Review Article Open Access
VEGF-A and VEGF-R1/2 Expression: Critical Importance in Different Stages of Human Gastric Adenocarcinoma
Christian Prinz*
Helios Klinikum Wuppertal, Medizinische Klinik, University of Witten, Germany
- Corresponding Author:
- Christian Prinz
Medizinische Klinik 2
Helios Klinikum Wuppertal
University of Witten/Herdecke
Germany, 42283 Wuppertal, Germany
Tel: 0049-202-896-2243
E-mail: christian.prinz@helios-kliniken.de
Received Date: November 18, 2013; Accepted Date: December 27, 2013; Published Date: January 07, 2014
Citation: Prinz C (2014) VEGF-A and VEGF-R1/2 Expression: Critical Importance in Different Stages of Human Gastric Adenocarcinoma. J Gastroint Dig Syst 3:160. doi: 10.4172/2161-069X.1000160
Copyright: © 2014 Prinz C. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
A key role for gastric micro vascularisation during cancer progression is the vascular growth factor VEGF-A. In gastric cancer, serum VEGF levels were also significantly higher in patients with advanced-stage cancer, higher lymph node ratio, and peritoneal invasion. Inhibition of VEGF or blockade of the corresponding VEGF-R1 and VEGF-R2 receptors has been investigated in the treatment of gastric adenocarcinoma. Treatment of VEGF-antibodies or VEGF-receptor antibodies, however, had no significant effect on overall survival. Most recently, however, a new and fully humanized IgG1 monoclonal antibody Ramucirumab (IMC-1121B) has been introduced, which targets the extracellular domain of VEGF receptor 2 (VEGFR2). The antibody, increasing the median overall survival compared to placebo. Thus, a more detailed analysis of VEGF and VEGF receptor expression in early compared to advanced stages is needed. The current review focuses on previous findings and describes own results in early compared to advanced stages of gastric adenocarcinoma. A special focus is given on VEGF-A expression as well as the expression of the corresponding receptors VEGF-R1 and VEGF-R2. Own data reveal that VEGF-R2 may be a better target since expression levels seem to be expressed at high levels in gastric cancer tissues
Keywords
Gastric cancer; VEGF-A; VEGFR-1; VEGFR-2; CD133 antigen; Microvessels
Introduction
Gastric adenocarcinoma is the second leading cause of cancerrelated death world- wide, but the overall survival rate from the disease still remains poor due to the lack of effective treatment strategies, especially in advanced situations. Approximately 650 000 people die worldwide from gastric cancer every year [1]. Epidemiological and interventional studies in humans, as well as experiments in rodents, have strongly linked H. pylori infection to the development of both types of distal gastric cancer [2]. Surgery, i.e. gastrectomy with extended lymphadenectomy is the only curative option in early stages of gastric adenocarcinomas, and this treatment option is reasonable when the tumor has exceeded the mucosal layer [3]. Tumor prognosis is favourable only in early stages of gastric cancer, and recent studies have revealed that tumors limited to the mucosa (T1m stages) can be resected endoscopically, since lymph nodes metastasis at this stage are extremely rare [4].
A key role for gastric micro vascularisation during cancer progression is the vascular growth factor VEGF-A [5]. Vascular endothelial growth factor (VEGF) is a multifunctional cellular factor which can induce neovascularization and increase capillary permeability. VEGF-A regulates important steps in angiogenesis and physiological vascularisation and controls a number of physiological processes like wound healing, ovulation, menstruation and pregnancy [6]. There are four active isoforms of the VEGF-A molecule generated by differential splicing of which VEGF-A165 is the most common. The biological activity of VEGF-A is mediated by binding on VEGFR-1 and VEGFR-2 receptors, that are expressed on vascular endothelial cells; but also by binding on neuropilins located on vascular endothelium or neurons [7]. VEGF-A expression has been shown to be of special importance for neovascularisation in gastric cancer [8]. Also, VEGFR-1 expression rate among gastric cancer patients is supposed to indicate a high risk group for metastasis [9]. Furthermore, neovascularisation may be a new target to treat advanced stages using new antibodies [10]. Indeed, a monoclonal antibody against VEGF-A, Bevacizumab, was approved for therapy of colon cancer in 2005 [11]. Clinical studies using this and other antibodies have revealed diverging results in gastric cancer.
Interestingly, most recent studies by Ebos et al. and Paez-Ribes et al. have pointed out that drugs inhibiting the VEGF pathway promote tumour invasiveness and metastasis in mice [12,13]. Thus, the molecular mechanisms underlying vascularisation in more advanced stages of gastric cancer, including molecular signals in different stages of cancer, are of special interest.
Most recently, ramucirumab, a monoclonal antibody VEGFR-2 antagonist, prolonged survival in patients with advanced gastric cancer. This international, randomised, double-blind, placebo-controlled, phase 3 trial was conducted between 2009 and 2012 in 29 countries including North America, Central and South America, Europe, Asia, Australia, and Africa. Ramucirumab showed survival benefits in patients with advanced gastric or gastro-oesophageal junction adenocarcinoma progressing after first-line chemotherapy. The findings validate VEGFR-2 signaling as an important therapeutic target in advanced gastric cancer [14].
Therefore, we investigated VEGF-A and VEGFR-1 and -2 expressions in gastric cancer tissue. We used specimens from different stages of gastric cancer in order to evaluate the importance of this factor during further tumor invasion and lymph node metastasis. Our data reveal a decreased expression of this factor in more advanced stages, and limit the potential use of such VEGF-A targets in modern therapy of gastric adenocarcinoma. However, VEGF-R2 receptors seem to be present in different stages of gastric cancer, and could serve as a potential target.
Materials and Methods
Study population and tissues
Gastric cancer tissues investigated were obtained from patients (n=52) who underwent curative gastrectomy between 2001 and 2006 at the Division of Surgery, Klinikum rechts der Isar, Technical University of Munich. Patients’ characteristics are listed in Table 1. Under RNAsefree conditions, formalin-fixed paraffin-embedded (FFPE) tissue samples were sectioned at 10 μm. Sections were dewaxed with xylene, rehydrated and stained with haematoxylin. Whole tissue or separated areas (tumour or normal mucosa) of the section were microdissected to extract total RNA. Tissue amounts per slide lay between 50 mm2 and 300 mm2 in most cases. Small tumour areas were balanced by increased numbers of serial sections used. Quantitative TaqMan® real-time(RT)- PCR was performed using the Step One Plus sequence detection system (Applied Biosystems, Foster City, CA) as described previously [15] (Table 2). A mastermix was prepared on ice using AbsoluteTM QPCR ROX Mix (ABgene, Hamburg, Germany), according to manufacturer’s instructions and mixed with 2 μl of cDNA (details of protocol reported previously in Besig et al., Neuroendocrinology 2009) [16].
| Number of patients | 52 |
| Age | |
| Average age at surgery | 63.45 years |
| Standard deviation | 11.55 years |
| Range | 25.6 – 84.7 years |
| Gender | |
| Male | 26 |
| Female | 26 |
| Penetration depth (pT staging) | |
| pT1 m | 16 |
| pT1 sm | 20 |
| pT2 | 8 |
| pT3 | 8 |
| Lymph node status | |
| pN0 | 35 |
| pN1 | 10 |
| pN2 | 6 |
| pN3 | 1 |
| Histological type according to Lauren’s classification | |
| Intestinal type | 24 |
| Diffuse type | 21 |
| Mixed type | 7 |
Table 1: List of Patients’ characteristics in the investigation of gastric cancer from patients (n=52) who underwent curative gastrectomy between 2001 and 2006 at the Division of Surgery by Technical University of Munich.
| VEGF-A | |
| Forward primer | 5’-tac ctc cac cat gcc aag tg-3’ |
| Reverse primer | 5’-gat gat tct gcc ctc ctc ctt-3’ |
| Probe | 5’FAM-tcc cag gct gca ccc atg gc-3’TAMRA |
| VEGFR-1 | |
| Forward primer | 5’-tca ctg cca ctc taa ttg tca atg t-3’ |
| Reverse primer | 5’-aaa cga tga cac ggc ctt tt-3’ |
| Probe | 5’FAM-aaa ccc cag att tac-3’TAMRA |
| VEGFR-2 | |
| Forward primer | 5’-caa gac agg aag acc aag aaa aga c-3’ |
| Reverse primer | 5’-ggt gcc aca cgc tct agg a-3’ |
| Probe | 5’FAM-ttg cgt ggt cag gca gct cac a-3’TAMRA |
| CD-133 | |
| Forward primer | 5’-GCC AAA CCA CGA CTG TCG TA-3’ |
| Reverse primer | 5’-CGA TAT CTG AAC CAA TGG AAT TCA-3’ |
| Probe | 5’FAM-CAG GTA TCA AAA GGG TC-3’TAMRA |
Table 2: Analysis of Quantitative TaqMan® real-time(RT)-PCR.
Immunohistochemistry and determination of microvessel density
Immunohistochemical staining for endothelial CD31 was performed on an automated staining system (Ventana BenchMark, Ventana Medical Systems Tucson, AZ). Antigen retrieval was performed by heating (CC1 mild, Ventana BenchMark). The primary antibody, a monoclonal mouse anti-human CD31 clone JC-70-A (Dako ChemMate, Glostrup, Denmark), was incubated at a dilution of 1:30 for 50min at RT. Antibody binding was visualized using the LSABPOX- method and DAB, which yields a brown staining. Hematoxylin (AppliChem, Darmstadt, Germany) was used for counterstaining. Microvessel density (MVD) was determined by counting CD31 positive structures according to Weidner [17]. Staining was considered immunoreactive when the cytoplasms of endothelial cells were stained brown, a lumen was not required. Branching structures were counted as a single vessel. At least eight 500x500μm sectors at a 200x magnification were counted. The average vessel count per mm2 was calculated as the MVD (details of protocol as reported previously in Besig et al., 2009) [16].
Results
Expression of the VEGF ligand VEGF-A and VEGF-R1/2 in gastric adenocarcinoma
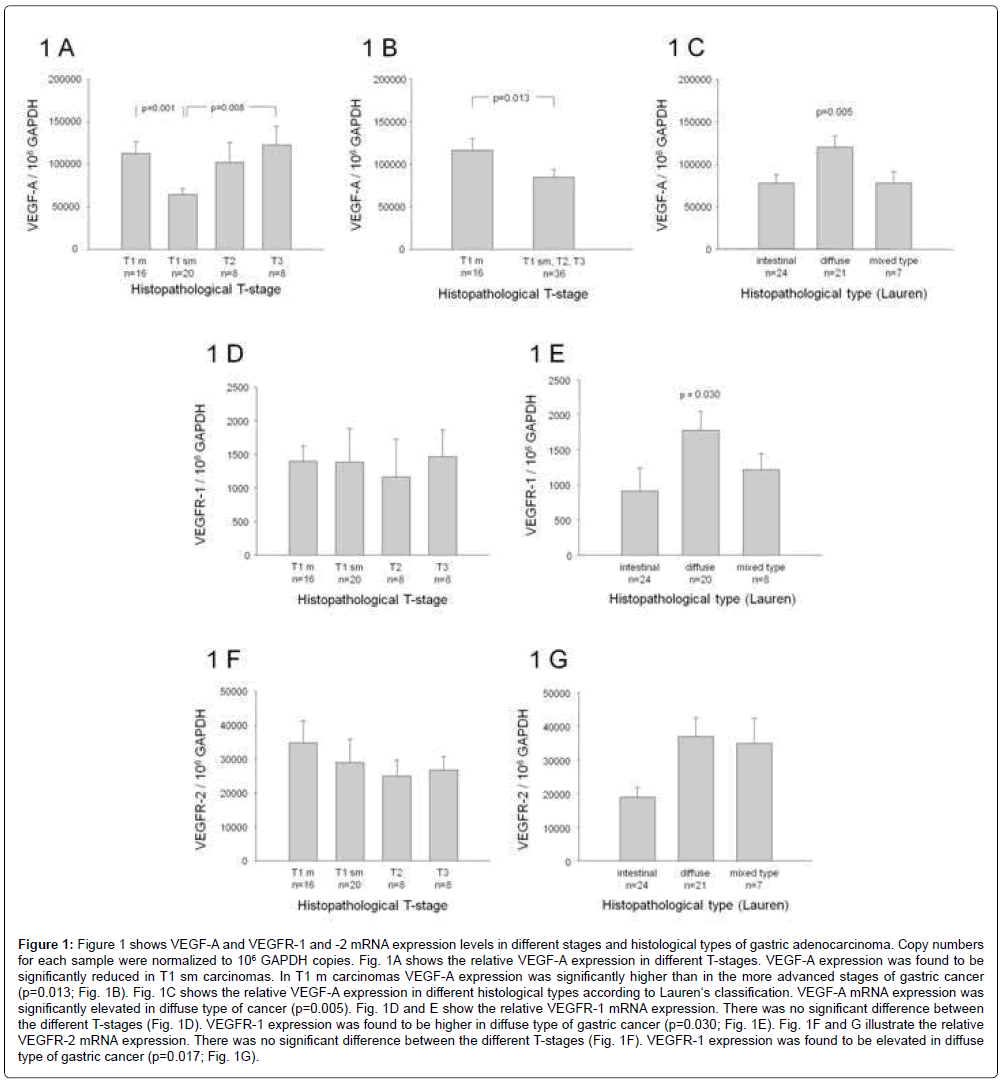
Gastric cancer tissues (n=52) were obtained from patients who underwent curative gastrectomy between 2001 and 2006 at the Division of Surgery, Klinikum rechts der Isar, Technical University of Munich. Gastric carcinomas were grouped according to Lauren’s classification and to the pathohistological T-stage. Initially, VEGF-A and VEGF-R1/2 mRNA levels were determined in gastric cancer tissue using TaqMan real-time PCR, and copy numbers of each gene were normalized to 106 GAPDH copies. Figure 1A shows the VEGF-mRNA expression in different T-stages. VEGF-A mRNA expression was significantly lower in carcinomas exceeding the mucosa compared to carcinomas limited to the mucosa (Figure 1B, p=0.013). Interestingly, VEGF-A expression was significantly elevated in diffuse type of gastric cancer compared to other cancer types (Figure 1C, p=0.017). A students t-test was used for statistical comparisons.
VEGFR-1 mRNA showed no significant difference between the different T-stages (Figure 1D). VEGFR-1 expression was significantly higher in the diffuse type of gastric carcinoma (Figure 1E, p=0.030).
Most interestingly, VEGFR-2 expression was expressed at a 100- fold higher level compared to VEGF-R1 expression levels, but revealed no significant difference between the different T-stages (Figure 1F). VEGFR-2 mRNA expression was significantly lower in intestinal type of gastric cancer (Figure 1G, p=0.017) compared to diffuse or mixed type. In average, the expression level of VEGF-R2 ranged at 10000- 50000 GAPDH copies, while VEGF-R1 was expressed at 500-1000 copies/GAPDH.-
Figure 1: Figure 1 shows VEGF-A and VEGFR-1 and -2 mRNA expression levels in different stages and histological types of gastric adenocarcinoma. Copy numbers for each sample were normalized to 106 GAPDH copies. Fig. 1A shows the relative VEGF-A expression in different T-stages. VEGF-A expression was found to be significantly reduced in T1 sm carcinomas. In T1 m carcinomas VEGF-A expression was significantly higher than in the more advanced stages of gastric cancer (p=0.013; Fig. 1B). Fig. 1C shows the relative VEGF-A expression in different histological types according to Lauren‘s classification. VEGF-A mRNA expression was significantly elevated in diffuse type of cancer (p=0.005). Fig. 1D and E show the relative VEGFR-1 mRNA expression. There was no significant difference between the different T-stages (Fig. 1D). VEGFR-1 expression was found to be higher in diffuse type of gastric cancer (p=0.030; Fig. 1E). Fig. 1F and G illustrate the relative VEGFR-2 mRNA expression. There was no significant difference between the different T-stages (Fig. 1F). VEGFR-1 expression was found to be elevated in diffuse type of gastric cancer (p=0.017; Fig. 1G).
Microvessel density in different stages of gastric cancer
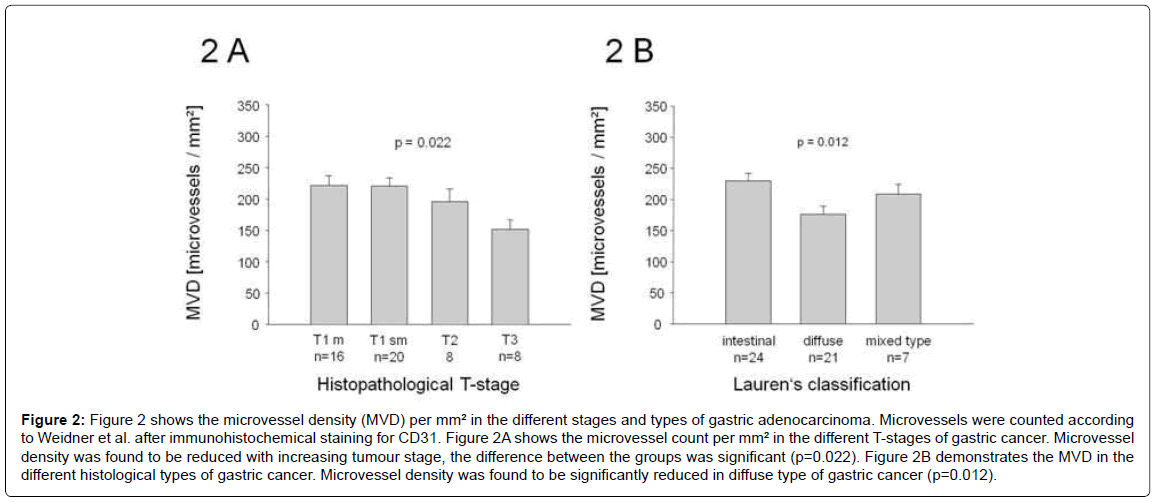
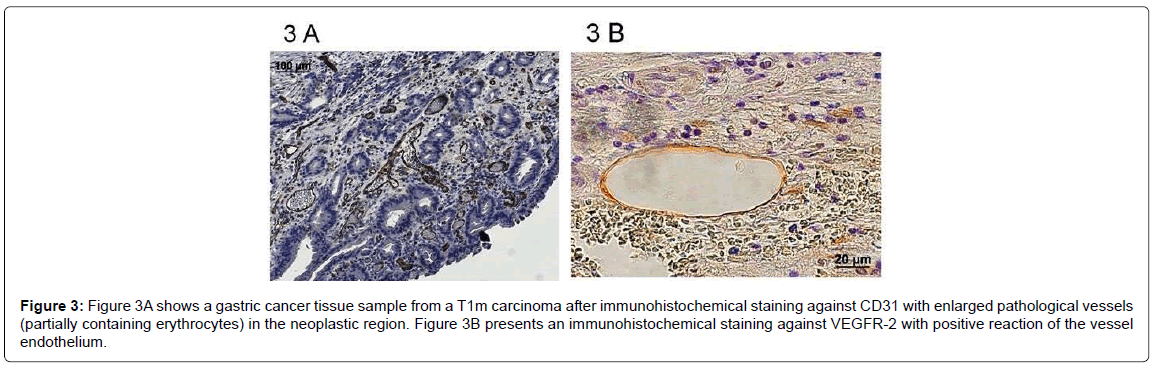
Microvessel density (MVD) in carcinoma tissue was determined by counting CD31 positive vessel structures, and results are shown in Figure 2. MVD in the carcinomas was decreased in more advanced stages of gastric cancer (p=0.022). MVD was also decreased in the diffuse type according to Lauren’s classification (p=0.012). Interestingly, the actual density of the vessels in tumor tissue was lower, but the vessels themselves were often larger. Figure 3A shows an immunohistochemical staining against the blood vessel endothelial marker CD31 and gives an illustration of the microvessel density in an intestinal type of early gastric cancer. Figure 3B is an image of an early gastric cancer stained with an antibody against the VEGF-R2. The receptor is detected on vessels.
Figure 2: Figure 2 shows the microvessel density (MVD) per mm² in the different stages and types of gastric adenocarcinoma. Microvessels were counted according to Weidner et al. after immunohistochemical staining for CD31. Figure 2A shows the microvessel count per mm² in the different T-stages of gastric cancer. Microvessel density was found to be reduced with increasing tumour stage, the difference between the groups was significant (p=0.022). Figure 2B demonstrates the MVD in the different histological types of gastric cancer. Microvessel density was found to be significantly reduced in diffuse type of gastric cancer (p=0.012).
Figure 3: Figure 3A shows a gastric cancer tissue sample from a T1m carcinoma after immunohistochemical staining against CD31 with enlarged pathological vessels (partially containing erythrocytes) in the neoplastic region. Figure 3B presents an immunohistochemical staining against VEGFR-2 with positive reaction of the vessel endothelium.
Expression of CD133 mRNA and correlation with VEGF-R1
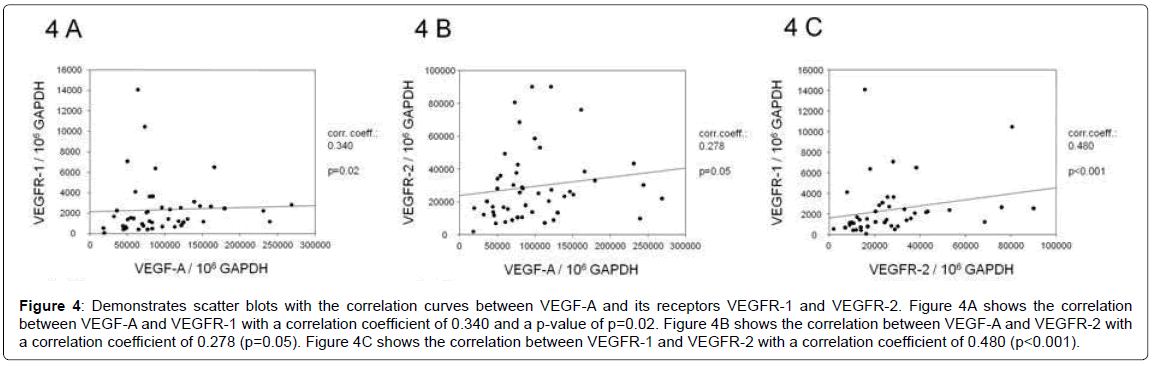
Since VEGF-A expression did not correlate with microvessel density, we sought to determine another parameter indicating presence of potential endothelial precursor that would be upregulated by this ligand. CD133 has been shown to be present on endothelial precursor cells [18]. We therefore investigated the relative CD133 mRNA expression in the present tumour specimens using TaqMan- PCR. CD133 was significantly reduced (p=0.008) in tumours with submucosal invasion (pT1sm, T2, T3), compared to those limited to the mucosa (pT1m, data not shown). VEGFR-1 (corr.coeff.: 0.515, p<0.001) but not VEGF-R2 (p=0.103) expression was significantly associated with CD133 expression; indicating a possible co-localisation on endothelial precursor cells (Figure 4A).
Figure 4: Demonstrates scatter blots with the correlation curves between VEGF-A and its receptors VEGFR-1 and VEGFR-2. Figure 4A shows the correlation between VEGF-A and VEGFR-1 with a correlation coefficient of 0.340 and a p-value of p=0.02. Figure 4B shows the correlation between VEGF-A and VEGFR-2 with a correlation coefficient of 0.278 (p=0.05). Figure 4C shows the correlation between VEGFR-1 and VEGFR-2 with a correlation coefficient of 0.480 (p<0.001).
Discussion
The current study investigated the expression of VEGF-A and the corresponding VEGFR-1 and -2 receptors in gastric adenocarcinomas. In parallel to other observations, VEGF-A expression was decreased in more advanced stages; limiting the potential use of VEGF-A antibodies in treatment of gastric cancer. In contrast, throughout the different tumor types and tumor stages, VEGF-R2 receptors were expressed at high levels. The new data thus suggest new implications of high clinical importance.
VEGF-A mRNA expression was significantly lower in carcinomas exceeding the mucosa compared to carcinomas limited to the mucosa, and was significantly elevated in diffuse type of gastric cancer. One explanation for this finding is the hypothesis that VEGF-A is mainly produced by the tumor cells themselves and although there is a progress of tumor cell mass, there is reduced expression or production of this growth factor, for example due to a loss of differentiation and chromosomal instability, leading to a generally reduced gene expression profile. It has been shown that VEGF-A is expressed in almost every kind of solid tumour and has been associated with progression and survival in different types of cancer [19-23]. However, it appears unlikely that especially gastric cancer plays a special role and resembles an exceptional tumor entity. Gene expression profiles of the divergent tumor cells were not available here; however, other authors have presented data in which a decreased VEGF-A expression in advanced stages was observed [24,25]. Our observation is in line with these last data, and may be explained by a more clear differentiation within the T-stages and by the accurate use of TaqMan PCR. In contrast, immunohistochemistry using antibodies against VEGF-A may be quite difficult to quantitate.
Another potential explanation for the reduced expression of VEGF-A in advanced stages is the idea that VEGF-A is mainly produced in endothelial cells and the vessels themselves. This idea, however, is in contrast to our own findings in diffuse type cancer, there is a #reduced presence of microvessel density. It has been proposed that hypoxia is a main reason for increased vessel formation in gastric cancer [26]. Other works have shown too that in advanced stages of cancer, a decrease in MVD can be observed [27]. This may be due to the fact that an increasing tumor volume is attended by an augmentation of the tumor matrix volume. Necrotic and desmoplastic reactions can be observed as well. Thus, the decline in vessel formation and VEGF-A expression may in fact reflect the increase in necrotic or desmoplastic tissue. At present there is only one phase-II study that shows a little benefit for patients with advanced gastric carcinoma receiving a combination therapy including Bevacizumab [10], which is in clear contrast to the observations made in patients with metastatic colon carcinoma [11]. Our and other observations thus question the benefit of antiangiogenetic therapies inhibiting the action of VEGF-A in advanced stages of gastric cancer. In contrast, our observations favor the role of anti-angiogenetic therapies in early neoplastic transformations limited to the mucosa.
In order to find a close correlation of the VEGF-A ligand with the receptors that are known to bind this ligand with high affinity, we determined presence of VEGFR-1 and VEGFR-2 mRNA expression in the tissue. VEGFR-1 (fms-like tyrosine kinase; Flt-1) binds VEGF-A with high affinity (Figure 4B). This receptor plays an important role in embryonal development and can not only be found on endothelial cells but also on osteoblasts, monocytes/macrophages and hematopoetic stem cells [28,29]. Experiments in animals have shown that VEGFR-1 is a critical factor in angiogenesis processes [29] (Figure 4C). VEGFR-2 (KDR, human; Flk-1, mouse) binds VEGF-A, VEGF-C, VEGF-D and VEGF-E and is expressed on endothelial cells, neuronal cells, osteoblasts, megakaryocytes and hematopoetic stem cells. VEGFR-2 activity is required for haematopoiesis and has antiapoptotic effects in endothelial cells, VEGFR-2 deficient mouse embryos die in utero [8].
VEGF-A on the one hand and VEGFR-1 and -2 on the other hand showed a close correlation, underlining the finding that VEGF-A may indeed be important for neo-vascularization of gastric cancer, especially in early T1m stages. One possibility for this finding may be that VEGF-A and the corresponding receptors are expressed by the same type of cells – tumor cells and/or endothelial cells. Another possibility is that high VEGF-A secretion from tumor cells, – induced for example in hypoxic areas of the tumor – may lead to higher VEGFR-1 and -2 expression in tumor vessel endothelial cells. In other works, VEGFR-2 receptors have been detected on vessels [30].
Here, our data observed in tumor specimens indicate that VEGFR-2 receptors are present on vessels present in the tumor tissue, but not the tumor cells themselves, as depicted in the Figure 3B. The findings are in line with current clinical reports which point out the targeting VEGF-R2 can be an relevant target. Ramucirumab, a monoclonal antibody VEGFR-2 antagonist, prolonged survival in patients with advanced gastric cancer. Ramucirumab showed survival benefits in patients with advanced gastric or gastro-oesophageal junction adenocarcinoma progressing after first-line chemotherapy. The findings validate VEGFR-2 signalling as an important therapeutic target in advanced gastric cancer [14].
No significant correlation was observable between VEGFR-2 mRNA levels and the MVD. This observation may appear divergent to the above data; however, it may be explained by the fact that the MVD only refers to the number of vessels in the visual field, but does not take into account the vessel size or quality. Small normal vessels are counted equally to abnormal tumor vessels. One must therefore mention that the different growth patterns may also affect on the MVD count: in diffuse type of gastric cancer there are often clusters of cells with a low cell and vessel density. The determination method of the MVD according to Weidner [17] does not compare the cell density to the vessel density, it only focuses on the absolute number of vessels. In our study we did not count vessels in exulcerated parts of the tumors, because in these cases granulation tissue could not be distinguished from vessels.
In order to further delineate the complex interaction between tumor cells and vessels, we used a novel marker of endothelial precursor cells (EPC) that may be of special interest in this context, CD133 is a pentaspan transmembrane glycoprotein of currently unknown function [31]. First reports describe that CD133 receptors are highly expressed on hematopoetic stem cells and related malignancies including myelogenous leukaemia, acute lymphocytic leukaemia, and chronic lymphatic leukaemia and myelodysplastic syndromes [32]. Recent studies by Asahara et al. [33] have shown that after bone marrow transplantation in mice, donor endothelial precursor cells (EPCs) were frequently found in sites of neovascularisation. These EPCs were characterized by the expression of CD133, CD34 and VEGFR-2 on their surface. CD133 expression is lost once these cells differentiate into more mature endothelial cells, and this differentiation is mediated by VEGF [18]. Thus, CD133 may also be related to angiogenesis processes since it is expressed on endothelial progenitor cells (EPCs). These cells derive from the bone marrow and have the potential to proliferate, migrate and to initialize endothelial growth.
When comparing CD133 expression with the above ligands and receptors, we found close correlation between CD133 and VEGF-R1. This finding suggests that EPCs are of special importance for neovascularisation in early stages of gastric cancer; once the cells differentiate into vessels, CD133 expression is lost and thus VEGF-1 is decreased simultaneously [34,35]. Thus, endothelial precursor cells appear to play a crucial role for neovascularisation in gastric cancer that deserves further investigation.
In summary, our findings clearly delineate the role of VEGF-A and its corresponding receptors in early stages of gastric stages. Advanced stages of gastric carcinoma however, seem to lose the expression, maybe due to a desmoplastic reaction, and are thus unsuitable for such directed molecular therapy. Further studies will investigate the role of other vascular growth patterns, such as lymphangiogenetic factors, neuropilins, and HIF-1alpha.
References
- Correa P (2004) Is gastric cancer preventable? Gut 53: 1217-1219.
- Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, et al. (2001) Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 345: 784-789.
- Siewert JR, Böttcher K, Stein HJ, Roder JD (1998) Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 228: 449-461.
- Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, et al. (2000) Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 3: 219-225.
- Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T, Horiuchi T, et al. (1996) Extent of tumor vascularization correlates with prognosis and hematogenous metastasis in gastric carcinomas. Cancer Res 56: 2671-2676.
- Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9: 653-660.
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M (1998) Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92: 735-745.
- Du JR, Jiang Y, Zhang YM, Fu H (2003) Vascular endothelial growth factor and microvascular density in esophageal and gastric carcinomas. World J Gastroenterol 9: 1604-1606.
- Kosaka Y, Mimori K, Fukagawa T, Ishikawa K, Etoh T, et al. (2007) Identification of the high-risk group for metastasis of gastric cancer cases by vascular endothelial growth factor receptor-1 overexpression in peripheral blood. Br J Cancer 96: 1723-1728.
- Shah MA, Ramanathan RK, Ilson DH, Levnor A, D'Adamo D, et al. (2006) Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 24: 5201-5206.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335-2342.
- Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, et al. (2009) Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15: 232-239.
- Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, et al. (2009) Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15: 220-231.
- Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, et al. (2013) for the REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 13: 61719-5.
- Rad R, Brenner L, Bauer S, Schwendy S, Layland L, et al. (2006) CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology 131: 525-537.
- Besig S, Voland P, Baur DM, Perren A, Prinz C (2009) Vascular endothelial growth factors, angiogenesis, and survival in human ileal enterochromaffin cell carcinoids. Neuroendocrinology 90: 402-415.
- Weidner N (1995) Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat 36: 169-180.
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, et al. (2000) Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95: 952-958.
- Goncharuk IV, Vorobjova LI, Lukyanova NY, Chekhun VF (2009) Vascular endothelial growth factor exression in uterine cervical cancer: correlation with clinicopathologic characteristics and survival. Exp Oncol 31: 179-181.
- Kolev Y, Uetake H, Iida S, Ishikawa T, Kawano T, et al. (2007) Prognostic significance of VEGF expression in correlation with COX-2, microvessel density, and clinicopathological characteristics in human gastric carcinoma. Ann Surg Oncol 14: 2738-2747.
- Lee JC, Chow NH, Wang ST, Huang SM (2000) Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer 36: 748-753.
- Nam DH, Park K, Suh YL, Kim JH (2004) Expression of VEGF and brain specific angiogenesis inhibitor-1 in glioblastoma: prognostic significance. Oncol Rep 11: 863-869.
- Sun HC, Qiu ZJ, Liu J, Sun J, Jiang T, et al. (2007) Expression of hypoxia-inducible factor-1 alpha and associated proteins in pancreatic ductal adenocarcinoma and their impact on prognosis. Int J Oncol 30: 1359-1367.
- Cabuk D, Basaran G, Celikel C, Dane F, Yumuk PF, et al. (2007) Vascular endothelial growth factor, hypoxia-inducible factor 1 alpha and CD34 expressions in early-stage gastric tumors: relationship with pathological factors and prognostic impact on survival. Oncology 72: 111-117.
- Kösem M, Tuncer I, Kotan C, Ibiloglu I, Oztürk M, et al. (2009) Significance of VEGF and microvascular density in gastric carcinoma. Hepatogastroenterology 56: 1236-1240.
- Harris AL (2002) Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer 2: 38-47.
- Cabuk D, Basaran G, Celikel C, Dane F, Yumuk PF, et al. (2007) Vascular endothelial growth factor, hypoxia-inducible factor 1 alpha and CD34 expressions in early-stage gastric tumors: relationship with pathological factors and prognostic impact on survival. Oncology 72: 111-117.
- Fong GH, Rossant J, Gertsenstein M, Breitman ML (1995a) Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. nature 376: 66-70.
- Sawano A, Iwai S, Sakurai Y, Ito M, Shitara K, et al. (2001) Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood 97: 785-791.
- Nakopoulou L, Stefanaki K, Panayotopoulou E, Giannopoulou I, Athanassiadou P, et al. (2002) Expression of the vascular endothelial growth factor receptor-2/Flk-1 in breast carcinomas: correlation with proliferation. Hum Pathol 33: 863-870.
- Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, et al. (1997) A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood 90: 5013-5021.
- Horn PA, Tesch H, Staib P, Kube D, Diehl V, et al. (1999) Expression of AC133, a novel hematopoietic precursor antigen, on acute myeloid leukemia cells. Blood 93: 1435-1437.
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, et al. (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85: 221-228.
- Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, et al. (1997) A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood 90: 5013-5021.
- Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, et al. (1997) AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 90: 5002-5012.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 14747
- [From(publication date):
December-2013 - Dec 23, 2025] - Breakdown by view type
- HTML page views : 10014
- PDF downloads : 4733