Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Research Article Open Access
Thickness limitation and cell viability of multi-layered cell sheets and overcoming the diffusion limit by a porous-membrane culture insert
| Waki Sekine1 Yuji Haraguchi2 Tatsuya Shimizu3 Akihiro Umezawa4 and Teruo Okano5* | |
| 1Institute of Advanced Biomedical Engineering and Science, TWIns, Tokyo Women’s Medical University, Tokyo, Japan | |
| 2Department of Reproductive Biology and Pathology, National Research Institute for Child Health and Development, Tokyo, Japan | |
| Corresponding Author : | Dr. Teruo Okano Institute of Advanced Biomedical Engineering and Science TWIns Tokyo Women’s Medical University 8-1 Kawada- Cho, Shinjuku-ku Tokyo 162-8666, Japan E-mail: tokano@abmes.twmu.ac.jp |
| Received August 30, 2011; Accepted October 14, 2011; Published October 29, 2011 | |
| Citation: Sekine W, Haraguchi Y, Shimizu T, Umezawa A, Okano T (2011) Thickness limitation and cell viability of multi-layered cell sheets and overcoming the diffusion limit by a porous-membrane culture insert. J Biochip Tissue chip S1:007. doi:10.4172/2153-0777.S1-007 | |
| Copyright: © 2011 Sekine W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Bioengineering and Bioelectronics
| Keywords |
| Cell sheet engineering; Cell-dense tissue; Cell viability; 3D culture; Diffusion limit; Oxygen and nutrients |
| Introduction |
| Cell-based therapy for tissue regeneration has a potential for treat- ing diseased organs and tissues which are unable to be cured by existing medical treatments including medicines and surgeries. Recently, the clinical application of regenerative medicine using artificial tissue made by scaffold tissue engineering, one of cell-based tissue regeneration technologies, has been launched [1-3]. On the other hand, our labora- tory has been developed an original scaffold-free technology called “cell sheet engineering” [4]. Both scaffold tissue engineering and cell sheet engineering have allowed regenerative medicine to advance significant- ly. Scaffold-based tissue engineering is suitable for extracellular matrix- rich tissues such as bone and cartilage. Cell sheet engineering allows the creation of cell-dense tissue. Upon in vivo transplantation, tissues fab- ricated by cell sheet engineering can adhere to the surface of host tissue without suture and form cell-dense structure, and these transplanted three-dimensional (3D) tissues can survive in living animals for long time [5-7]. The clinical applications of cell sheet engineering to regen- erative medicine have already been performed [8-10]. Although both scaffold tissue engineering and cell sheet engineering have significantly contributed to regenerative medicine, the clinical applications of both are limited to the transplantation of thin tissues. These days, a major challenge for tissue engineering is the creation of 3D and functional tis- sues. However, the tissue ischemic environments make the production of thicker 3D tissues difficult, because the construction of thicker tissue requires blood vessels supplying oxygen and essential nutrients. In cell sheet engineering, thicker and high cell-dense 3D tissues without blood vessels are reported to be unable to survive in vivo [11]. Therefore, the thickness limitation of viable tissue depends on oxygen and nutrient gradients. It is important to know the thickness limitation of surviv- ing tissue for fabricating thicker 3D tissue. Although there are several reports describing the diffusion limitation or gradients of tissue, these reports dealt with tissues in scaffolds. Due to possible complex rela- tions among oxygen and nutrient gradients, scaffold material proper- ties, and cell density, there are experimental difficulties in the detailed quantitative analysis of the relationship between the thickness of 3D tissue and their biological functions. Therefore, this study clarified the thickness limitation of cultured cell-dense tissues by investigating nu- trient metabolism, cell damage, and cell viability in a 3D tissue model, which composed of multi-layered cell sheets. Cell sheet engineering al- lowed us to control the thickness of engineered tissues by adjusting the number of cell sheets. In addition, an improved method for enhancing cell viability in multi-layered cell sheets was also investigated |
| Materials and Methods |
| Temperature-responsive culture dishes: Cell sheets were harvested from 35-mm diameter temperature-responsive culture dishes (Upcell™) (CellSeed, Tokyo, Japan). The procedures for preparing the culture dishes have been previously described [12,13]. Briefly, temperature- responsive monomer solution containing N-isopropylacrylamide (IPAAm) was spread on polystyrene culture dishes. Electron beam ir- radiation onto IPAAm monomer on these dishes allowed the mono- mers to polymerize and covalently bond to the dish surfaces. The poly (IPAAm)-grafted dishes were rinsed with cold distilled water to remove ungrafted monomer and were finally sterilized. |
| Cell culture: Human endometrial-derived mesenchymal cells (EMCs) [14] were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma-Aldrich Japan, Tokyo) supplemented with 10% fe- tal bovine serum (FBS) (Japan Bio Serum, Nagoya), 1% penicillin and streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified incubator with 5% CO2. EMCs showed adherent spindle shape mor- phology. |
| Preparation of multi-layered EMC sheets: EMCs (1.0 × 106 cells) were cultured in DMEM supplemented with 10% FBS, 1% penicillin and streptomycin on 35-mm diameter temperature-responsive culture dishes for 4 days at 37°C in a humidified incubator with 5% CO2. To harvest intact confluent cells as contiguous cell sheets, the culture dish- es were placed in a CO2 incubator at 20°C. EMC sheets detached them- selves spontaneously within 30 min and became slightly shrunken due to their cytoskeletal reorganization. The entire cell sheet with media was gently aspirated into the tip of a pipette. The first sheet was transferred onto a 35-mm diameter normal culture dish, which was pretreated with FBS for more than 30 min. After EMC sheet was spread, the media was aspirated, and the dish was incubated at 37°C for 20 min for allowing the cell sheet to fully adhere to the culture dish. To layer EMC sheets, the second EMC sheet harvested from another temperature-responsive culture dish was stacked onto the first cell sheet. By the same fashion, the harvested cell sheets were successfully layered up to sextuple-layer. Eventually, 2 mL fresh culture media (DMEM supplemented with 10% FBS, 1% penicillin and streptomycin) were added to the dish. Individ- ual single- to sextuple-layered EMC sheets were cultured for 1, 3, or 7 days in a humidified atmosphere (37°C, 5% CO2). The culture medium was changed every day. |
| Histological analysis: After being incubated for 7 days, multi-lay- ered cell sheets were mechanically recovered from dishes and fixed with 4% paraformaldehyde solution (Muto Pure Chemicals, Tokyo) at least for 24 h. Specimens were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (HE). Prepared specimens were observed by an optical microscope (ECLIPSE TE2000-U) (Nikon, Tokyo). The images were processed using an AxioVision imaging system (Carl Zeiss, Hallbergmoos, Germany). Thicknesses of multi-layered cell sheets were measured from the digitalized images using ImageJ software (National Institute of Health, Bethesda, MD). |
| Immunohistochemistry: For detecting cell proliferation, the par- affin sections were also stained with mouse monoclonal anti-human Ki-67 antibody (Dako Denmark, Glostrup, Denmark) diluted at 1:250. Hematoxylin was used for nuclear counterstaining. Prepared specimens were observed by an optical microscope (ECLIPSE TE2000-U). The im- ages were processed using an AxioVision imaging system. The percent- age of Ki-67 positive cells was calculated by dividing the number of Ki-67 positive cells by the number of total cells and multiplied by 100. |
| Glucose consumption and lactate production: Metabolic activi- ties of the layered cell sheets were monitored by measuring glucose con- sumption and lactate production for 24 h in culture medium. Culture medium samples were collected before medium change on Day 1, 3, and 7. Glucose consumption and lactate production were determined by hexokinase UV method [15] and lactic oxidase method [16], respec- tively. The coefficient of variation (CV) of lactate-to-glucose ratio was also calculated. |
| Measurement of released lactate dehydrogenase (LDH) activity: LDH is used as a common index of cell injury and cell death. The cul- ture medium samples were collected before medium change on Day 1, 3, and 7. The release of LDH from multi-layered cell sheets for 24 h in the condition media were measured with LDH assay kit (Sicaliquid LDH J) (KANTO CHEMICAL, Tokyo). |
| LIVE/DEAD cell viability assay: Cell viability of layered cell sheet was also examined by LIVE/DEAD Viability/Cytotoxicity Kit (Invit- rogen, Carlsbad, CA), which contains calcein AM and ethidium ho- modimer-1. Membrane-permeant calcein AM is cleaved by esterases in live cells to yield cytoplasmic green fluorescence, and membrane- impermeant ethidium homodimer-1 labels the nucleic acids of mem- brane-injured cells with red fluorescence. Briefly, layered cell sheets were treated with 0.5% trypsin / 0.2% EDTA (Sigma-Aldrich Japan) for dispersing them and obtaining single cells. The disassembled cells were stained with calcein AM and ethidium homodimer-1 for 30 min at 37°C. And the fluorescent images of the cells were photographed by a fluorescence microscope (ECLIPSE TE2000-U) with a CCD camera (Axio Cam HRc) (Carl Zeiss). The green and red fluorescent cells were counted for calculating the percentage of live cells. |
| Measurement of oxygen level between multi-layered cell sheets and culture surface: Oxygen concentration between multi-layered cell sheets and the culture surface of dish was monitored by a fiber-optical oxygen microsensor (Oxygen Sensor PSt3) (PreSens Precision Sensing, Regensburg, Germany). Before the measurement, the oxygen sensor was calibrated at zero oxygen and air-saturated levels in culture me- dium. The sensor was fixed on the bottom of a culture dish. A multi- layered cell sheet up to sextuple-layer was put over the sensor, and oxy- gen concentration was recorded for a minimum of 20 min until meas- urements was stabilized. All measurements were carried out at 37°C in humidified CO2 incubator of which gas phase was set at atmospheric oxygen levels (21% oxygen). |
| Culturing on porous membranes: Multi-layered cell sheets were also cultured on cell culture inserts (Becton, Dickinson and Company, Franklin Lakes, NJ) having porous polyethylene terephthalate (PET) track-etched membranes (the membrane pore size: 1 mm). Inserts were set in a 6-well cell culture insert companion plate (Becton, Dickinson and Company). The cell sheets were put on cell culture inserts in the aforementioned way, and the multi-layered cell sheets were cultured for 7 days in a humidified atmosphere of 5% CO2 at 37°C with 2 mL me- dium (DMEM supplemented with 10% FBS, 1% penicillin and strepto- mycin) in both insert and well. At the end of incubation, cell viabilities in the multi-layered cell sheets were examined by the method described above with LIVE/DEAD Viability/Cytotoxicity Kit (Invitrogen). The layered cell sheets were fixed with 4% paraformaldehyde solution (Muto Pure Chemicals) for histological analysis and immunohistochemistry as described above. Oxygen concentration between multi-layered cell sheets and the culture surface of insert was monitored by a fiber-optical oxygen microsensor (Oxygen Sensor PSt1). Before the measurement, the oxygen sensor was calibrated at zero oxygen and air-saturated levels in culture medium. The sensor was fixed on the bottom of a insert. A multi-layered cell sheet up to sextuple-layer was put over the sensor, and oxygen concentration was recorded for a minimum of 20 min un- til measurements was stabilized. All measurements were carried out at 37°C in humidified CO2 incubator of which gas phase was set at atmos- pheric oxygen levels (21% oxygen). |
| Statistical analysis: All data are expressed as mean ± SD. The sta- tistical analyses were performed by unpaired Student’s t test or one-way analysis of variance followed by Dunnett’s multiple comparison test. A probability value of p < 0.05 was considered statistically significant. |
| Results |
| Culturing on normal culture dish |
| Morphological analysis of the layered cell sheets: Upon the prep- aration of a single-layered cell sheet, EMCs (1.0 × 106 cells) were plated initially in a 35-mm temperature-responsive culture dish, and upon the harvest after 4 days incubation, the number of cells in a cell sheet increased to be 2.5 × 106 ± 0.09 × 106 cells with the coefficient of vari- ance of 3.6% (n = 6). Therefore, the number of cells in double-layer cell sheets was calculated to be 5.0 × 106 cells; the triple-layered cell sheets, 7.5 × 106 cells; the quadruple-layer cell sheets, 1.0 × 107 cells; quintuple- layered cell sheets, 1.25 × 107 cells; sextuple-layered cell sheets, 1.5 × 107 cells. |
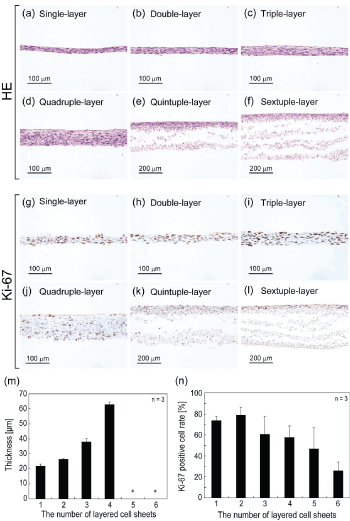
| After the incubation of layered cell sheets for 7 days, the cross-sec- tions of layered cell sheets were observed (Figure 1a - f). With increas- ing the number of stratified cell sheets (single to quadruple), the thick- ness of the layered cell sheets increased (Figure 1m). In single- to triple- layered cell sheets, stratified viable tissues were observed. The thickness of triple-layered cell sheets was approximately 40 mm. However, quin- tuple- and sextuple-layered cell sheets showed damaged tissues with the delamination of cell sheets in the bottom part, which directly contacted with the dish (Figure 1e, f). |
| Ki-67 staining: The nuclear activity of the cells in the layered cell sheets was investigated by staining with Ki-67 (Figure 1g - l). Ki-67 pos- itive cells were found in all layered cell sheets and observed throughout layered cell sheets in single- to quadruple-layer. On the other hand, in quintuple- and sextuple-layer, positive cells were localized in the upper side of layered cell sheets. With increasing the number of layered cell sheets, the percentage of Ki-67 positive cells decreased (Figure 1n). |
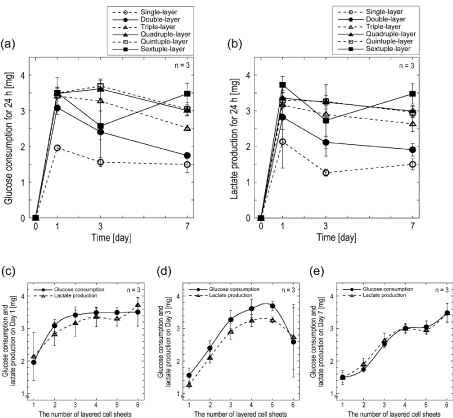
| Nutrient metabolism of layered cell sheets: For monitoring the metabolic activities of multi-layered cell sheets, the glucose consump- tion and lactate production of layered cell sheets were examined. The total glucose consumption (Figure 2a) and total lactate production (Figure 2b) in condition media for 24 h were measured on Day 1, 3, and 7 after the start of incubation. Total glucose consumption and lactate production of single- to quintuple-layered cell sheets decreased over the incubation period. However, those of sextuple-layered cell sheets decreased at Day 3 and increased at Day 7. Both glucose consumption and lactate production increased in accordance with the number of lay- ered cell sheets up to quadruple-layer. However, those amounts showed small increase over quadruple-layered cell sheets (Figure 2c-e). The ra- tio of the lactate production to glucose consumption (YL/G) of all layered cell sheets showed approximately 2 throughout the cultivation (Table 1), indicating anaerobic cell metabolism. High YL/G ratios in all layered cell sheets were also obtained on Day 7; the average YL/G value was 2.34 ± 0.09 with the coefficient of variance of 4.39%. YL/G ratios in the cell sheets from single to quintuple at Day 3 showed less than 2. |
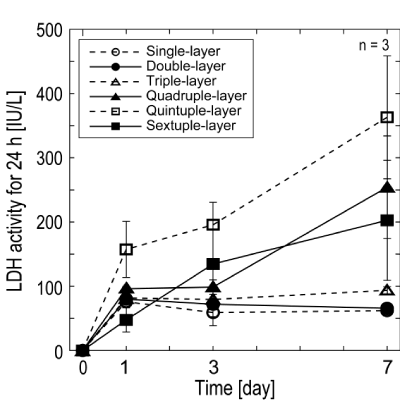
| Detection of LDH release: The total release of LDH for 24 h in the condition media was measured on Day 1, 3, and 7, because LDH is known as a common index of cell injury and cell death (Figure 3). Although single-, double-, and triple-layered cell sheets released con- stantly low amounts of LDH in the medium over the cultivation period, a steep increase in LDH release was observed in quadruple- to sextuple- layered cell sheets. |
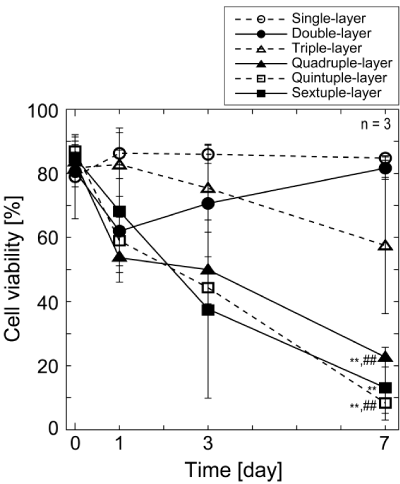
| Cell viability in layered EMC sheets: Cell viabilities in multi- layered cell sheets were examined quantitatively using calcein AM and ethidium homodymer-1. The cell viabilities decreased time-dependent- ly in proportion to the number of cell sheets except for double-layered cell sheets (Figure 4). The viabilities of quadruple-, quintuple-, and sextuple-layered cell sheets were already less than 70% on Day 1. Es- pecially, severe cell deaths were found in quadruple-, quintuple-, and sextuple-layered cell sheet after culture for 7 days. The viabilities of quadruple-, quintuple-, and sextuple-layered cell sheets were 23%, 8%, and 13% at Day 7, respectively. On the other hand, many survival cells were found single-, double-, and triple-layered cell sheets even after in- cubation for 7 days. There was a gap between the viabilities of cells in below triple-layered and over quadruple-layered cell sheets at Day 7. |
| Oxygen concentrations: Control measurements in the absence of multi-layered cell sheets with medium yielded output signals of ~21% O2 equal to signal in air-saturated culture medium. When multi-lay- ered cell sheets were immersed in medium during measurement, ox- ygen concentrations just under the layered cell sheets were found to decrease quickly (< 20 min) to 0.45 ± 0.28% O2 under single-layered cell sheets; 0.41 ± 0.18% O2, double-layer; 0.43 ± 0.15% O2, triple-layer; 0.45 ± 0.14% O2, quadruple-layer; 0.47 ± 0.13% O2, quintuple-layer; and 0.50 ± 0.13% O2, sextuple-layer (n = 3). It was confirmed that oxygen concentration under layered cell sheets was quite low regardless of the number of layers. |
| Thickness limitation of cultured cell-dense tissue: From these re- sults, it seems reasonable to conclude that the thickness limitation of layered cell sheets was triple-layered cell sheets with a thickness of ap- proximately 40 mm. |
| Improvement of cell condition in layered EMC sheets using porous-membrane culture inserts |
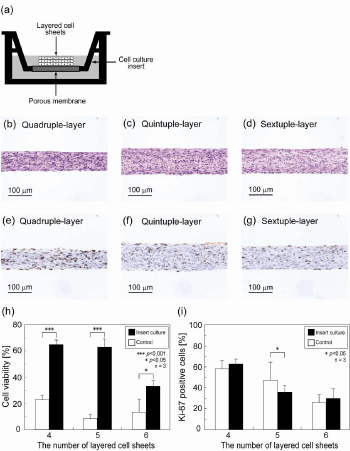
| For overcoming the thickness limitation, a special method for cul- turing layered cell sheets in vitro was also investigated. Layered cell sheets were incubated on a porous-membrane culture insert (Figure 5a). The device can supply nutrients to the both side of layered cell sheets and remove waste products. Cell viabilities of quadruple-, quintuple-, and sextuple-layered cell sheets on Day 7 were 65%, 63%, and 33%, re- spectively (Figure 5h). Viabilities of the layered cell sheets showed sta- tistically significant differences between the insert cultured sheets and the controls. Hematoxylin and eosin staining showed that the stratified viable tissues were observed even in quadruple-, quintuple-, and sextu- ple-layered cell sheets (Figure 5b - d). Thickness of quintuple-layered cell sheet was measured to be approximately 100 mm. Ki-67 positive cells were found throughout layered cell sheets homogeneously (Figure 5e-g). Percentage of Ki-67 positive cell decreased with increasing the number of layered cell sheets and showed no statistically significant dif- ferences between the insert cultured sheets and the dish cultured sheets (Figure 5i). Oxygen concentrations between multi-layered cell sheets and the insert membrane were quickly (< 50 min) decrease to 0.27 ± 0.22% O2 under single-layered cell sheet; 0.05 ± 0.03% O2, double-layer; 0.03 ± 0% O2, triple-layer; 0.02 ± 0.02% O2, quadruple-layer; 0.14 ± 0.21% O2, quintuple-layer; and 0.02 ± 0.02% O2, sextuple-layer (n = 3). |
| Discussion |
| The goal of this study was to determine the thickness limitation of cultured cell-dense tissue prepared by layering cell sheet. Furthermore, this study proposed a culture method for 3D tissues with porous-mem- brane culture inserts for overcoming this limitation. The metabolism, cell-damage, and viability of the cells in layered cell sheets were inves- tigated quantitatively, and the histological analysis of layered cell sheets was also performed for obtaining the biological limitation of stratified cell sheets. In addition, the measurements of oxygen concentration were also performed. As one of the advantages, multi-layered cell-sheet tissue can allow their high cell-dense tissue containing only cell com- ponent to be evaluated without the possible effects of scaffold. There- fore, this study investigated the thickness limitation of prepared multi- layered cell-sheet tissue, which was cultured with the simple diffusion of oxygen and nutrients, by controlling the number of cell-sheet layers. |
| The cell sheets from single- to quadruple-layered cell sheets showed no internal separation or delamination in the layered cell sheets (Figure 1a-d). However, although the measurement of the thickness of histo- logical specimen showed the thickness of layered cell sheets increased, the increase was not linear (Figure 1m). The thicknesses were less than the expected values. By stratifying cell-sheets, the thickness of cell-sheet tissue was found to be thinner than that of expected, because the total physical size of tissue became compact during cultivation. Nuclear ac- tive cells recognized as Ki-67 positive cells were observed homogene- ously distributed in single- to quadruple-layered cell sheets (Figure 1g- j). However, in over quintuple-layered cell sheets, the cell sheets showed damaged tissues with delamination and many Ki-67 positive cells were also found at the upper side of layered cell sheets (Figure 1 k,l). |
| Although the glucose consumption and lactate production of lay- ered cell sheets were expected to increase proportionally with the in- crease of the number of layers, the increases were limited after quadru- ple-layered cell sheets (Figure 2a,b). To understand this limitation of the increase and the relationship between the glucose consumption and the lactate production, the data on Day 1, 3 and 7 were plotted in the func- tion of the number of layered cell sheets in (Figure 2c,d,e) respectively. Glucose consumption and lactate production increased almost linearly from single- to triple-layered cell sheets and gradually from quadruple- to sextuple-layered cell sheets. Since LDH analysis showed that there were no dead cells in single- to triple-layered cell sheets (Figure 3), the linear relationship between the number of sheets and the glucose and lactate metabolisms was thought to be understandable. Because the cell sheets over quadruple-layered could contain significant numbers of unviable cells (Figure 1,3), the glucose consumption and lactate pro- duction decreased. In sextuple-layered cell sheets, glucose consumption and lactate production decreased at Day 3. LDH activity increased and cell viability decreased from Day 1 to 3 (Figure 3,4). Since the total met- abolic activity of sextuple-layered cell sheets decreased from Day 1 to 3 due to the large amounts of acute cell death, glucose consumption and lactate production decreased steeply. On the other hand, at Day 7, glu- cose consumption and lactate production increased in sextuple-layered cell sheets. LDH activity increased, and the cell viability decreased from Day 3 to 7 (Figure 3,4). Therefore, for explaining the increasing of glu- cose consumption and lactate production at Day 7, surviving cells were speculated to proliferate while dying cells were found. In the exami- nation of the permeability of glucose across multi-layered cell sheets, almost the complete inhibition of glucose permeability was observed on over quadruple-layered cell sheets [17]. Glucose consumption also might show no increase in accordance with the number of cell sheets due to the insufficient supply of glucose. |
| Unexpectedly, the amount of glucose consumption was almost identical to that of lactate production (Figure 2c-e). In the table 1, YL/G of layered cell sheets showed approximately 2. It is well known that under anaerobic condition, one glucose molecule is metabolized to produce two lactate molecules for producing two ATP molecules without using oxygen. Therefore, the energy production of the layered cell sheets using glucose is speculated to be performed under anaero- bic condition in spite of aerobic culture condition with 21% O2. Some tissues and cell types are known to produce lactate from glucose even under aerobic conditions [18]. Skeletal muscles contain relatively small number of mitochondria because of their high tolerance against low ox- ygen circumstances. Therefore, the cells in these organs are recognized to have a high glycolytic metabolism system. Having no mitochondria, erythrocytes are unable to oxidize pyruvate to CO2. Cultured human Müller cells oxidize glucose at a low rate and generate most of their ATP from glycolysis. Therefore, they can resist to mitochondrial inhibi- tion that have relevance to certain manifestations of retinal ischemia [19]. In EMC of layered cell sheets, although enough number of mito- chondria might be found, the metabolic rate of mitochondria might be practically zero because of hypoxic condition in layered cell sheets. For obtaining the sufficient amount of energy for surviving, the cells in the sheet were thought to upregulate their glycolytic pathway. |
| The experiment of cell viability showed a gap in below triple-layered and over quadruple-layered cell sheets at Day 7 (Figure 4). The average of the former group (single-, double-, and triple-layered cell sheets) and the latter groups (quadruple-, quintuple-, and sextuple-layered cell sheets) were 74.7 ± 14.9% and 14.7 ± 7.3%, respectively. There is a statis- tical difference between two groups (p = 0.003). The result of measure- ment of LDH activity, a marker of cell damage, also showed practically no dead cell of single- to triple-layered cell sheets, and the increase of dead cell was found in quadruple- to sextuple-layered cell sheets (Figure 3). Therefore, the gap found in the experiment of cell viability was speculated to be due to no dead cells of single- to triple-layered cell sheets. Although triple-layered cell sheets showed a decrease in the cell viability during cultivation, both average cell survival ratio (72 ± 13%) and LDH activity were comparable to double layers (72 ± 10%). Mean- while, quadruple- to sextuple-layered cell sheets reduced in the cell vi- ability with the progress of incubation. Especially, there was a signifi- cantly reduction in the cell viability from Day 3 to Day 7. Overall results obtained in this study can allow us it seems reasonably to conclude that the thickness limitation of layered cell sheets is approximately 40 mm, which is equivalence to the thickness of triple-layered cell sheets. |
| When oxygen distribution in animal isolated islet organ was exam- ined, the thickness of viable tissue was reported to be 235 ± 36 mm [20]. Due to the lack of supply of oxygen and nutrients by a vascular system, the necessary amounts of oxygen and nutrients for isolated organ can be supplied only by diffusion. Diffusional gradients of oxygen in stati- cally cultured cardiac constructs based on neonatal rat cardiomyocytes cultured on collagen scaffolds were measured [21]. After 16 days of cul- tivation in static dish, the physiological density of live cells was found only within the top 128 mm of construct thickness. A tissue fabricated from suspended cells in microcapsules for cell-based drug delivery and tumor models shows its critical size (approximately 200 – 300 mm) [22]. In these methods, oxygen concentration and cell viability decrease with the distance from the construct surface. In this experiment, oxygen concentration just below layered cell sheets was approximately 0% O2 regardless of the number of sheets. Therefore, instead of extremely low oxygen condition, glucose depletion in the cells was speculated to af- fect the cell survivability. Many viable cells with minimum damage were found near the surface of multi-layered cell sheets. Due to glucose diffu- sion limitation, cell damage was found to increase in the dish-contact- ing side of the sheet with increasing sheet thickness. The critical thick- ness of the layered cell sheets in this study was concluded to be 40 mm, which is thinner than that of scaffold-based tissue. Since 3D cell-dense tissues made by layering cell sheets are thought to have a higher cell density than that of a tissue made from cells with scaffold, the former may have a difficulty in keeping its glycolytic metabolism due to the in- sufficient supplies of nutrients and build up of waste products. Eventu- ally, cell-dense multi-layered cell sheets can reach its critical thickness, which is far thin compared with that of scaffold-based tissue. |
| The thickness of layered cell sheet is known to depend on the type of cell. Naturally larger cells can make a thicker cell sheet. However, it has to be investigated whether the thickness limitation of layered cell sheet depends on the type of cells forming cell sheet. In the case of neo- natal rat cardiomyocyte, the thickness of triple-layered cell sheets was 100 – 150 mm immediately after the preparation and 20 mm after 1-day incubation (data not shown). When single- to quintuple-layered neo- natal rat cardiomyocyte sheets were stacked in vitro and transplanted into the dorsal subcutaneous tissue of nude rats, the thickness of the transplanted layered cell sheets were found to be thinner for short pe- riod after the transplantation (e.g. the thickness of triple-layered cell sheet was approximately 60 mm on 7 days after the transplantation) and became thicker several weeks later [23]. One month after the transplan- tation, the thickness of recovered tissue showed linearly increase up to the triple-layered constructs (80 ± 16 mm thick), but no clear increases of the thickness over quadruple-layered constructs were observed [11]. Layered cell sheets that were transplanted in living body was thought to receive the necessary amount of oxygen and nutrients from the host tissue by simple diffusion until neovascularization occurs within the transplanted tissue and supplied the sufficient amount of blood to the whole tissue. Therefore, over quadruple-layered cell sheets became necrotic after the transplantation due to the diffusion limit. |
| For improving cell culture circumstances, layered cell sheets were incubated on porous-membrane culture inserts, which are expected to provide nutrients to the both side of constructs (Figure 5a). Oxygen concentration at the contact surface between layered cell sheets and membrane was almost 0% O2, which was the same condition on dish culture, indicating that oxygen supply was insufficient for the needs of multi-layered cell sheets through the membrane of the insert. After 7 days incubation, quadruple- to sextuple-layered cell sheets showed remarkable increases in cell viability, indicating that the porous-mem- brane culture insert was effective for supplying nutrients (Figure 5h). Histological analysis of the quadruple- to sextuple-layered cell sheets incubated on the inserts also showed healthy cell status compared with that incubated on cell culture dishes (Figure 5 b- d). Although extreme- ly low-oxygen condition was found under the layered cell sheets on cul- ture insert, the cells were able to survive by glucose supplied though the membrane. Multi-layered over quadruple layers should be cultured on porous-membrane culture inserts that can supply adequate amount of nutrients. Although many Ki-67 positive cells were homogeneously distributed in the layered cell sheets, the positive rate over the total number of cells was found to tend to increase compared with that of cell sheets cultured on dishes (Figure 5 e- g, i). Since the porous-mem- brane culture insert can provide good cell survivability though a good nutrients supply, the cells in the layered cell sheets were speculated to be able to form a high cell-dense and thick tissue. As a result, cells exited the cell cycle of cell proliferation by the contact inhibition. Therefore, no Ki-67-positive-cell rate was found to increase. This culture method gave an adequate adhesion among cell sheets, resulting in better cell vi- ability than that on normal culture dishes. These results suggest that cell death in multi-layered cell sheets is induced only by the insufficiency of nutrients and the accumulation of waste products. Thicker 3D tissues (approximately 100 mm) are able to be cultured in vitro using porous- membrane culture inserts instead of normal culture dishes. |
| However, both supply routes through porous-membrane of the cul- ture insert and direct contact with the medium were unable to be effec- tive over sextuple-layered cell sheets. Therefore, for obtaining thicker viable layered cell sheets, a perfusion bioreactor system or a new tech- nology to control vascularization will be needed. Endothelial cells in myocardial cell sheet were reported to form a blood vessel like network during cultivation [24]. The network may induce a capillary formation and allow us to create thicker tissue constructs in vitro without the in- sufficiency of oxygen and nutrients. |
| Recently, a focus on 3D cell culture has developed both in cell and molecular biology as well as tissue engineering. Cell morphology, cell- to-cell interactions, and surrounding extracellular matrix of 2D cul- tured cells show significant differences from those of 3D cultured cells. The differences may affect gene expression and other biological activity [25-27]. Therefore, this multi-layered cell sheets can provide new find- ings in 3D culture system and ischemic tissues, and contribute to tissue engineering. |
| In conclusion, this work reveals (1) the thickness limitation of 3D cell-dense tissue which fabricated by layering cell sheets and (2) a pos- sible solution to break the limitation for fabricating thicker cell-dense tissue through a both side culture system using a porous-membrane culture insert. |
| Acknowledgements |
| We appreciate the useful comments and technical criticism from Dr. Norio Ueno (Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University) and human endometrial-derived mesenchymal cells were kind- ly supplied from Dr. Shunichiro Miyoshi (Keio University School of Medicine). This research was granted by the Japan Society for the Promotion of Science (JSPS) through the “Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program)”, initiated by the Council for Science and Technology Policy (CSTP) and the Ministry of Education, Culture, Sports, Science, and Tech- nology through the “High-Tech Research Center Program”. |
| References |
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Post your comment
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 18348
- [From(publication date):
specialissue-2011 - Dec 15, 2025] - Breakdown by view type
- HTML page views : 13187
- PDF downloads : 5161
