Research Article Open Access
Trans-Lymphatic Metastasis in Peritoneal Dissemination
| Yutaka Yonemura1,5,6*, Emel Canbay1, Yan Liu1, Ayman Elnemr2, Yoshio Endo3, Masahiro Miura4, Haruaki Ishibashi5, Yoshiaki Mizumoto6 and Masamitsu Hirano6 |
|
| 1NPO to support Peritoneal Surface Malignancy Treatment, Japan | |
| 2Department of Surgery, Tanta Faculty of Medicine, Tanta, Egypt | |
| 3Department of Molecular Targeting Therapy, Cancer Institute, Kanazawa University, Japan | |
| 4Department of Human Anatomy, School of Medicine, Ooita University, Japan | |
| 5Departmen of Surgery, Kishiwada Tokushukai Hospital, Japan | |
| 6Department of Surgery, Kusatsu General Hospital, Japan | |
| Corresponding Author : | Yutaka Yonemura Regional CTherapies Carcinomatosis Peritoneal Cancer Center NPO to support Peritoneal Surface Malignancy Treatment 1-26 Haruki-MotoMachi, Kishiwada City Oosaka, Japan E-mail: y.yonemura@coda.ocn.ne.jp |
| Received April 28, 2013; Accepted May 21, 2013; Published May 23, 2013 | |
| Citation: Yonemura Y, Canbay E, Liu Y, Elnemr A, Endo Y, et al. (2013) Trans-Lymphatic Metastasis in Peritoneal Dissemination. J Gastroint Dig Syst S12:007. doi: 10.4172/2161-069X.S12-007 | |
| Copyright: © 2013 Yonemura Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Mechanism of the formation of peritoneal metastasis (PM) through lymphatic vessels was studied. Materials and methods: Parietal peritoneum was divided into 8 regions, and specimens of each zone were removed from patients with PM. The specimens were stained with enzyme histochemical staining for alkaline phoshatase (ALPase) and 5-Nase activity, and with immunohistochemical staining with D2-40. Surface of the peritoneum and subperitoneal tissue were observed by a scanning electron mcirosopy. Results: Well-developed lymphatic lacunae were found in the shallow submesothelial layer of 7 regions except for the anterior abdominal wall. Lymphatic vessels were found in the deep submesothelial layer up to 200 micrometer from the peritoneal surface. The mesothelial stomata directly connect with the submesothelial lymphatic vessels through holes of the macula cribrifolmis. Migration of cancer cells through stoma was found, and cancer cells were detected in the submesothelial lymphatic lacunae. Lymphatic vessels are not found in the center of established PM, but were found in the adjacent normal tissue. In the subperitoneal tissue outside the PM, morphological findings suggesting lymphangiogenesis designated as cystic Lymphatic Island, ladder formation, budding, and extension of lymphatic vessels were found. Conclusion: The triplet structure consisting of mesothelial stomata, holes on macula cribriformis and submesothelial lymphatic lacunae is essential for the migration of peritoneal free cancer cells into the submesothelial lymphatic lacunae. The rout of the formation of PM through peritoneal lymphatic vessels was named as translymphatic metastasis.
| Keywords |
| Peritoneal metastasis; Trans-lymphatic metastasis; Angiogenesis; Peritoneal lymphatic vessels |
| Introduction |
| Traditionally, Peritoneal Metastasis (PM) has been considered to be established by a multi-step processes, consisting of the tumor cell detachment from the primary tumor, attachment on the peritoneum, invasion into the submesothelial tissue, and proliferation by the induction of angiogenesis and stromal tissue [1]. The concept was called as a trans-mesothelial metastasis to complete the process; however, concerted expression of metastasis-related genes must be required. In contrast, cancer cells having low biological behavior fail to establish peritoneal metastasis, because they cannot produce enough kinds of metastasis-ralated gene expression for the invasion and proliferation. Recently, Yonemura et al. reported that the peritoneal lymphatic network has an important role on the formation of peritoneal dissemination [1], and the concept was designated as trans-lymphatic metastasis. Milky spots on the greater omentum have been well known as a rout of the trans-lymphatic metastasis [2]. However, the mechanisms of the trans-lymphatic metastasis except through milky spots have not been clarified. The present study demonstrated the novel findings of the trans-lymphatic metastasis in the human peritoneal tissue bearing peritoneal metatstasis. |
| Materials and Methods |
| Human peritoneum |
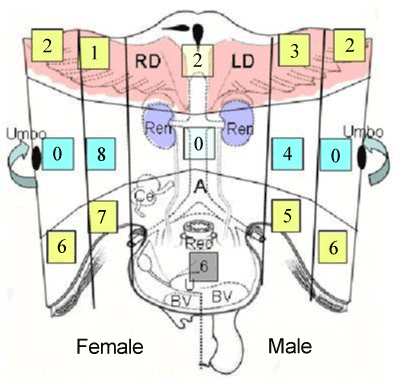
| Parietal peritoneum was divided into 9 regions from region 0 to region 8 (Figure 1). Surgical specimens of the peritoneal tissues of each zone were removed from patients with PM from six and five patients with gastric cancer and pseudomyoma peritonei. Specimens were fixed with formalin, and embedded in paraffin. |
| Staining of lymphatic vessels |
| Peritoneum were removed and fixed with folrmaldehyde-CaCl2 fixative (2% paraformaldehyde, 1% CaCl2) in 0.1M cacodylate buffer containing 7% sucrose for 24 hours. Then, the tissues were embedded in OCT compound (Miles, Diagnostic Division, Elkhalt, IN, USA), and the sections were cut at 15-μm thickness with a cryostat [3]. For the observation of whole mount specimens, 2 cm by 2 cm of peritoneum was removed. After complete removal of the fatty tissues from the sub peritoneal tissue, they were extended on the board and fixed with the same fixation methods. Staining of blood and lymphatic vessels was done by an enzyme - histochemical staining using alkaline phoshatase (ALPase) and 5-Nase activity [3]. Lymphatic vessels showing positive staining for 5‘-Nase activity revealed the lead sulfate reaction product, colored dark brown. In contrast, no 5’-Nase activity was recognized in the blood vessels [3]. After staining for alkaline phosphatase (ALPase), a blue reaction product was restricted to blood vessels. The whole mount specimens were observed by stereoscopic microscopy. D2-40 is a monoclonal antibody, which specifically reacts with O-linked sialoglycoprotein on the lymphatic endothelial cells. Immunohistochemistry was performed using deparafinized sections by D2-40 antibody (DAKO, Tokyo, Japan) [4]. |
| Scanning electron microscopy |
| Surface of peritoneum and the subperitoneal tissue after removal of mesothelial clells by a chemical digestion using 6N-KOH was observed by a scanning electron mcirosopy (Hitachi, S-4800, Tokyo, Japan) [3]. This study was approved by the ethical committee of Kishiwada Tokushukai Hospital and Kusatsu general hospital and signed written informed consent was obtained from all patients. |
| Results |
| Lymphati vessels in human peritoneum |
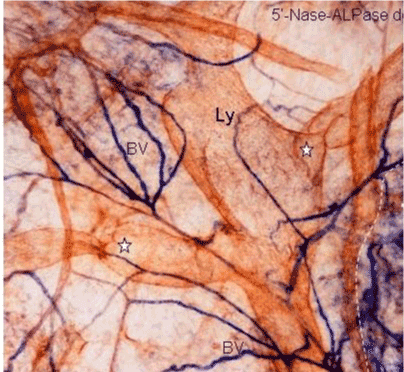
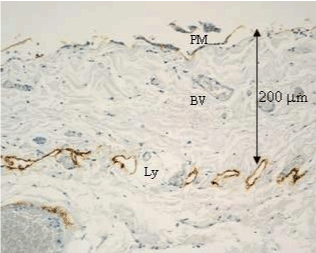
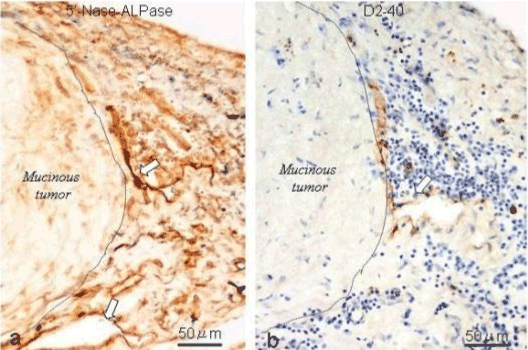
| In human subperitoneal tissue, lymphatic vessels were found in the shallow subperitoneal tissues between one to 200 micrometer from the peritoneal surface (Figures 2 and 4). By the observation for the whole mount specimens, well-developed lymphatic vessels and lacunae were found in the Morrison’s pouch, diaphragm, pelvic peritoneum, falciform ligament, lesser omentum, and greater omentum (Figure 3). As shown in figure 4, the lymphatic vessels in these regions locate in the submesothelial tissue shallower than the blood vessels. In contrast, subperitoneal lymphatic vessels in the anterior abdominal wall locate (Figure 1, region 1) in the deep subperitoneal tissue in 200 micrometer from the peritoneal surface (Figure 5). |
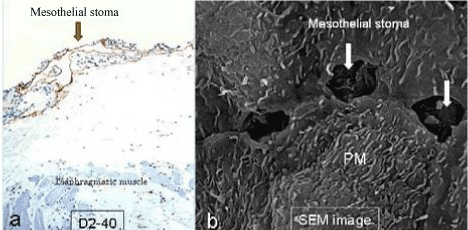
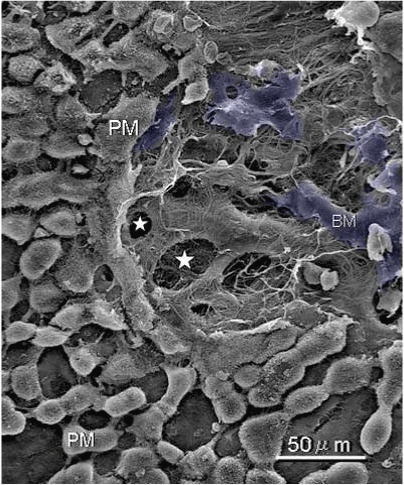
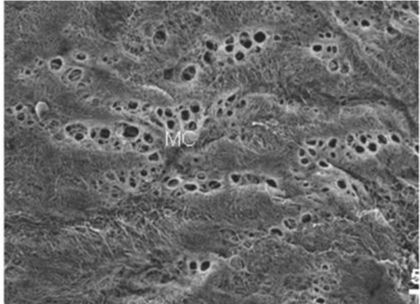
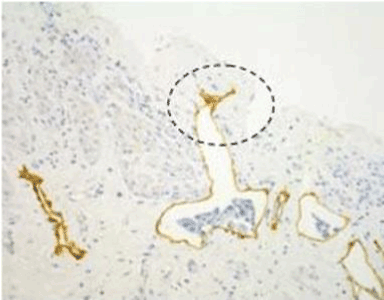
| Figure 6 shows mesothelial stoma opened between mesothelial cells. Just below the mesothelial cells, basement membrane and macula cribrifolmis were found (Figure 7). The macula cribriformis is a plate consisted of collagen, and has holes in some area (Figure 8). Submesothelial lymphatic vessels locate just below the macula cribrifolmis and the mesothelial stomata directly connect with the submesothelial lymphatic vessels through holes of the macula cribrifolmis. |
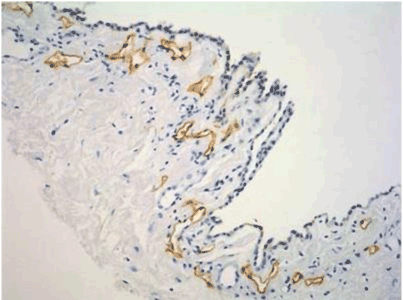
| Figure 9 shows that a gastric cancer cell migrates into the submesothelial lymphatic vessels through a hole of macula cribriformis. |
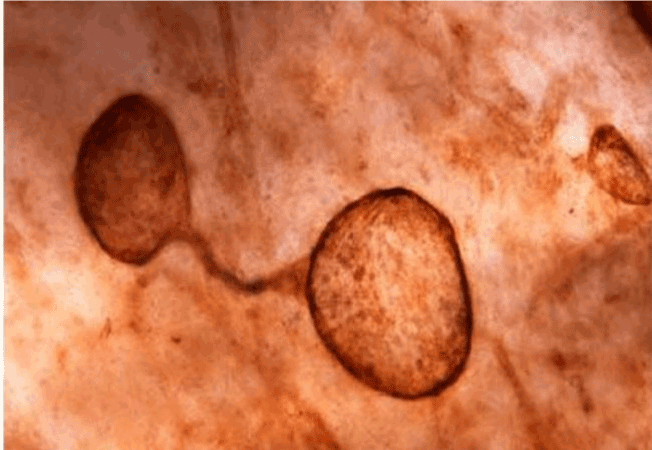
| Figure 10 shows the micrometastasis in the submesothelial lymphatic vessel on the pelvic peritoneum, and clusters of gastric cancer cells were found in the lymphatic lacuna. |
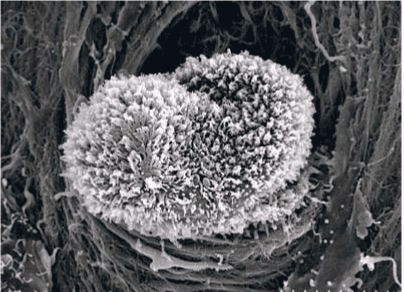
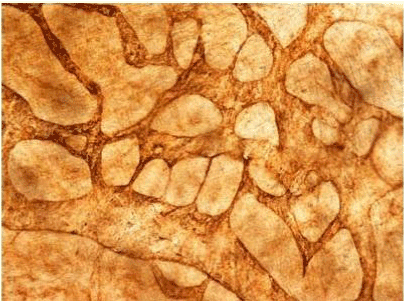
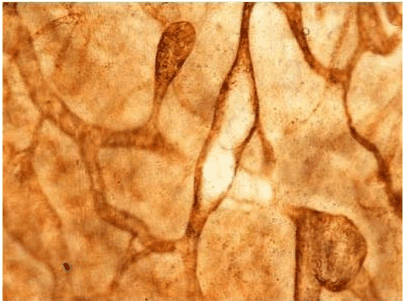
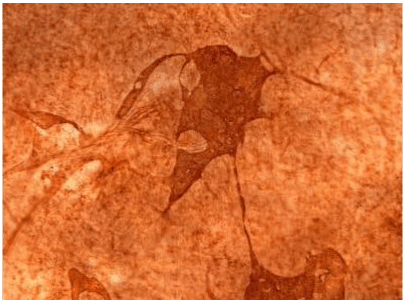
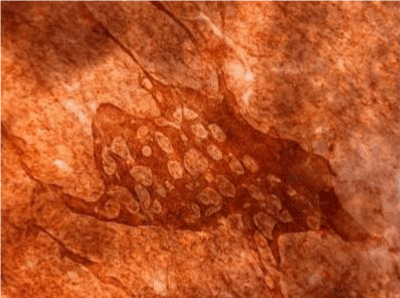
| Figure 11 shows the established peritoneal metastasis with angiogenesis and stromal formation. The lymphatic vessels are not found in the center of the tumor, but were found in the adjacent normal tissue around the tumor nodule. In the subperitoneal tissue outside the peritoneal metastasis, several morphological findings suggesting lymphangiogenesis were observed. Cystic Lymphatic Island (Figure 12), ladder formation (Figure 13), budding from preexisting lymphatic vessels (Figure 14), and extension of the blind loop of lymphatic vessels were found (Figure 15). Furthermore, enlarged cystic lymphatic islands connected with fine lymphatic vessels (Figure 16). Figure 17 showed the cystic lymph sac which was separated by septum and showed lacework like structure, suggesting to form lymphatic plexus. |
| Discussion |
| The milky spots on the greater omentum are open lymphatic lacunae that absorb fluid and thereby bring tumor cells in large quantity to the structure [2]. In contrast, peritoneal dissemination except on the greater omentum have been considered to be established through the trans-mesotherial metastasis, which consists of direct attachment of peritoneal free cancer cells (PFCCs) on the peritoneal surface, invasion into subperitoneal tissue and proliferation with stromal induction. |
| The present study first demonstrated the mechanisms of trans-lymphatic metastasis on the parietal peritoneum except on the greater omentum. Stereological analysis of lymphatic vessels cannot be done by the immunohistochemical study using thin section of paraffin-embedded specimens. This hampered the development of the study of the lymphatic distribution and lymphatic spread of animal and human cancer. |
| We used a method to stain the peritoneal lymphatic and blood vessels in the human peritoneum using the double-enzyme staining method (5’-nucleotidase [5’-Nase] and alkaline phosphatase [ALPase]) [3]. Since lymphatic endothelial cells express 5’-Nase, the lymphatic vessels are stained brown. In contrast, the arterial blood capillaries are stained blue by the ALPase reaction. Accordingly, the lymphatic and arterial blood capillaries can be visualized using this method. This method is also useful to investigate stereological network of peritoneal lymphatic system, when the whole mount specimens are stained and observed by a stereoscopic microscopy. |
| The present study demonstrated that PFCCs migrated into the submesothelial lymphatic lacunae through mesotherial gaps (stomata) and holes on the macula cribriformis. In the omental milky spots, stomata can be detected on the surfaces of such milky spots, and these stomata connect with holes on the macula cribriformis beneath the mesothelial basement membrane. The lymphatic lacunae attach to the holes on the cribriform plate. Many arterial blood capillaries are distributed around the lymphatic lacuna at milky spots [5]. PFCCs can easily migrate into the lymphatic lacunae through the stomata and then proliferate in the lymphatic lacunae. |
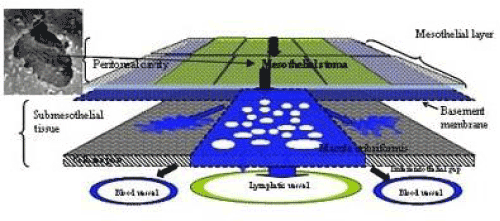
| The triplet structure consisting of mesothelial stoma, holes on macula cribriformis and submesothelial lymphatic lacunae is essential for the migration of PFCCs into the lymphatic lacunae (Figure 18). The present study demonstrated that these structures are detected not only in the milky spots but also in the parietal peritoneum except anterior parietal peritoneum (Figure 1, region 0). |
| The peritoneum of diaphragm, pelvis, paracolic gutter, and Morrison’s pouch and perihepatic ligaments does not have any milky spots, but it does have the triplet structure. Under normal conditions, the mean diameter of stoma is 1.2 mm (ranging from 1 – 30 mm) [6]. The mean stomata density of the rabbit diaphragm is 250/mm2 [6-8]. The size of the stomata changes by the contraction of mesothelial cells. In the normal condition, mesothelial cells show a characteristic rounded morphology, and several cytokines produced from cancer cells cause the separation of cell-to-cell contacts of mesothepial cells, resulting in the exposure of the macula cribriformis [9-11]. The macula cribriformis is a collagen plate with numerous holes. The surface is covered with basement membrane, to which the mesothelial cells attach. Beneath the macula cribriformis, a rich network of diaphragmatic submesothelial lymphatic vessels exists. The PFCCs move from the peritoneal cavity to the subperitoneal lymphatic vessels through the stomata and holes in the macula cribriformis (Figure 18). In an experimental study, intraperitoneal inoculation of a highly metastatic cell line induces mesothelail cell contraction, and cancer cells were detected in the submesothelial lymphatic vessels on day 3 [11]. |
| PFCCs migrated in the lymphatic lacunae proliferate by supplying the oxygen and nutrients from the arterial capillary. Rapid tumor growth generates intratumoral stromal pressure and may induce the collapse of lymphatic vessels. Because the lumen of lymphatic vessels is maintained by the traction of fine anchoring filament due to the low intraluminal pressure of the lymphatic vessels [3], the intratumoral pressure may collapse the lumen of lymphatic vessels, resulting in the dysfunction and destruction of intratumoral lymphatic vessels. The present study demonstrated the lymphangiogenesis in the subperitoneal tissue adjacent to the established PM or in the peritoneum without PM. Two types of lymphangiogenesis were found. One is the budding from preexisting lymphatic vessels, which were designated as budding, ladder formation and extension. Another type was the lymphatic vessel neoformation from lymphatic islands, which were detected as cystic structure in the the stroma. Asahara have proved that human peripheral blood contains endothelial (EC) progenitors, which can differentiate endothelial cells [7]. These progenitor cells may be incorporated in the sites of active lymphangiogenesis. Lee reported a contribution of podoplanin positive bone marrow cells to lymphatic vessel formation [8]. These podoplanin positive cells highly express markers for lymphatic endothelial cells, hematopoietic lineages, and stem/progenitor cells, and on further cultivation, they generate lymphatic endothelial cells. In addition, these cells exist in small numbers in peripheral blood of normal mice, but are significantly augmented after tumor inoculation. After injection of these cells, they were incorporated into the lymphatic vasculature and increased lymphatic vascular density in tissue [8]. Lymphatic Island is isolated from the preexisting lymphatic vessels, and may be derived from lymphatic endothelial progenitor cells in bone marrow or macrophages [10,12]. VEGF-C produced from tumor cells may induce lymphangiogenesis in the subperitoneal tissue [13-20]. |
References
- Yonemura Y, Endo Y, Obata T, Sasaki T (2007) Recent advances in the treatment of peritoneal dissemination of gastrointestinal cancers by nucleoside antimetabolites. Cancer Sci 98: 11-18.
- Shimotsuma M, Shields JW, Simpson-Morgan MW, Sakuyama A, Shirasu M, et al. (1993) Morpho-physiological function and role of omental milky spots as omentum-associated lymphoid tissue (OALT) in the peritoneal cavity. Lymphology 26: 90-101.
- M. Miura, Yonemura Y (2011) “Morphological study of human omental milky spotsand their morphological changes in omental disseminated metastasis”. Jap J Lymphology 34: 2-6.
- Yonemura Y, Endo Y, Fujita H, Fushida S, Ninomiya I, et al. (1999) Role of vascular endothelial growth factor C expression in the development of lymph node metastasis in gastric cancer. Clin Cancer Res 5: 1823-1829.
- Yonemura Y (2012) Atlas and principles of peritonectomy, Published by NPO to Support Peritoneal Surface Malignancy Treatment 125-127.
- Bettendorf U (1978) Lymph flow mechanism of the subperitoneal diaphragmatic lymphatics. Lymphology 11: 111-116.
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964-967.
- Lee JY, Park C, Cho YP, Lee E, Kim H, et al. (2010) Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation 122: 1413-1425.
- Yonemura Y, Endou Y, Nojima M, Kawamura T, Fujita H, et al. (1997) A possible role of cytokines in the formation of peritoneal dissemination. Int J Oncol 11: 349-358.
- Yonemura Y, Endou Y, Sasaki T (2002) “VEGF-C/VEGFRS and cancer metastasis,” Growth factors and their receptors in cancer metastasis. Kluwer Acad 223-239.
- Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, et al. (1997) Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276: 1423-1425.
- Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, et al. (1996) A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 15: 290-298.
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, et al. (2001) Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 7: 192-198.
- Yonemura Y, Endo Y, Tabata K, Kawamura T, Yun HY, et al. (2005) Role of VEGF-C and VEGF-D in lymphangiogenesis in gastric cancer. Int J Clin Oncol 10: 318-327.
- Conboy JG, Chan J, Mohandas N, Kan YW (1988) Multiple protein 4.1 isoforms produced by alternative splicing in human erythroid cells. Proc Natl Acad Sci U S A 85: 9062-9065.
- Miura M, Yonemura Y (2011) Morphological study of human omental milky spotsand their morphological changes in omental disseminated metastasis. Jap J Lymphology 34: 2-6.
- Noguchitakino M, Endo Y, Yonemura Y, Sasaki T (1996) Relationship between expression of plasminogen activator system and metastatic ability in human cancers. Int J Oncol 8: 97-105.
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, et al. (2001) Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 7: 192-198.
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964-967.
- Gordon EJ, Rao S, Pollard JW, Nutt SL, Lang RA, et al. (2010) Macrophages define dermal lymphatic vessel calibre during development by regulating lymphatic endothelial cell proliferation. Development 137: 3899-3910.
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
 |
 |
 |
 |
 |
| Figure 6 | Figure 7 | Figure 8 | Figure 9 | Figure 10 |
 |
 |
 |
 |
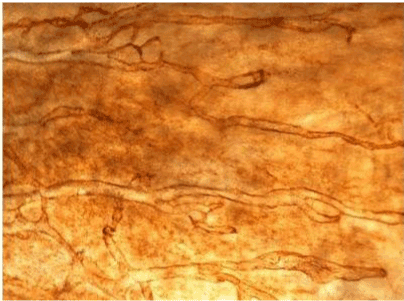 |
| Figure 11 | Figure 12 | Figure 13 | Figure 14 | Figure 15 |
 |
 |
 |
| Figure 16 | Figure 17 | Figure 18 |
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15686
- [From(publication date):
specialissue-2013 - Dec 20, 2025] - Breakdown by view type
- HTML page views : 10791
- PDF downloads : 4895
