The Raman Effect was discovered in 1928 by the Indian physicist Sir Chandrasekhara Venkata Raman [6,7], who won the Nobel Prize in 1930 due to this discovery. Raman spectroscopy represents a non-mutagenic and label-free optical technology that utilizes inelastic light scattering. When incident laser light induces a polarization change of molecules, a small proportion of the incident photons (˜1 in 108) are scattered with a change in frequency [8-16]. The absorbed energy corresponds to the specific Raman active vibrational modes of the molecules. The inelastic scattered light therefore represents a spectral molecular “fingerprint”. For tissue and cells, Raman spectroscopy is able to harvest a wealth of information from the myriad of interand/ or intra- cellular components (i.e., proteins, lipids and deoxyribonucleic acid) and has therefore been considered a promising technique for noninvasive histopathological assessments (i.e., optical biopsy) in medicine [5,7,17-23]. Figure 1 shows representative examples of Raman spectra from gastric normal, high-grade dysplasia and adenocarcinoma as confirmed by histopathological examinations. The distinct Raman features specific to the various biomolecules (i.e., proteins, lipids and deoxyribonucleic acids) are also highlighted in the Figure 1. These highly specific spectra directly reflect the carcinogenic onset at the biomolecular level [24-29]. Encouraged by extensive in vitro Raman spectroscopy studies on excised gastrointestinal tissue [8-16], there have recently been major efforts to translate Raman spectroscopy into clinical endoscopic examinations [17-23]. in vivo Raman clinical endoscopic applications is, however, extremely challenging and have been limited not only by the difficulty in capturing inherently weak tissue Raman signals, but also by the slow speed of spectral measurements (>5 sec). The miniaturization of flexible fiber-optic Raman probes that can pass down the accessory channel of medical endoscopes for effective tissue Raman light collections presents another great challenge in clinical Raman spectroscopy [23]. Recent progress in instrumentation and translational research of Raman spectroscopy has lead to a wide range of in vivo applications in biomedicine. In this article, we will review the applications of novel Raman endoscopy technology in the gastrointestinal system and discuss the potential function and role in gastrointestinal endoscopy and therapeutics. The results of latest Raman clinical trials are summarized, illustrating the great potential of this unique Raman technology for non-invasive, in vivo diagnosis and detection of precancer and early cancer in the gastrointestinal system.
Brief Overview of Raman Spectroscopy Studies on the Gastrointestinal System
A large number of in vitro Raman spectroscopy studies on excised tissues and cells have been reported on the gastrointestinal system [8-16]. Stone and co-workers [8-9] have characterized esophageal cells and tissue specimens associated with Barrett’s disease. Raman microspectroscopy has been used to map the biomolecular distribution of protein, lipid and DNA in sectioned esophageal tissue samples, revealing the distinct biomolecular features associated with carcinogenesis [9]. Huang and co-workers reported a series of in vitro tissue Raman studies on gastric tissues [11-15]. For instance, differentiation among different types of pathologic gastric tissues (e.g. Helicobactor Pylori (HP) infection, intestinal metaplasia, dysplasia, neoplasia, diffuse and intestinal-type adenocarcinoma) with good accuracies (of ˜85-96%) was demonstrated using Raman spectroscopy technique. Raman spectroscopy has also shown promising results for differential diagnosis of adenomatous and hyperplastic polyps in the colon in vitro [16,21]. Wilson and co-workers [21] reported a Raman study on eight patients, indicating that Raman spectroscopy could distinguish adenomatous from hyperplastic polyps ex vivo with 91% sensitivity and 95% specificity. To move the Raman technique for medical diagnosis at endoscopy, Shim et al. developed in vivo fiberoptic Raman spectroscopy technique for gastrointestinal endoscopic applications [19,20], and demonstrated the capability of Raman spectroscopy for the in vivo differential diagnosis between adenomatous and hyperplastic polyps in the colon in vivo with a sensitivity of 100% and specificity of 89% [21]. However, Raman acquisition time in their Raman system design was lengthy (>5 sec), which was impractical for routine real-time endoscopic applications [23].
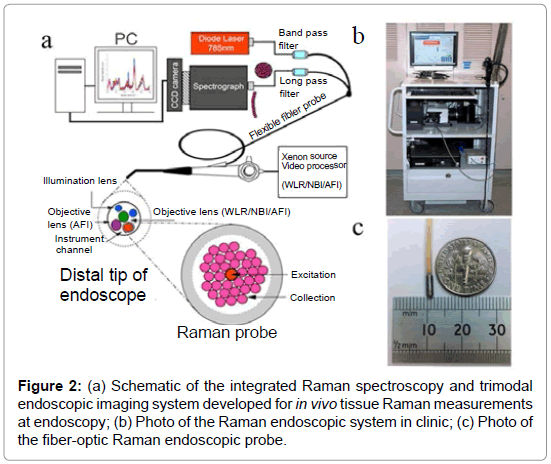
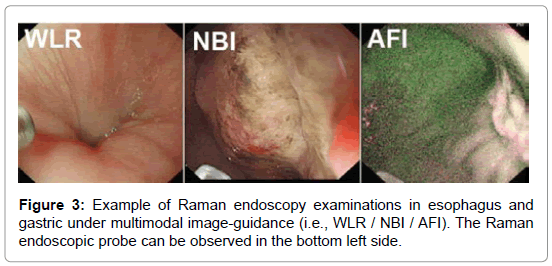
With very recent technological advancements in Raman spectroscopy instrumentation, including high-throughput spectrographs, NIR lasers, sensitive CCD cameras, personal computers and fiber-optic Raman probes, substantial progress has been made in translating Raman spectroscopy into real-time in vivo routine endoscopic applications [22-32]. Our group has developed a rapid trimodal image guided (WLR / NBI / AFI) Raman endoscopy system for in vivo tissue diagnosis during gastrointestinal endoscopy [22,23]. The Raman endoscopy platform (Figure 2) consists of a spectrumstabilized 785 nm diode laser (maximum output: 300 mW, B&W TEK Incorporated, Newark, Delaware), a transmissive imaging spectrograph (Holospec f/1.8, Kaiser Optical Systems, Ann Arbor, Michigan), a liquid nitrogencooled, near-infrared (NIR)-optimized, back-illuminated deep depletion charge coupled device (CCD) camera (Spec-10: 400BR/LN, Princeton Instruments, Trenton, New Jersey), and a specially designed Raman endoscopic probe for both laser light delivery and in vivo tissue Raman signal collection. The 1.8 mm fiber optic Raman endoscopic probe (Figure 2), compatible with flexible medical endoscopes, consists of 32 Raman collection fibers (core diameter of 200 μm) surrounding the central laser light delivery fiber (core diameter of 200 μm), with two stages of optical filtering incorporated at the proximal and distal ends of the probe for maximizing the collection of tissue Raman signals while reducing the interference of Rayleigh scattered light, fiber fluorescence, and silica Raman signals [23]. The fiber-optic Raman probe can be inserted into the instrument channel of conventional endoscopes and placed in gentle contact with the gastrointestinal mucosa for real-time in vivo tissue diagnosis. This Raman endoscopy platform is one of the most advanced diagnostic systems for in vivo Raman endoscopic diagnosis. Examples of Raman endoscopy examinations in esophagus and gastric under multimodal image-guidance (i.e., WLR / NBI / AFI) are shown in Figure 3. The clinical Raman endoscopy system can be used to measure in vivo Raman spectra in the gastric and esophagus within 0.1-0.5 sec at endoscopy. With the use of this novel Raman endoscopy technology developed, we have carried out a nationwide gastric cancer screening program, focusing on early diagnosis and treatment of gastroesophageal malignancies run by the Singapore gastric cancer epidemiology, clinical and genetic program (GCEP) [31]. Since August 2008 till June 2013, a total of 450 patients have been enrolled in in vivo Raman endoscopic examinations for screening various gastric indications, including dyspepsia, bleeding and upper gastrointestinal neoplasia. Over 12.000 in vivo Raman spectra have been measured from different pathological tissue types of gastric tissue to build a comprehensive Raman spectral database [30]. Accordingly, Raman endoscopy in the gastrointestinal system now enables clinicians to obtain a probabilistic measure of the risk associated with suspicious lesions, thereby improving the guidance of physical biopsies. We have also conducted a preliminary study in the esophagus to evaluate the diagnostic capability of Raman endoscopy for cancer diagnosis [26]. Twenty-seven esophageal patients with suspicious symptoms were recruited and 42 in vivo Raman spectra were measured from normal and cancerous tissues (i.e., squamous cell carcinoma and adenocarcinoma). Semi-quantitative biochemical analysis showed that cancerous tissue was associated with major changes in biochemical compositions (e.g., protein, DNA, lipid and glycogen) [26]. Cancer can be detected in vivo with sensitivity of 97% and specificity of 95%. Another retrospective analysis using Raman endoscopy under NBI guidance with 30 recruited patients showed sensitivity of 94% and specificity of 96% for diagnosis of gastric dysplasia in vivo [33]. We have also elucidated the biomolecular profile of esophageal and gastric carcinogenesis sequence by modeling the biochemical compositions using reference biomolecules (e.g., proteins, deoxyribonucleic acids and lipids) of gastrointestinal mucosa [26,27]. The study based on 83 patients uncovered the progressive changes of biochemical constituents in gastric tissue associated with multi-step pathways of gastric intestinal type carcinogenesis. The semi-quantitative spectral modeling identified a gradual disease transformation from normal, intestinal metaplasia to dysplasia and ultimately adenocarcinoma that reflected a multitude of oncogenes, cell cycle regulators, growth factors, mitoses, and nuclear abnormalities etc. (i.e. progressive accumulation of biochemical and biomolecular changes associated with neoplastic transformation). Multivariate statistical analysis showed that Raman endoscopy provided a good discriminative capability among normal tissue (sensitivity of 76% and specificity of 87%), intestinal metaplasia (sensitivity of 47% and specificity of 88%), dysplasia (sensitivity of 83% and specificity of 96%) and cancer (sensitivity of 85% and specificity of 96%). The positive predictive value (PPV) and negative predictive value (NPV) were 85% and 97%, respectively, for high-risk lesions (i.e., dysplasia and cancer), confirming that Raman endoscopy can play a key role in endoscopic examinations of the gastrointestinal system. The differential diagnosis between benign and malignant gastric ulcers remains another great challenge in clinical endoscopy [29]. A study based on 71 patients was used to evaluate the differential diagnosis between benign and malignant gastric ulcerations. The study showed diagnostic sensitivities of 90.8%, 84.7%, 82.1%, and specificities of 93.8%, 94.5%, 95.3%, respectively, for identification of normal mucosa, benign and malignant ulcerous lesions in the stomach [29].
The Raman spectroscopy technique has also shown promising potential in other endoscopic accessible organs. For instance, Zeng and co-workers have developed an image-guided Raman endoscopic system for bronchoscopic applications [32]. They conducted a preliminary study based on 46 patients, and reported that Raman endoscopy complements WLR and AFI by reducing the false positives biopsies. WLR+AFI and Raman endoscopy showed the sensitivity and specificity of 96% and 91% respectively for detecting the preneoplastic lesions [32]. Draga et al. conducted an in vivo Raman study on 38 patients in the bladder during the procedure of transurethral resection of bladder tumors (TURBT), and found that Raman spectroscopy could differentiate bladder cancer from normal tissue with a sensitivity of 85% and a specificity of 79% [34]. Very recently, our group has also reported on the implementation of transnasal Raman endoscopy of nasopharyngeal and laryngeal tissues [35]. We conducted a study on 39 patients and measured a total of 94 Raman spectra using transnasal image-guided high-wavenumber Raman endoscopy [36]. Raman spectra from tumors showed distinct spectral characteristics, and could be diagnosed on spectrum basis with a sensitivity of 90.3% and a specificity of 90.9%. Overall, the above literature studies have demonstrated the high diagnostic accuracy of Raman technique for precancer and cancer detection in vivo. Hence, in vivo Raman endoscope technology has prospect to revolutionize clinical endoscopy in healthcare.
Discussion
Early diagnosis directly affects the morbidity and mortality rates in patients with gastrointestinal cancers. The ability to accurately diagnose gastrointestinal cancers in the early stages would allow curative treatment options (e.g., endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), polypectomy) [3]. For more than a hundred years, endoscopists have relied on the visualization of gross morphological manifestations of tissue with histopathological assessment of suspicious lesions in order to establish the clinical diagnosis. In general, this approach is labor intensive and prone to human errors. With ever-aging populations, the need for accurate and efficient cancer screening technologies is highly desirable. The development of a new objective endoscopic modality for in vivo diagnosis (i.e., optical biopsy) at the biomolecular level would therefore represent one of the major advances since the invention of modern endoscopes [3].
Raman endoscopy represents an advanced spectroscopic tool that is able to harvest a wealth of biomolecular information from tissue without labelling [5,7]. This objective technology therefore enables accurate optical biopsy exclusively based on functional and biomolecular manifestations of the tissue. There has been profound agreement on the diagnostic capability of Raman spectroscopy through extensive in vitro and in vivo studies [8-16,18-30]. In the forthcoming years, the precise role and applications of Raman endoscopy in biomedicine must be defined. The current indications for the use of Raman endoscopy may include all clinical scenarios that require histopathological biopsy samplings in the esophagus, gastric and colon. Therefore, the general applications of Raman endoscopy include the detection and diagnosis of premalignant and malignant mucosa. As premalignant foci are prone to biopsy sampling errors, Raman endoscopy can be used to accurately pinpoint the high-risk tissue sites and objectively stratify those patients who have higher risk of developing cancer and prompt increased frequency of surveillance or therapeutic intervention to the individual patient. We hence foresee that Raman endoscopy can have a pivotal role in the screening and management of GI patients. For instance, current clinical guidelines in patients with Barrett’s esophagus adopt four-quadrant random biopsy protocols that have intrinsically low diagnostic yield but associated with a vast number of negative biopsies [2]. In Barrett’s esophagus, Raman endoscopy could be used to target the high-yield (i.e., high-grade dysplasia) regions as well as detect those lesions that are indefinite for dysplasia or presenting with extensive inflammation, where histopathological manifestations are insufficient for final diagnostic decision. It may even be possible to detect biochemical and biomolecular changes in high-risk patients occurring before histopathological manifestations [37]. This new endoscopy modality could become a game-changer to the current resource-intensive random biopsy protocol, thereby altering the clinical pathways [17].
Therapeutic interventions are another scenarios where Raman endoscopy could play a key role in biomedicine. Raman endoscopy offers the surgeon a novel tool, which allows real-time assessment of tissue pathology and could be used to accurately define the resection margins of macroscopically invisible dysplasia or cancer in patients undergoing surgical intervention (e.g., EMR, ESD etc.). This will enable complete excision, and subsequent margin assessment, so that malignant progression can be efficiently prevented. With the second generation of this technology, this might open the possibility of combining final diagnosis and therapeutic eradication (e.g., EMR, ESD) in one single procedure. Raman endoscopy could also have important applications in the monitoring of chemotherapeutic response in tissue and cells [38]. The monitoring of the response of tumor cells could possible allow a deeper understanding of the molecular and biochemical mechanisms associated with anti-cancer agents in vivo. There are several other exciting areas to explore in translational clinical research of Raman endoscopy. The combination of Raman endoscopy with other optical imaging techniques (e.g. optical coherence tomography (OCT), confocal endomicroscopy imaging) and/or conventional medical imaging (e.g. ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI)) may also be the direction to explore for functional imaging at the molecular, cellular, tissue and even organ levels. Moreover, the correlation of Raman spectra with other fields of biomolecular research (e.g., genomics, proteomics) could further deepen the insight into the pathway of gastrointestinal carcinogenesis in situ.
There are room for further improvement in Raman endoscope instrumentation. All the in vivo literature studies so far have utilized volume-type fiber-optic Raman endoscopic probes which interrogates a relatively large tissue volume ˜1 mm3 [20,23-30,32]. Very recently, novel confocal Raman probes have been developed to selectively interrogate the gastrointestinal epithelium <200 μm associated with early carcinogenic onset, thereby enhancing the sensitivity to epithelial premalignant lesions (e.g., dysplasia) [39-41]. Moreover, the second generation Raman endoscopy system has very recently been developed which utilizes a broader range of biomolecular frequencies simultaneously (i.e., 800-1800 and 2800-3600 cm-1) for improved tissue characterization and diagnosis [42]. In terms of clinical merit and cost effective analysis, there are large prospective randomized multicenter studies underway to assess the performance of Raman endoscopy in gastrointestinal examinations. It is expected that these technical advancements have great impact on further pushing the frontier of Raman endoscopy into routine clinical applications. Raman endoscopy has undoubtedly opened up new horizons in the field of medicine that enables biopsy-free, real-time, in vivo diagnosis of premalignant lesions as well as assessments of tumor margins for surgical operation. Raman endoscopy is a versatile tool for research, diagnostic and therapeutic, and could benefit the patients and medical society with non-invasive diagnosis, reduction of the vast number of biopsies, surgery guidance as well as significantly low cost of endoscopic procedures.
Summary
Gastrointestinal malignancies remain one of the most common causes of cancer deaths, as well as one of the largest burdens on healthcare systems throughout the world. Raman endoscopy has opened up an exciting avenue to assist the endoscopists in improving in vivo cancer and precancer diagnosis and therapeutic follow-up in the gastrointestinal system. We envisage that with further technology advancements in the coming years, the Raman endoscopy technique will be emerging as a new routine endoscopic tool for shaping in vivo medical diagnosis in hospitals. Undoubtedly, fiber-optic Raman endoscopy for tissue optical biopsies will tremendously benefit both the patients and the healthcare systems, as it enables the noninvasive, functional and biomolecular assessment of gastrointestinal tissue in vivo, thereby providing the clinicians with critical diagnostic information for medical decision making in real-time during clinical endoscopic examination.
Acknowledgements
This research was supported by the National Medical Research Council and the Biomedical Research Council, Singapore.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69-90.
- Shaheen NJ, Richter JE (2009) Barrett's oesophagus. Lancet 373: 850-861.
- Li QL, Zhong YS, Chen WF, He MJ, Zhou PH, et al. (2012) New EndoscopicDiagnosis and Treatment Options for Early Esophageal Cancer. J Gastroint Dig Syst 2: 1-8.
- Reid BJ, Haggitt RC, Rubin CE, Roth G, Surawicz CM, et al. (1988) Observer variation in the diagnosis of dysplasia in Barrett's esophagus. Hum Pathol 19: 166-178.
- Nijssen A, Koljenovic S, Bakker Schut TC, Caspers PJ, Puppels GJ (2009) Towards oncological application of Raman spectroscopy. J Biophotonics 2: 29-36.
- Raman CV, Krishnan KS (1928) A new type of secondary radiation. Nature 121: 501-502.
- Stone N, Kendall C, Smith J, Crow P, Barr H (2004) Raman spectroscopy for identification of epithelial cancers. Faraday Discuss 126: 141-157.
- Hutchings J, Kendall C, Smith B, Shepherd N, Barr H, et al. (2009) The potential for histological screening using a combination of rapid Raman mapping and principal component analysis. J Biophotonics 2: 91-103.
- Shetty G, Kendall C, Shepherd N, Stone N, Barr H (2006) Raman spectroscopy: elucidation of biochemical changes in carcinogenesis of oesophagus. Br J Cancer 94: 1460-1464.
- Kawabata T, Kikuchi H, Okazaki S, Yamamoto M, Hiramatsu Y, et al. (2011) Near-infrared multichannel Raman spectroscopy with a 1064 nm excitation wavelength for ex vivo diagnosis of gastric cancer. J Surg Res 169: e137-143.
- Teh SK, Zheng W, Ho KY, Teh M, Yeoh KG, et al. (2008) Diagnostic potential of near-infrared Raman spectroscopy in the stomach: differentiating dysplasia from normal tissue. Br J Cancer 98: 457-465.
- Teh SK, Zheng W, Ho KY, Teh M, Yeoh KG, et al. (2010) Near-infrared Raman spectroscopy for early diagnosis and typing of adenocarcinoma in the stomach. Br J Surg 97: 550-557.
- Teh SK, Zheng W, Ho KY, Teh M, Yeoh KG, et al. (2010) Near-infrared Raman spectroscopy for optical diagnosis in the stomach: identification of Helicobacter-pylori infection and intestinal metaplasia. Int J Cancer 126: 1920-1927.
- Teh SK, Zheng W, Ho KY, Teh M, Yeoh KG, et al. (2009) Nearinfrared Raman spectroscopy for gastric precancer diagnosis. J Raman Spectrosc 40: 908-914.
- Teh SK, Zheng W, Ho KY, Teh M, Yeoh KG, et al. (2008) Diagnosis of gastric cancer using near-infrared Raman spectroscopy and classification and regression tree techniques. J Biomed Opt 13: 034013.
- Widjaja E, Zheng W, Huang Z (2008) Classification of colonic tissues using near-infrared Raman spectroscopy and support vector machines. Int J Oncol 32: 653-662.
- Almond LM, Hutchings J, Shepherd N, Barr H, Stone N, et al. (2011) Raman spectroscopy: a potential tool for early objective diagnosis of neoplasia in the oesophagus. J Biophotonics 4: 685-695.
- Shim MG, Wilson BC (1997) Development of an in vivo Raman spectroscopic system for diagnostic applications. J Raman Spectrosc 28: 131-142.
- Shim MG, Wilson BC, Marple E, Wach M (1999) Study of fiberoptic probes for in vivo medical Raman spectroscopy. Appl Spectrosc 53: 619-627.
- Shim MG, Song LM, Marcon NE, Wilson BC (2000) in vivo near-infrared Raman spectroscopy: demonstration of feasibility during clinical gastrointestinal endoscopy. Photochem Photobiol 72: 146-150.
- Molckovsky A, Song LM, Shim MG, Marcon NE, Wilson BC (2003) Diagnostic potential of near-infrared Raman spectroscopy in the colon: differentiating adenomatous from hyperplastic polyps. Gastrointest Endosc 57: 396-402.
- Huang Z, Zeng H, Hamzavi I, McLean DI, Lui H (2001) Rapid near-infrared Raman spectroscopy system for real-time in vivo skin measurements. Opt Lett 26: 1782-1784.
- Huang Z, Teh SK, Zheng W, Mo J, Lin K, et al. (2009) Integrated Raman spectroscopy and trimodal wide-field imaging techniques for real-time in vivo tissue Raman measurements at endoscopy. Opt Lett 34: 758-760.
- Bergholt MS, Zheng W, Lin K, Ho KY, Teh M, et al. (2011) in vivo diagnosis of gastric cancer using Raman endoscopy and ant colony optimization techniques. Int J Cancer 128: 2673-2680.
- Bergholt MS, Zheng W, Lin K, Ho KY, Teh M, et al. (2011) Characterizing variability in in vivo Raman spectra of different anatomical locations in the upper gastrointestinal tract toward cancer detection. J Biomed Opt 16: 037003.
- Bergholt MS, Zheng W, Lin K, Ho KY, Teh M, et al. (2011) in vivo diagnosis of esophageal cancer using image-guided Raman endoscopy and biomolecular modeling. Technol Cancer Res Treat 10: 103-112.
- Bergholt MS, Zheng W, Ho KY, Teh M, Yeoh KG, et al. (2013) Fiber-optic Raman spectroscopy probes gastric carcinogenesis in vivo at endoscopy. J Biophotonics 6: 49-59.
- Bergholt MS, Zheng W, Lin K, Ho KY, Teh M, et al. (2011) Combining near-infrared-excited autofluorescence and Raman spectroscopy improves in vivo diagnosis of gastric cancer. Biosens Bioelectron 26: 4104-4110.
- Bergholt MS, Zheng W, Lin K, Ho KY, Teh M, et al. (2010) Raman endoscopy for in vivo differentiation between benign and malignant ulcers in the stomach. Analyst 135: 3162-3168.
- Duraipandian S, Sylvest Bergholt M, Zheng W, Yu Ho K, Teh M, et al. (2012) Real-time Raman spectroscopy for in vivo, online gastric cancer diagnosis during clinical endoscopic examination. J Biomed Opt 17: 081418.
- Yeoh KG (2007) How do we improve outcomes for gastric cancer? J Gastroenterol Hepatol 22: 970-972.
- Short MA, Lam S, McWilliams AM, Ionescu DN, Zeng H (2011) Using laser Raman spectroscopy to reduce false positives of autofluorescence bronchoscopies: a pilot study. J Thorac Oncol 6: 1206-1214.
- Huang Z, Bergholt MS, Zheng W, Lin K, Ho KY, et al. (2010) in vivo early diagnosis of gastric dysplasia using narrow-band image-guided Raman endoscopy. J Biomed Opt 15: 037017.
- Draga RO, Grimbergen MC, Vijverberg PL, van Swol CF, Jonges TG, et al. (2010) in vivo bladder cancer diagnosis by high-volume Raman spectroscopy. Anal Chem 82: 5993-5999.
- Bergholt MS, Lin K, Zheng W, Lau DP, Huang Z (2012) in vivo, real-time, transnasal, image-guided Raman endoscopy: defining spectral properties in the nasopharynx and larynx. J Biomed Opt 17: 077002.
- Lin K, Cheng DL, Huang Z (2012) Optical diagnosis of laryngeal cancer using high wavenumber Raman spectroscopy. Biosens Bioelectron 35: 213-217.
- Nawaz H, Bonnier F, Knief P, Howe O, Lyng FM, et al. (2010) Evaluation of the potential of Raman microspectroscopy for prediction of chemotherapeutic response to cisplatin in lung adenocarcinoma. Analyst 135: 3070-3076.
- Lieber CA, Nethercott HE, Kabeer MH (2010) Cancer field effects in normal tissues revealed by Raman spectroscopy. Biomed Opt Express 1: 975-982.
- Day JC, Bennett R, Smith B, Kendall C, Hutchings J, et al. (2009) A miniature confocal Raman probe for endoscopic use. Phys Med Biol 54: 7077-7087.
- Kendall C, Day J, Hutchings J, Smith B, Shepherd N, et al. (2010) Evaluation of Raman probe for oesophageal cancer diagnostics. Analyst 135: 3038-3041.
- Wang J, Bergholt MS, Zheng W, Huang Z (2013) Development of a beveled fiber-optic confocal Raman probe for enhancing in vivo epithelial tissue Raman measurements at endoscopy. Opt Lett 38: 2321-2323.
- Bergholt MS, Zheng W, Huang Z (2013) Development of a multiplexing fingerprint and high wavenumber Raman spectroscopy technique for real-time in vivo tissue Raman measurements at endoscopy. J Biomed Opt 18: 030502.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 17483
- [From(publication date):
specialissue-2012 - Dec 21, 2025] - Breakdown by view type
- HTML page views : 12422
- PDF downloads : 5061