Associated Complex of Urine Creatinine, Serum Creatinine, and Chronic Kidney Disease
Received: 14-Mar-2016 / Accepted Date: 28-Mar-2016 / Published Date: 04-Apr-2016 DOI: 10.4172/2161-1165.1000234
Abstract
Background: Impaired kidney function can affect levels of urine (UCR) and serum creatinine (SCR).
Objective: Data from National Health and Nutrition Examination Survey for the years 1999-2014 were used to study associations between UCR, SCR, and chronic kidney disease (CKD) stages 1-5.
Results: Levels of SCR and UCR were positively correlated for CKD Stages 1-3 and negatively correlated for CKD Stages 4 and 5. Males had higher levels of UCR than females but male-female differences were narrower for CKD Stages 1-3 than for CKD Stages 4-5. Males had higher levels of SCR than females but male-female differences were 30% for CKD Stages 1-3 and 20% for CKD Stages 4-5. NHB had higher levels of UCR than NHW but these differences were 27.5 mg/dL for CKD Stages 1-3 and 17.9 mg/dL for CKD Stage 4 and 5. NHB had about 11% higher SCR levels than NHW for CKD Stages 1-3 and about 20% higher levels than NHW for CKD Stages 4-5. For CKD Stages 1-3, levels of UCR decreased with increase in CKD Stage but levels of SCR increased with increase in CKD Stage. Levels of both SCR and UCR were higher for CKD Sage 5 than for CKD Stage 4.
Conclusions: CKD stages affect association between SCR and UCR as well as gender and racial/ethnic differences in both UCR and SCR.
Keywords: Chronic kidney disease; Albumin creatinine ratio; Diabetes; Serum creatinine; Gender; Race/Ethnicity; Ageing
Introduction
Barr et al. [1] showed that age, gender, race/ethnicity, and body mass index (BMI) affect urine creatinine levels (UCR). Persons with diabetes had lower UCR than persons without diabetes; and kidney function was also shown to affect UCR [1]. Levels of serum creatinine are substantially affected by impaired kidney function. In addition, they can also be affected by age, gender, race/ethnicity, body habitus, diet (vegetarian vs. meat eater) and use of certain medications like cimetidine, ketoacids, bilirubin, flucytosine, and metamizole [2].
When analyte concentrations can be measured in both blood and urine, usually, there should be a positive correlation between blood and urine concentrations. However, in the presence of impaired kidney function, this association may change from being positive to negative. Kidney function is usually represented as glomerular filtration rate (GFR) as well as urinary albumin creatinine ratio (ACR). GFR ≥ 90 mL/min/1.73 m2 body surface area (BSA) is considered to represent normal kidney function (Stage 1) and GFR between 60 and 90 mL/min/1.73 m2 BSA is considered to represent mildly reduced kidney function (Stage 2). GFR between 45 and 60 mL/min/1.73 m2 BSA (Stage 3A) and between 30 and 45 mL/min/1.73 m2 BSA (Stage 3B) represents moderately reduced kidney function. GFR between 15 and 30 mL/min/1.73 m2 BSA (Stage 4) represents severely reduced kidney function and GFR < 15 mL/min/1.73 m2 BSA is considered to represent very severe or end-stage kidney failure (https:// www.renal.org/information-resources/the-uk-eckd-guide/ckdckd stages# sthash.LrKN6RB6.dpbs). GFR is not directly measured but is estimated using one of the several available equations that use serum creatinine measurements with gender, race/ethnicity, and age as input. For adults, these equations include Modification of Diet in Renal Disease (MDRD) Study equation [3] and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [4]. Separate equations are available for Americans and others. Equations that use serum cystain C alone or in combination with serum creatinine have also been proposed to estimate GFR [5]. Albuminuria or ACR > 30 mg/g is considered to be a marker of chronic kidney disease or CKD. For those under 18 years of age, Bedside Schwartz equation is used [6,7].
Data from NHANES have often been used to estimate the prevalence of CKD and albuminuria in U.S. population [8-17] as well as to evaluate association between reduced GFR and albuminuria with adverse health conditions and events [10,18-21], including mortality [10,22-26]. However, an investigation evaluating multivariate interacting associations among UCR, SCR, CKD, and other factors over time does not exist. This study was undertaken to fill this gap by analyzing relevant NHANES data for 1999-2014.
The objectives of this study were: (i) evaluate correlations between UCR and SCR by the stages of CKD, (ii) fit regression model to evaluate factors that affect observed levels of UCR for CKD stages 1-5, and (iii) fit regression model to evaluate factors that affect observed levels of SCR for CKD stages 1-5. Data from National Health and Nutrition Examination Survey (www.cdc.gov/nchs/nhanes.htm, NHANES) for the years 1999-2014 were selected to conduct this study.
Participants and Methods
Data source and data description
Data from NHANES for the years 1999-2014 from demographic, body measures, serum cotinine, UCR, and standard biochemistry files for all those aged ≥ 20 years were downloaded and match merged. NHANES uses a complex, stratified, multistage, probability sampling designed to be representative of the civilian, non-institutionalized U.S. population based on age, sex, and race/ethnicity.
Sampling weights are created in NHANES to account for the probabilities of selection and response as well as U.S. population for certain combinations of gender, age, and race/ethnicity.
Total number of participants available for analysis for 39276 (19516 males, 19760 females, 18305 non-Hispanic whites or NHW, 8172 non- Hispanic blacks or NHB, 7021 Mexican Americans, 5778 belonging to unclassified race/ethnicities or OTH). Number of participants by age was 6451, 6535, 6938, 5936, 6380, and 7036 for those aged 20-29, 30-39, 40-49, 50-59, 60-69, and ≥ 70 years respectively.
Derived variables and definitions
Those with serum cotinine levels ≥ 10 mg/mL were defined as smokers and those with serum cotinine levels below 10 mg/mL were defined as nonsmokers. Those who had glycohemoglobin levels ≥ 6.5% were defined as diabetics and those with glycohemoglobin levels < 6.5% were defined as nondiabetics.
CKD stages were based on the computed GFR. GFR expressed as mL/min/1.73 m2 of BSA were computed based on the formula given in [4]. The formula to compute GFR by [4] was based on The Chronic Kidney Epidemiology Collaboration study. Five CKD stages were defined as: Stage 1 if GFR ≥ 90 mL/min/1.73 m2 of BSA, Stage 2 if 60 ≤ GFR < 90, Stage 3A if 45 ≤ GFR < 60, Stage 3B if 30 ≤ GFR < 45, Stage 4 if 15 ≤ GFR < 30, and Stage 5 if GFR < 15 mL/min/1.73 m2 BSA.
Software
All analyses were done by using SAS University Edition (www.sas.com) software.
Statistical analysis of correlations between UCR and SCR
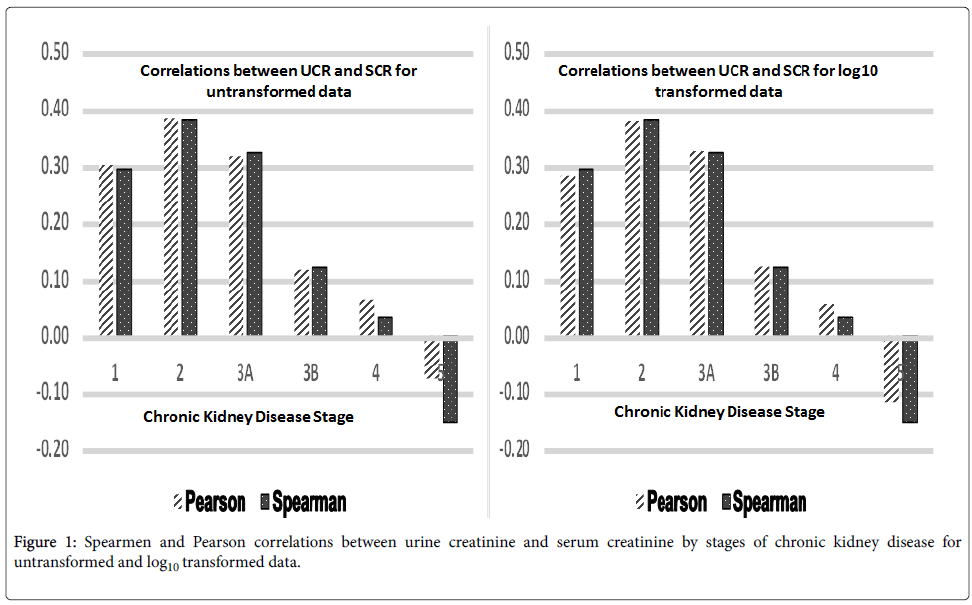
Both Pearson and Spearman correlations between UCR and SCR were computed by using SAS Proc CORR. Correlations were computed both between un-transformed values of UCR and SCR as well as log10 transformed values of UCR and SCR. Values of UCR and SCR were log10 transformed because of their positively skewed distributions. Results are given in Figure 1.
Statistical analysis of factors affecting UCR values
As explained in the next Section, data were split in two separate databases. First database included all data when CKD stages were 1, 2, and 3. Second database included all data when CKD stages were 4 and 5.
In Model I, which analyzed database 1, log10 transformed values of UCR were used as the dependent variable, and gender (male, female), age (20-29 years or A20, 30-39 years or A30, 40-49 years or A40, 50-59 years or A50, 60-69 years or A60, and ≥ 70 years or A70), race/ ethnicity (non-Hispanic White or NHW, non-Hispanic Black or NHB, Mexican Americans or MA, other unclassified race/ethnicities or OTH), CKD stages (1, 2, 3A, 3B), smoking status (nonsmoker or NSM, smoker or SM), diabetes status (diabetic or DIAB, non-diabetic or NDIAB) were used as categorical independent variables, and log10 transformed values of SCR, log10 transformed values of body mass index (BMI) and survey year were used as continuous independent variables.
First order interaction terms between gender, age, race/ethnicity, CKD stages, smoking status, and diabetes status were considered but were included in the final model if they were found to be statistically significant at α = 0.05. In Model II, which analyzed database 2, log10 transformed values of UCR were used as the dependent variable. However, because of small sample sizes, only 3 age categories and only 3 race/ethnicity categories were used.
The categorical independent variables used were: gender (male, female), age (20-59 years or A20++, 60-69 years or A60, and ≥ 70 years or A70), race/ethnicity (non-Hispanic White or NHW, non-Hispanic Black or NHB, other race/ethnicities including Mexican Americans or OTH+), CKD stages (1, 2, 3A, 3B), smoking status (nonsmoker or NSM, smoker or SM), diabetes status (diabetic or DIAB, non-diabetic or NDIAB). log10 transformed values of SCR, log10 transformed values of body mass index (BMI) and survey year were used as continuous independent variables. Because of small sample sizes, interaction terms were not considered for Model II.
SAS Proc SURVEYREG was used to fit both regression models I and II, compute adjusted geometric means (AGM) with 95% confidence intervals, and estimate regression slopes. Pairwise comparisons were tested for statistical significance by t-test. Only those who were aged ≥ 20 years were included for analyzing data for Models I and II.
Statistical analysis of factors affecting SCR values
With log10 transformed values of SCR as dependent variables and independent variables as used for Model I and Model II were fitted to evaluate that affected the observed values of SCR when CKD stages were 1, 2, 3A, and 3B (Model III) and when CKD stages were 4 or 5 (Model IV). UCR vales were not used in either Model III or Model IV.
SAS Proc SURVEYREG was used to fit both regression models III and IV, compute adjusted geometric means (AGM) with 95% confidence intervals, and estimate regression slopes. Pairwise comparisons were tested for statistical significance by t-test. Only those who were aged ≥ 20 years were included for analyzing data for Models III and IV.
Results
Correlations between UCR and SCR
There was a decreasing trend of positive correlations from over 0.25 for CKD Stage 1 to just over 0.10 for CKD Stage 3B between UCR and SCR (Figure 1).
For CKD Stage 4, this correlation was < 0.10 and for CKD Stage 5, it became negative of the order 0.10 to 0.15.
Factors affecting UCR – model I
Actual N used for this model was 30980 and R2 was 17.3%. Statistically significant interactions between gender and race/ethnicity, gender and age, gender and CKD stage, and gender and smoking status were observed at α = 0.05.
Statistically significant interaction between age and race/ethnicity, age and CKD stages, age and smoking status, and race/ethnicity and diabetes status were also observed.
SCR was found to be positively associated with UCR (β = 0.475, p < 0.01, Table 1). UCR was also found to be positively associated with BMI (β = 0.505, p < 0.01, Table 1).
| Group | AGM (95% CI) | Statistically Significant Differences |
|---|---|---|
| Male (M) | 96.1 (90-102.6) | M>F (p<0.01) |
| Female (F) | 84.8 (80.1-89.7) | |
| Age: 20-29 Years (A20) | 107.9 (96.1-121.3) | A20>A40 (p = 0.01), A20>A50 (p<0.01, A20>A60 (p<0.01), A20>A70 (p<0.01) |
| Age: 30-39 Years (A30) | 109.3 (97.2-122.8) | A30>A40 (p = 0.02), A30>A50 (p<0.01), A30>A60(p<0.01), A30>A70 (p<0.01) |
| Age: 40-49 Years (A40) | 87.8 (76.3-100.9) | |
| Age: 50-59 Years (A50) | 80.2 (73.4-87.5) | |
| Age: 60-69 Years (A60) | 77.8 (73.2-82.7) | |
| Age: ≥ 70 Years (A70) | 83.8 (80.1-87.7) | |
| Non-Hispanic White (NHW) | 80 (75.2-85.1) | NHW<NHB (p<0.01), NHW<MA (p<0.01), NHW<OTH (p<0.01) |
| Non-Hispanic Black (NHB) | 107.5 (101.2-114.1) | NHB>MA (p<0.01), NHB>OTH (p<0.01) |
| Mexican American (MA) | 89.5 (85.1-94.1) | |
| Oher unclassified race/ethnicities (OTH) | 86.3 (80-93) | |
| CKD Stage 1 (CKD1) | 102.4 (99.9-105) | CKD1>CKD3B (p<0.01) |
| CKD Stage 2 (CKD2) | 105.4 (102.4-108.5) | CKD2>CKD3B (p<0.01) |
| CKD Stage 3A (CKD3A) | 100.9 (92-110.7) | CKD3A>CKD3B (p<0.01) |
| CKD Stage 3B (CKD3B) | 60.9 (52.2-71.1) | |
| Non-Diabetic (NDIAB) | 93.8 (89.3-98.6) | NDIAB>DIAB (p<0.01) |
| Diabetic (DIAB) | 86.8 (81.5-92.5) | |
| Nonsmoker (NSM) | 88.5 (83.8-93.5) | NSM<SM (p<0.01) |
| Smoker (SM) | 92 (86.9-97.5) |
Table 1: Adjusted geometric means (AGM) with 95% confidence intervals in mg/dL for urine creatinine for Model I** by gender, age, race/ ethnicity, diabetes status, and chronic kidney disease (CKD) status*. Data from National Health and Nutrition Examination Survey 1999-2014.*CKD stages were defined on the basis of glomerular filtration rate (GFR) as: Stage 1: GFR ≥ 90, Stage 2: GFR ≥ 60 but less than 90, Stage 3A: GFR ≥ 45 but less than 60, Stage 3B: GFR ≥ 30 but less than 45. **Actual N used for the model was 30980. R2 for the fitted model was 17.3%. Regression slopes (β) with log10 transformed values of urine creatinine was -0.0117 (p < 0.01) for survey year, 0.475 (p < 0.01) for log10 transformed values of serum creatinine, and 0.505 for log10 transformed values of body mass index.
UCR levels decreased over time (β = -0.0117, p < 0.01, Table 2).
| Group | AGM (95% CI) | Statistically Significant Differences |
|---|---|---|
| Male (M) | 113.3 (95.4-134.5) | M>F (p<0.01) |
| Female (F) | 88.4 (76.5-102.3) | |
| Age: 20-59 Years (A20++) | 112.7 (89.4-142.1) | |
| Age: 60-69 Years (A60) | 100.2 (80.3-125.2) | |
| Age: ³ 70 Years (A70) | 88.7 (75.5-104.3) | |
| Non-Hispanic White (NHW) | 102.3 (87.4-119.8) | |
| Non-Hispanic Black (NHB) | 120.2 (98.6-146.4) | NHB>OTH+ (p<0.01) |
| Oher race/ethnicities including Mexican Americans (OTH+) | 81.5 (66-100.8) | |
| CKD Stage 4 (CKD4) | 82.8 (71-96.5) | CKD4<CKD5 (p = 0.03) |
| CKD Stage 5 (CKD5) | 121 (92-159.3) | |
| Non-Diabetic (NDIAB) | 103.9 (90.5-119.4) | |
| Diabetic (DIAB) | 96.4 (79.4-117) | |
| Nonsmoker (NSM) | 96.9 (81-116) | |
| Smoker (SM) | 103.3 (87.5-122) |
Table 2: Adjusted geometric means (AGM) with 95% confidence intervals in mg/dL for urine creatinine for Model II** by gender, age, race/ethnicity, diabetes status, and chronic kidney disease (CKD) status*. Data from National Health and Nutrition Examination Survey 1999-2014.*CKD stages were defined on the basis of glomerular filtration rate (GFR) as: Stage 5: GFR ≥ 15 but less than 30, Stage 5: GFR < 15. **Actual Nused for the model was 279. R2 for the fitted model was 10.7%. Regression slopes (β) with log10 transformed values of urine creatinine was -0.0134 (p = 0.14) for survey year, -0.5583 (p < 0.01) for log10 transformed values of serum creatinine, and -0.0759 (p = 0.72) for log10 transformed values of body mass index.
Effect of gender
Males were found to have statistically significantly higher UCR AGM than females (96.1 vs. 84.8 mg/dL, p < 0.01, Table 1).
However, male-female differences in AGM varied by race/ethnicity (10.0, 6.6, 8.1, 15.0 mg/dL for NHW, NHB, MA, and OTH respectively, Table S1), age (1.1, 1.7, 11.1, 16.8, 15.9, 15.6 mg/dL for A20, A30, A40, A50, A60, and A70 respectively, Table S1), CKD stages (24.0, 22.6, 13.2 mg/dL for CKD stages 1,2, and 3A respectively, Table S1), and smoking status (13.4 vs. 9.1 mg/dL for nonsmokers and smokers respectively, supplementary Table S1).
Effect of age
Both A20 and A30 had statistically significantly higher UCR AGM than almost every other age group (p < 0.01, Table 1). Statistically significant differences among A40, A50, A60, and A70 were not observed. In addition, some of the age differences among males were smaller than among females.
For example, while AGM difference between A20 and A40 for males was 15.0 mg/dL, this difference for females was 25.0 mg/dL (Table S1). Similarly, age differences across various racial/ethnic groups were not same (Table S1). For example, for NHW, the difference between A20 and A70 was 18.9 mg/dL, this difference for NHB was 38.1 mg/dL, Table S1).
Effect of race/ethnicity
The order in which UGMs by race/ethnicity was observed was: NHB > MA > OTH > NHW and all pairwise differences except between MA and OTH were statistically significant (p < 0.01, Table 1). However, racial/ethnic differences varied with gender, age, and diabetes status (Table S1).
For example, NHB-NHW differences were 23.5 mg/dL for males and 30.9 m/dL for females, 34.0 mg/dL for nondiabetics and 21.5 mg/dL for diabetics (Table S1). By age, NHB-NHW differences in mg/dL were: 35.0 for A20, 39.4 for A30, 30.0 for A40, 27.3 for A50, 20.9 for A60, and 15.8 for A70 (Table S1).
Effect of CKD stages
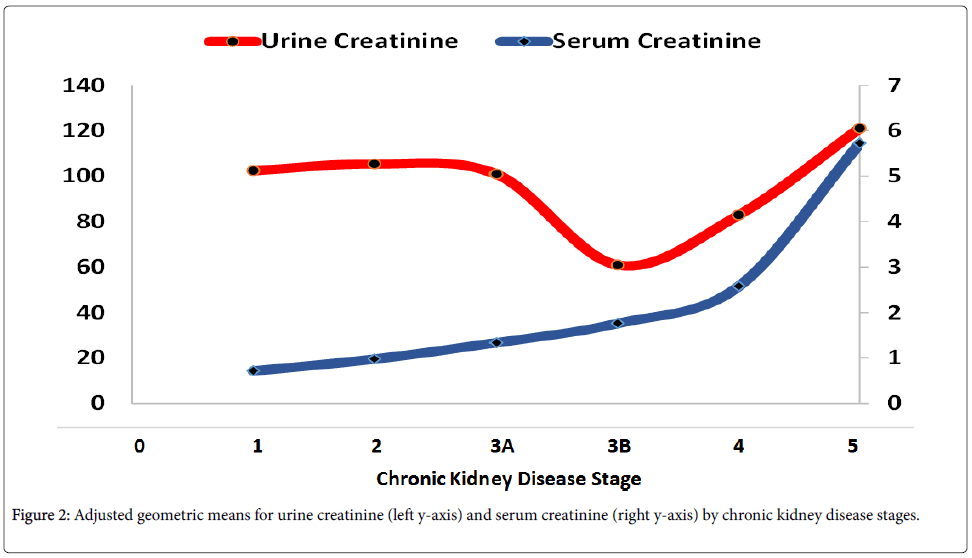
UCR AGMs were statistically significantly lower for CKD3B than other stages (p < 0.01, Table 1, Figure 2). There were minimal differences in UCR for CKD stages 1, 2, and 3A. However, these differences varied by gender and age (Table S1). CKD3A-CKD3B differences for males were 49.2 mg/dL and 31. 2 mg/dL for females (Table S1). By age, CKD3A-CKD3B differences in mg/dL were: 114.1 for A20, 23.7 for A30, 34.3 for A40, 37.3 for A50, 27.0 for A60, and 10.8 for A70 (Table S1).
Effect of Diabetes
Nondiabetics had higher UCR AGM than diabetics (93.8 vs. 86.8 mg/dL, p < 0.01, Table 1). Nondiabetic-diabetic differences by race/ ethnicity were: 4.5, 17.0, 6.5, and 2.0 mg/dL for NHW, NHB, MA, and OTH respectively (Table S1).
Effect of smoking status
Smokers had higher UCR than nonsmokers (92.0 vs. 88.5 mg/dL, p < 0.01, Table 1). These differences for males were 1.2 mg/dL and 5.5 mg/dL for females. By age, smoker-nonsmoker differences in mg/dL were: 9.3 for A20, 10.6 for A30, 2.1 for A40, -1.7 for A50, -2.1 for A60, and 6.3 for A70 (Table S1).
Factors affecting UCR – model II
Actual sample size used to analyze data for Model II was 279 and model R2 was 10.7%. Statistically significant negative association between log10 (UCR) and log10 (SCR) (β = -0.5583, p < 0.01) was observed (Table 3). However, no statistically significant association between log10 (UCR) and BMI (p = 0.72) as well as survey year (p = 0.14) was discovered (Table 2). Males had about 25% higher levels of UCR when compared with females (113.3 vs. 88.4 mg/dL, p < 0.01, Table 3). UCR levels did not vary by age (Table 2). No statistically significant differences in the levels of UCR between NHW and NHB were observed but NHB did have higher levels of UCR than OTH+ (120.2 vs. 81.5 mg/dL, p < 0.01, Table 2).
| Group | AGM (95% CI) | Statistically Significant Differences |
|---|---|---|
| Male (M) | 1.28 (1.27-1.29) | M>F (p<0.01) |
| Female (F) | 0.99 (0.98-1) | |
| Age: 20-29 Years (A20) | 1.23 (1.18-1.29) | A20>A40 (p = 0.01), A20>A50 (p<0.01, A20>A60 (p<0.01), A20>A70 (p<0.01) |
| Age: 30-39 Years (A30) | 1.19 (1.16-1.22) | A30>A40 (p = 0.03), A30>A50 (p<0.01), A30>A60(p<0.01), A30>A70 (p<0.01) |
| Age: 40-49 Years (A40) | 1.14 (1.12-1.17) | A40>A60 (p<0.01), A40>A70 (p<0.01) |
| Age: 50-59 Years (A50) | 1.13 (1.11-1.14) | A50>A60 (p<0.01), A50>A70 (p<0.01) |
| Age: 60-69 Years (A60) | 1.08 (1.07-1.09) | A60>A70 (p<0.01) |
| Age: ≥ 70 Years (A70) | 1.01 (1-1.01) | |
| Non-Hispanic White (NHW) | 1.1 (1.09-1.12) | NHW<NHB (p<0.01), NHW>MA (p<0.01) |
| Non-Hispanic Black (NHB) | 1.24 (1.23-1.26) | NHB>MA (p<0.01), NHB>OTH (p<0.01) |
| Mexican American (MA) | 1.08 (1.06-1.09) | MA>OTH (p = 0.04) |
| Oher unclassified race/ethnicities (OTH) | 1.1 (1.08-1.11) | |
| CKD Stage 1 (CKD1) | 0.71 (0.71-0.72) | CKD1<CKD2 (p<0.01), CKD1<CKD3A (p<0.01), CKD1<CKD3B (p<0.01) |
| CKD Stage 2 (CKD2) | 0.97 (0.96-0.98) | CKD2<CKD3A (p<0.01), CKD2<CKD3B (p<0.01) |
| CKD Stage 3A (CKD3A) | 1.33 (1.31-1.35) | CKD3A<CKD3B (p<0.01) |
| CKD Stage 3B (CKD3B) | 1.76 (1.7-1.83) | |
| Non-Diabetic (NDIAB) | 1.15 (1.14-1.16) | NDIAB>DIAB (p<0.01) |
| Diabetic (DIAB) | 1.1 (1.09-1.12) | |
| Nonsmoker (NSM) | 1.13 (1.12-1.14) | |
| Smoker (SM) | 1.13 (1.11-1.14) |
Table 3: Adjusted geometric means (AGM) with 95% confidence intervals in mg/dL for serum creatinine for Model III** by gender, age, race/ethnicity, diabetes status, and chroic kidney disease (CKD) status*. Data from National Health and Nutrition Examination Survey 1999-2014. *CKD stages were defined on the basis of glomerular filtration rate (GFR) as: Stage 1: GFR ≥ 90, Stage 2: GFR ≥ 60 but less than 90, Stage 3A: GFR ≥ 45 but less than 60, Stage 3B: GFR ≥ 30 but less than 45. **Actual N used for the model was 30980. R2 for the fitted model was 73.6%. Regression slopes (β) with log10 transformed values of serum creatinine was 0.0047 (p < 0.01) for survey year and 0.012 (p = 0.04) for log10 transformed values of body mass index.
Diabetes and smoking status also did not affect the levels of UCR. Those who were in CKD Stage 5 had statistically significantly higher levels of UCR than those who were in CKD Stage 4 (121.0 vs. 82.8 mg/dL, p < 0.01, Table 2).
Factors affecting SCR – model III
Actual sample size used to analyze data for this model was 30980 and the model R2 was 73.6% (Table 3). Adjusted SCR levels were found to increase over 1999-2014 (β = 0.0047, p < 0.01, Table 3). BMI was found to have a positive association with SCR (β = 0.012, p = 0.04, Table 3). Statistically significant interactions between gender and race/ ethnicity, gender and age, gender and CKD stage, and gender and smoking status were observed at α = 0.05. Statistically significant interaction between age and race/ethnicity, age and CKD stages, age and diabetes status, and race/ethnicity and CKD Stages were also observed.
Effect of gender
Males had statistically significantly higher SCR AGM than females (1.28 vs. 0.99 mg/dL, p < 0.01, Table 3). However, male-females differences varied by race/ethnicity (0.26 for NHW, 0.32 for NHB, 0.28 for MA, 0.29 for OTH, Table S2), age (0.33 for A20, 0.31 for A30, 0.26 for A60, 0.25 for A70), and CKD stages (0.20 for CKD1, 0.23 for CKD3A, 0.44 for CKD3B).
Effect of age
SCR AGM decreased with increase in age and almost all pairwise differences were statistically significant (p ≤ 0.03, Table 3). However, age differences varied with gender, race/ethnicity, CKD stages, and diabetes status (Table S2).
Effect of race/ethnicity
NHB had the highest SCR AGM and there were almost no differences in SCR among NHW, MA, and OTH (NHW: 1.1 mg/dL, NHB: 1.24 mg/dL, MA: 1.08 mg/dL, OTH: 1.1 mg/dL, Table 3). However, within racial/ethnic differences varied by gender (NHWNHB difference 0.12 for males, 0.11 for females), age (NHW-NHB difference for A20 0.11, A40 0.14, A70 0.17), and CKD stages (CKD 1 vs 3B, 0.98 for NHW, 1.15 for NHB, 1.02 for MA, 1.05 for OTH, Table S2).
Effect of CKD Stages 1, 2, 3A, and 3B
SCR levels increased with increase in CKD stages and all pairwise differences were statistically significant (p < 0.01, Table 3, Figure 2). Differences in SCR among CKD stages varied by gender (CKD 1 vs. 3B, 1.18 for males, 0.94 for females), age (CKD 1 vs. 3B, 1.26 for A20, 1.10 for A30, 0.97 for A60, 0.91 for A70, Table S2).
Effect of diabetes status
Nondiabetics had higher SCR AGM than diabetics (1.15 vs. 1.10 mg/dL, p < 0.01, Table 3). Nondiabetic-diabetic differences decreased with increase in age (0.13 for A20, 0.10 for A30, 0.06 for A40, 0.02 for A50 and A60, 0 for A70, Table S2).
Effect of smoking status
Overall, smoking did not affect the levels of SCR (Table 3).
Factors affecting SCR – model IV
Actual sample size used for Model IV was 279 and model R2 was 83.9% (Table 4). Adjusted SCR levels did not vary by survey year (β = -0.0003, p = 96, Table 4) or BMI (β = -0.0621, p = 0.36, Table 4). Males had higher UCR levels than females (4.31 vs. 3.43 mg/dL, p < 0.01, Table 4). There was a statistically significant decrease in SCR levels with age and all three pairwise differences were statistically significant (p < 0.01, Table 4). NHW had lower SCR levels than NHB (3.68 vs. 4.20 mg/dL, p < 0.01, Table 4) and NHB had higher SCR levels than OTH (4.20 vs. 3.68 mg/dL, p < 0.01, b). Higher SCR levels were associated with CKD-5 when compared with CKD-4 (5.72 vs. 2.58 mg/dL, p < 0.01, Table 4, Figure 2). Smoking and diabetes did not affect the levels of SCR when CKD stage was 4 or 5 (Table 4).
| Group | AGM (95% CI) | Statistically Significant Differences |
|---|---|---|
| Male (M) | 4.31 (4.05 - 4.59) | M>F (p<0.01) |
| Female (F) | 3.43 (3.23 - 3.63) | |
| Age: 20-59 Years (A20++) | 4.35 (3.93 - 4.82) | A20++>A60 (p<0.01), A20++>A70 (p<0.01) |
| Age: 60-69 Years (A60) | 3.82 (3.58 - 4.08) | A60>A70 (p<0.01) |
| Age: ≥ 70 Years (A70) | 3.42 (3.19 - 3.65) | |
| Non-Hispanic White (NHW) | 3.68 (3.4 - 3.97) | NHW<NHB (p<0.01) |
| Non-Hispanic Black (NHB) | 4.2 (3.93 - 4.48) | NHB>OTH+ (p<0.01) |
| Oher race/ethnicities including Mexican Americans (OTH+) | 3.68 (3.45 - 3.92) | |
| CKD Stage 4 (CKD4) | 2.58 (2.47 - 2.7) | CKD4<CKD5 (p =0.03) |
| CKD Stage 5 (CKD5) | 5.72 (5.21 - 6.27) | |
| Non-Diabetic (NDIAB) | 3.77 (3.59 - 3.96) | |
| Diabetic (DIAB) | 3.92 (3.62 - 4.24) | |
| Nonsmoker (NSM) | 3.8 (3.6 - 4) | |
| Smoker (SM) | 3.89 (3.6 - 4.2) |
Table 4: Adjusted geometric means (AGM) with 95% confidence intervals in mg/dL for serum creatinine for Model IV** by gender, age, race/ethnicity, diabetes status, and chronic kidney disease (CKD) status*. Data from National Health and Nutrition Examination Survey 1999-2014.*CKD stages were defined on the basis of glomerular filtration rate (GFR) as: Stage 5: GFR ≥ 15 but less than 30, Stage 5: GFR < 15. **Actual Nused for the model was 279. R2 for the fitted model was 83.9%. Regression slopes (β) with log10 transformed values of serum creatinine was -0.0003 (p = 0.96) for survey year and -0.0621 (p = 0.36) for log10 transformed values of body mass index.
Discussion
Factors affecting UCR concentrations including CKD
Because of correlations between SCR and UCR in different directions when CKD Stage was 1-3 vs. 4-5, different regression models were fitted (Model I and II). Differences in UCR levels between males and females, NHW and NHB, 20-29 years vs. ≥ 70 years old when adjusted for the associations with SCR, CKD, diabetes and smoking status, age, gender, race/ethnicity, and BMI were found to be in the same direction as reported by Barr et al. (2005). However, when CKD was in Stages 1-3, male-female differences were 11.3 mg/dL and 24.9 mg/dL when CKD Stages was 4 or 5 while unadjusted malefemale difference reported by [1] was 34.8 mg/dL for all those aged ≥ 6 years. Similarly, NHB-NHW differences for this study were 27.5 mg/dL when CKD Stages were 1-3 and 17.9 mg/dL when CKD Stage was 4 or 5 which as reported by [1] was 40.8 mg/dL for all those aged ≥ 6 years. Because of the age range covered by these two studies, these differences are not directly comparable but adjusted levels do seem to decrease compared with unadjusted levels. This is what was seen when differences between 20-29 years were compared with ≥ 70 years old. Barr et al. [1] reported an unadjusted difference of 63.8 mg/dL between these two age groups. For this study this difference was found to be 24.1 mg/dL when CKD Stages was 1-3 and 24.0 mg/dL when CKD Stage was 4 or 5. To the best of our knowledge, this was the first time that effect of smoking on the levels of UCR was evaluated using a nationally representative sample U.S. population. Smokers were found to have higher adjusted levels of UCR than nonsmokers (Table 1 and 3).
Adjusted UCR levels decreased with decrease in CKD Stages as long as CKD Stages were 1-3 (Table 1) but UCR levels increased when CKD Stage increased from Stage 4 to 5. This is reflective of positive correlation between SCR and UCR when CKD Sates were 1-3 and negative when CKD Stages were 4 and 5.
Factors affecting SCR concentrations including CKD
To the best of our knowledge, this is the first time that the adjusted levels of SCR by age, gender, and race/ethnicity are being presented. Irrespective of the Model (III or IV) fitted, males had higher levels of adjusted SCR than females but while males had about 30% higher levels than females for CKD 1-3, males had about 20% higher levels than females for CKD 4-5. However, NHB had about 11% higher levels than NHW for CKD Stages 1-3, NHB had about 20% higher levels than NHW for CKD Stages 4-5. In other words, decreasing GFR levels narrow male-female differences but they widen NHW-NHB differences.
When CKD Stages were between 1 and 3, levels of UCR decreased with increased in CKD Stage but levels of SCR increased with increase in CKD Stage. However, for CKD Stages 4-5, levels of both SCR and UCR were higher for Stage 5 than for Stage 4. The explanation for this lies in the positive correlation between SCR and URC for CKD Stages 1-3 and negative correlation for CKD Stages 4-5.
UCR, SCR, and CKD stages
Impaired kidney function is reflected in elevated SCR levels. In other words, SCR should increase with increase in CKD stages. This is exactly what was observed from the data generated in this study. With CKD stages between 1 and 3B, adjusted mean SCR levels increased from 0.71 to 1.33 mg/dL or almost a 90% increase in SCR levels from being in CKD stage 1 to CKD stage 3B (Table 3, Figure 2). The increase in adjusted mean levels in CKD satge 4 and 5 was even more remarkable (Table 4, Figure 2). From CKD stage 3B to CKD stage 4, there was another 47% increase from 1.76 to 2.58 mg/dL (Table 4, Figure 2). From CKD stage 4 to CKD stage 5, there was another 122% increase from 2.58 to 5.72 mg/dL (Table 4, Figure 2). Consequently, adjusted SCR level could easily separate at least CKD stages 3, 4, and 5.
Impaired kidney function should also be reflected in decreased UCR levels, at least in CKD stages 4 and 5. For this study, adjusted UCR levels stayed relatively flat during CKD stages 1, 2, and 3A (Table 3, Figure 2) at 102.4, 105.4, and 100.9 mg/dL respectively and decreased by about 40% from CKD stage 3A to 3B at 60.9 mg/dL (Table 2, Figure 2). However, for reasons that need further explanation, UCR levels increased to 82.8 mg/dL for CKD stage 4 and to 121.0 mg/dL for CKD stage 5. The reason for increase in UCR for CKD stages 4 and 5 may be because UCR levels have also been adjusted for SCR levels. A relatively lower sample sizes for CKD stages 4 and 5 as compared to stages 1, 2, 3A, and 3B may also provide part of the explanation but more work will be needed in this area.
Limitations of the study
Creatinine excretion is subject to wide fluctuations due to several internal and external factors [27] and as such it may adversely affect computations of GFR because of the association between UCR and SCR. Association between GFR and urinary creatinine excretion has also been described by [28] in the context of identifying acute kidney injury.
These analyses were based on cross-sectional data. A true effect of CKD on SCR and UCR can only be assessed if the same cohort of subjects is followed while they move from CKD Stage 1 to Stage 5. A study of this nature can only be done on a small scale, for example, patients undergoing treatment for kidney related issues. Even in these studies, there may be a conflict between treatment and research needs. Samples of the sizes of about 30000+ that were available to analyze data for this study, may never be available in follow up cohort studies.
There were hundreds of pairwise comparisons that were made to test for differences between the levels of UCR and SCR by age, race/ ethnicity, gender, CKD Stages, smoking status, and diabetes status. When the number of comparisons that needed to be made are that large, statisticians usually advise to adjust for multiple comparisons by such methods as Tukey’s Least Significant Difference method, Neuman-Keuls method, Scheffe’s method, Bonferroni correction, Dunnett’s method, and so on. Unfortunately these procedures do not always result in the same conclusions. Hence, the success of using adjustment for multiple comparison also dependents up on the adjustment procedure selected for use. In clinical practice as compared to statistical practice, not all pairwise comparisons may be of interest, and even among those comparisons that are of interest, some may carry greater weight or clinical importance than others. Consequently, instead of statistical software divide total α level of 0.05 among all comparisons, it may be more appropriate to divide total α level of 0.05 among comparisons of interest. For this reason, in this study no adjustments were made for multiple comparisons.
Summary and Conclusion
In summary, the primary findings of this study were: (i) levels of SCR and UCR were positively correlated for CKD Stages 1-3 and negatively correlated for CKD Stages 4 and 5, (ii) males had higher levels of UCR than females but male-female differences were narrower for CKD Stages 1-3 than for CKD Stages 4-5, (iii) males had higher levels of SCR than females but male-female differences were 30% for CKD Stages 1-3 and 20% for CKD Stages 4-5, (iv) NHB had higher levels of UCR than NHW but these differences were 27.5 mg/dL for CKD Stages 1-3 and 17.9 mg/dL for CKD Stage 4 and 5, (v) NHB had about 11% higher SCR levels than NHW for CKD Stages 1-3 and about 20% higher levels than NHW for CKD Stages 4-5, (vi) for CKD Stages 1-3, levels of UCR decreased with increased in CKD Stage but levels of SCR increased with increase in CKD Stage, and (viii) levels of both SCR and UCR were higher for CKD Sage 5 than for CHD Stage 5.
Acknowledgements
Author declares that he did not receive any funds to conduct this research and all data used in this research are available free of charge from www.cdc.gov/nchs/nhanes.htm. Author had no financial or other conflicts including employment that could have affected the conclusions arrived at in this communication.
References
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, et al. (2005) Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environmental Health Perspectives. 113:192-200.
- Lascano ME, Poggio ED (2010) Kidney function assessment by creatinine-based estimation equations.
- Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, et al. (2006) Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247-254
- Levey AS, Stevens LA, Schmid CS, Zhang Y, Castro III AS, et al. (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med150:604-612.
- Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, et al. (2008) Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51:395-406.
- Schwartz GJ, Muñoz A, Schneider MF, Mak RH, KaskelF,et al. (2009) New equations to estimate GFR in children with CKD. J Am SocNephrol 20:629-637.
- Schwartz GJ, Work DF (2009) Measurement and estimation of GFR in children and adolescents. Clin J Am SocNephrol 4:1832-1843.
- Odutayo A, Hsiao AJ, Emdin CA (2015) Prevalence of Albuminuria in a General Population Cohort of Patients With Established Chronic Heart Failure. J Card Fail 22: 33–37.
- Ricardo AC, Flessner MF, Eckfeldt JH, Eggers PW, Franceschini N, et al. (2015) Prevalence and Correlates of CKD in Hispanics/Latinos in the United States. Clin J Am SocNephrol. 10:1757-1766.
- Ricardo AC, Athavale A, Chen J, Hampole H, Garside D,et al. (2015) Periodontal disease, chronic kidney disease and mortality: results from the third National Health and Nutrition Examination Survey. BMC Nephrol16:97
- Grams ME, Juraschek SP, Selvin E, Foster MC, Inker LA, et al. (2013) Trends in the prevalence of reduced GFR in the United States: a comparison of creatinine- and cystatin C-based estimates. Am J Kidney Dis 62:253-260.
- Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, et al. (2011) Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 305:2532-2539.
- Gambaro G, Yabarek T, Graziani MS, Gemelli A, Abaterusso C, et al. (2010) Prevalence of CKD in northeastern Italy: results of the INCIPE study and comparison with NHANES. Clin J Am SocNephrol 5:1946-1953.
- Stevens LA, Li S, Wang C, Huang C, Becker BN, et al. (2010) Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis55:S23-33.
- Foley RN (2010) Temporal trends in the burden of chronic kidney disease in the United States. CurrOpinNephrolHypertens 19:273-277.
- Duru OK, Vargas RB, Kermah D, Nissenson AR, Norris KC (2009) High prevalence of stage 3 chronic kidney disease in older adults despite normal serum creatinine. J Gen Intern Med 24:86-92
- Xu R, Zhang L, Zhang P, Wang F, Zuo L, et al. (2009) Comparison of the prevalence of chronic kidney disease among different ethnicities: Beijing CKD survey and American NHANES. Nephrol Dial Transplant 24:1220-1226.
- Ricardo AC, Fischer MJ, Peck A, Turyk M, Lash JP (2010) Depressive symptoms and chronic kidney disease: results from the National Health and Nutrition Examination Survey (NHANES) 2005-2006. IntUrolNephrol 42:1063-1068.
- Targher G, Bosworth C, Kendrick J, Smits G, Lippi G, et al. (2009) Relationship of serum bilirubin concentrations to kidney function and albuminuria in the United States adult population. Findings from the National Health and Nutrition Examination Survey 2001-2006. ClinChem Lab Med 47:1055-1062.
- Whaley-Connell A, Sowers JR, McCullough PA, Roberts T, McFarlane SI, et al. (2009) Diabetes mellitus and CKD awareness: the Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES). Am J Kidney Dis 53: S11-S21.
- McCullough PA, Li S, Jurkovitz CT, Stevens LA, Wang C, et al. (2008) Kidney Early Evaluation Program Investigators. CKD and cardiovascular disease in screened high-risk volunteer and general populations: the Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999-2004. Am J Kidney Dis 51:S38-S45.
- Ricardo AC, Grunwald JE, Parvathaneni S, Goodin S, Ching A, et al. (2014) Retinopathy and CKD as predictors of all-cause and cardiovascular mortality: National Health and Nutrition Examination Survey (NHANES) 1988-1994. Am J Kidney Dis 64:198-203.
- Odden MC, Amadu AR, Smit E, Lo L, Peralta CA (2014) Uric acid levels, kidney function, and cardiovascular mortality in US adults: National Health and Nutrition Examination Survey (NHANES) 1988-1994 and 1999-2002. Am J Kidney Dis 64:550-557.
- Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, et al. (2013) Kidney disease and increased mortality risk in type 2 diabetes. J Am SocNephrol 24:302-308.
- Wang HE, Gamboa C, Warnock DG, Muntner P (2011) Chronic kidney disease and risk of death from infection. Am J Nephrol 34:330-336.
- Wu CK, Chang MH, Lin JW, Caffrey JL, Lin YS (2011) Renal-related biomarkers and long-term mortality in the US subjects with different coronary risks. Atherosclerosis 216:226-236.
- Boeniger MF, Lowry LK, Rosenberg J (1993) Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: A review. Am IndHygAsso J54:615-627.
- Waikar SS, Sabisetti VS, Bonventre JV (2010) Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int78:486-494.
Citation: Jain RB (2016) Associated Complex of Urine Creatinine, Serum Creatinine, and Chronic Kidney Disease. Epidemiology (Sunnyvale) 6:234. Doi: 10.4172/2161-1165.1000234
Copyright: © 2016 Jain RB. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 16157
- [From(publication date): 4-2016 - Apr 26, 2024]
- Breakdown by view type
- HTML page views: 15316
- PDF downloads: 841