Research Article Open Access
Decline and Die-Back of Woody Plants in a Physiognomic and Floristically Complex Sebungwe Region and the Factors Modifying Mosaic Patch Landscapes at Sengwa Wildlife Area, Zimbabwe
Clifford Tafangenyasha1*, Blessing Kavhu2and Knowledge Vingi21Scientific Services, Zimparks, Causeway, Harare, Zimbabwe
2University of Zimbabwe, Department of Geography and Environmental Science, Geo-information and Earth Observation Centre, Mount Pleasant, Harare, Zimbabwe
- *Corresponding Author:
- Tafangenyasha C
Scientific Services, Zimparks
P O Box CY 140, Causeway
Harare, Zimbabwe
Tel: 07767739071/0735607804
E-mail: cliffordtafa@gmail.com
Received date May 19, 2016; Accepted date June 21, 2016; Published date June 28, 2016
Citation: Tafangenyasha C, Kavhu B, Vingi K (2016) Decline and Die-Back of Woody Plants in a Physiognomic and Floristically Complex Sebungwe Region and the Factors Modifying Mosaic Patch Landscapes at Sengwa Wildlife Area, Zimbabwe. J Ecosys Ecograph 6:194. doi:10.4172/2157-7625.1000194
Copyright: © 2016 Tafangenyasha C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
An investigation of the status of woodlands at Sengwa Wildlife Research Area (SWRA) revealed factors influencing decline and die-back of woody plants at a time of low elephant densities. The vegetation condition of the study area in the elephant range area of the semi-arid area of northwest Zimbabwe was randomly assessed in 50 × 20 m permanently marked degraded and undegraded plots in a study area approximately 80 km2. Diversity of woody plants increased towards downstream. Percentage elephant damage was slight on undegraded plots and termite damage was three times greater on undegraded plots than degraded plots. Density of woody plants was high following a period of coppice regeneration. The results suggest regeneration of woody plants following a long period of elephant culls between 1960 and 1992 that altered forest structures. It is conceivable that other environmental agents may play the role of elephant damage in the presence of elephant densities < 1 individuals/ km2. Significant differences (p<0.05) in elephant densities (no/km2) were recorded in SWRA between 1958 and 1996, 1958 and 1993 suggesting that increasing elephant densities were negatively impacting on woody cover (%). Woody cover increased from 60% in 1993 to 70% in 1996 inside SWRA. SWRA vegetation may be on regeneration path if no adverse impacts are recorded from other environmental agents including termite activity, fungal attack (Fusarium oxysporum), Lantana camara L. invasions and drought. Epidemic die-back is not yet a common feature in the protected area. The results refute the postulation that elephant alone prevent woodland regeneration and recruitment into larger size classes by feeding on small trees. The findings suggest need to consider end to end cycle of each disturbance factor in order to accurately predict scale of vegetation change in savanna ecosystems.
Keywords
Wildlife Research Area; Elephant; Density; Woody plants; Zimbabwe
Introduction
Woody plant disappearances, loss of woody plant vigour, branch dieback and tree mortality for reasons unknown or difficult to determine is a common phenomenon confronting rangeland managers and ecologists from the turn of 20th century. Several workers [1,2] have attempted to throw light on what drives tree losses in protected areas but not much progress has been achieved given new confounding factors of climate change and growing aridity in semi-arid areas. Tree disappearances, decline and die back from unknown or complex ecological causes are factors often leading to changes in land cover in the landscape. Trees can remain in varying degrees of decline for a number of years.
Trees die when they no longer have the ability to acquire or mobilize sufficient resources to support life. Trees must fulfill several essential life functions: reproduction, maintenance, growth, storage and defence. If trees lack the resources to achieve this, they must die back to a size they can support. Thus, while dieback appears alarming, it can serve as a survival mechanism. Trees cannot get up and move when environmental conditions change, so their only recourse is to alter their size to conform to the resources available. A natural forest typically begins as a large group of small trees, perhaps as many as several thousand to the acre. Over a period of many years, the numbers are reduced to only a few large trees per acre. Periods of drought, early and late frosts, wide fluctuations in temperature, insect and disease epidemics, wildfire and other forces may speed up the natural selection process.
Various reasons for vegetation change in rangeland areas with elephant in the Sebungwe region: exposure to extremes of weather, heat, cold and wind mega herbivory attack old age (senescence) insect attack (bark beetles, wood borers) windstorms, floods or wet conditions, drought for extended periods, lightning, soil compaction, soil salinity, high water table, pollution, nutrient deficiency, competition, fire, narrow genetic base, fungal attack (Gall fungus), Fusarium oxysporium and Mistletoe (Loranthus spp).
Although endogenous and exogenous factors may negatively attack a wide spectrum of species the most notably woody plants affected include Acacia spp, Terminalia spp and Pterocarpus angolensis among other species.
Land cover change inflicting the SWRA has probably led to a progressive deterioration in the health of a tree or shrub as a result of a combination of factors. A number of authors attribute endogenous and exogenous as key to woody plant mortality. The elephant (Loxodonta Africana Blumenbach) management plan of Zimbabwe is implicit in monitoring habitats in order to preserve a wide range of habitats for the conservation of biodiversity. The mosaic of vegetation has been attributed to edaphic, climatic, geomorphology, management decisions and stocking densities [3]. Several authors (Mapaure [1,4,5], Mukwashi et al. [6], Gandiwa et al. [7], Zisadza et al. [8], Furley et al. [9], Dublin et al. [10], Frost et al. [11]) cite fire, herbivory, drought, disease, proximity to watering points, temperature and edaphic factors as key drivers of vegetation change. African savanna are typified by the coexistence of woody and herbaceous species, with relative proportions of each being influenced by soil moisture availability, soil nutrient status, fire and herbivory [12]. The African elephant is regarded as the main force driving change in woody cover in the range area because of the very large body size that enables it to kill mature trees [13-15].
Elephants alone at a density of 0.27 km-2 will convert the woodland into coppice in 120 years due to resulting massive declines of large trees [16,17]. The same result is achieved in only 10 years if elephant density is at 2 km-2 [1]. The problem of elephant numbers first surfaced in the early 1960 in the range areas. Population reductions were carried out through culling operations [18,19]. The first cull of 500 elephants was carried out in 1966/67 [18]. The last large cull was conducted in 1989 and the period after has been characterized by minor disturbance culls and regeneration and coppice regrowth [20]. Destruction of trees leading to unacceptable habitat change is accelerating [2]. One elephant can knock down 1 500 trees in a year [21]. In the period 1974 to 1978, due to elephant activities, there was a 4% reduction in woodland per year in the Sengwa area (Figure 1) [21,22]. The response of vegetation to elephants is difficult to interpret but may often lead to difficult problems of dredging water reservoirs in protected areas [23]. New effort is required to consider end to end cycle of each disturbance factor in order to predict scale of vegetation change in savanna ecosystems. There is concern that patterns of tree population change may be due to endogenous and exogenous factors in the predicted era of growing aridity and climate change. This study takes the opportunity to investigate factors modifying vegetation further in the SWRA after a long period of purposeful elephant population reductions.
Study Area
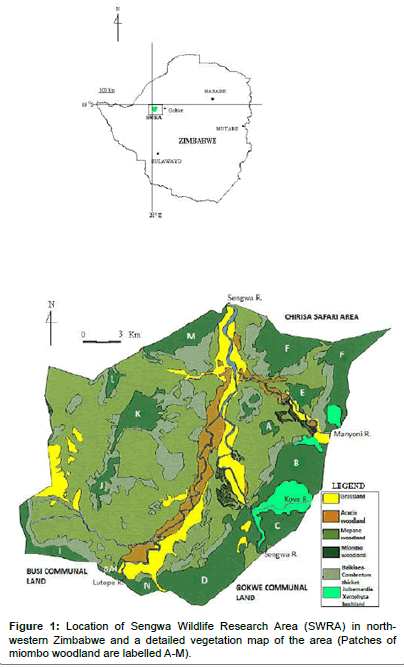
SWRA is situated at the southern end of Chirisa Safari Area and lies between 28° 03′ and 28° 20’E and 18°0′ and 18°13′ in Gokwe South District, north-western Zimbabwe (Figure 1). Covering an area of about 373 km2, the area was set aside in the late 1960s for long-term ecological integrity monitoring.
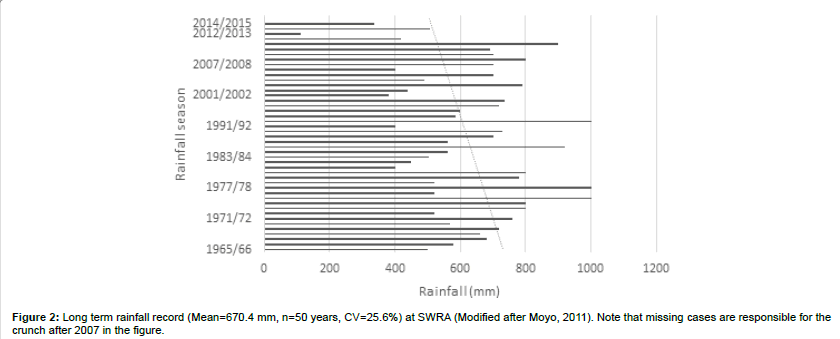
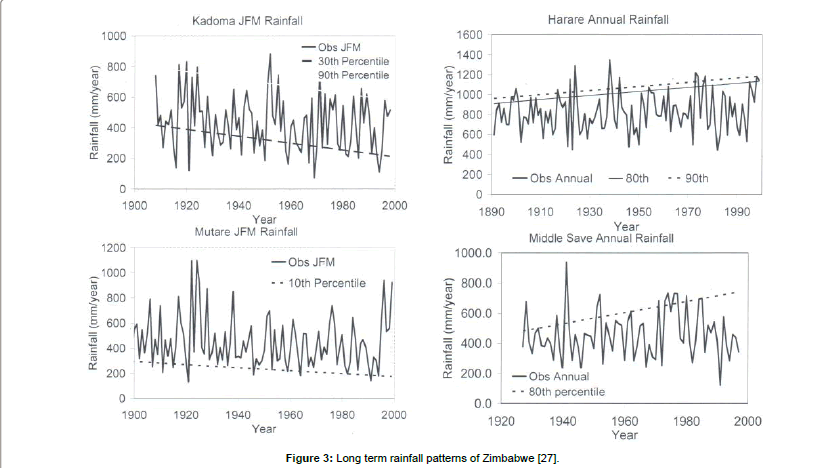
The SWRA has a diverse large animal mammal community of 7 species of large carnivores and 18 species of large herbivorys. The biodiversity includes diverse communities of birds, small mammals and reptiles. There is a wide variety of vegetation types, 26 types having been described and mapped by Craig [24] (Figure 1). Cumming [18] recognized three vegetation types characterizing major elephant woodland damage patterns as including Miombo, Acacia and Mopane types. The soils are derived from weathering Karoo sedimentary deposits. The area is generally regarded as hot and dry. It experiences three climatic seasons, a single rainy season that extends from November to April, a cool season from May to July and a hot dry season from August to October [25-27]. Annual precipitation is between 600 – 700 mm, but usually 400 – 600 mm may be expected (Figure 2). Mean annual temperature is 22.2°C. The longterm rainfall pattern of Zimbabwe (Figure 3) and in particular the study area suggests polycyclic incidences of drought.
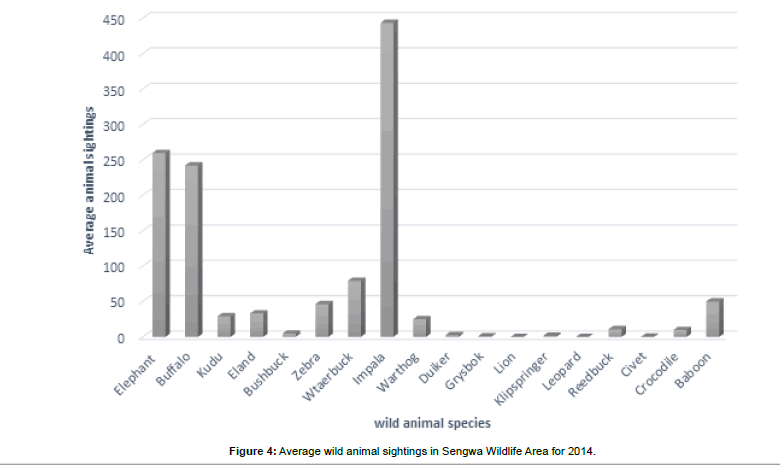
The vegetation type is a complex mosaic of canopy trees, shrubs, young woody plants on the forest floor, creeping and spreading herbaceous plants and grasslands adapted to variable soil moisture regimes and catena conditions. Wild, Fernandez et al. [28] ruled that the flora of the Zambesian basin comprised many varieties of native plant species. Thomson [29] and Anderson et al. [13] observed that abiotic and biotic factors in particular the elephant, fire and soil conditions controlled the stand size and structure of the vegetation in the study area. Timberlake determined that human factors, fire, climate and soil factors were important factors shaping the physiognomy and floristics of the Zambesian flora. The vegetation of SWRA may be seen to be struggling in the series of succession to reach climax potential. The vegetation of the SWRA is related to the soil catena, geology and soils which are mainly sands of various textures, derived from numerous sandstone beds and mudstones of various Karoo ages, sequences and landforms [24]. The soils are generally low in plant nutrients, frequently shallow and sandy with low moisture retention capacity; some are sodic, and many of them, particularly on steeper land, will erode easily [30]. The semi-arid nature of SWRA has unpredictable direction changes to the fragile ecosystems in the event of sudden episodic environmental disturbances. The presence or the lack of precipitation influences the type of wildlife in the study area (Figure 4).
Methods
To assess the vegetation status species were identified, density, girth, height, canopy cover, elephant damage, termite damage, distance to seasonal water and distance to permanent water were recorded in randomly selected 18 plots each measuring 50 × 20 m. The 18 plots were assigned according to categories that included degraded (formerly over utilized patches) and undegraded plots (no obvious recent damage) at a site. Woody species were identified according to a criteria established by Anderson et al. [13]. Density of woody species was established by enumerating all standing woody species encountered in study plots. Woody species girth (cm) was measured according to a criterion set by Anderson et al. [13]. Small tree girth was measured approximately 5 cm above ground and large trees at waist height. Height (cm) was estimated after an initial calibration with a 5 m graduated pole. Canopy cover was estimated using the Braun-Blanquet scale on a 0-100% scale. Elephant damage was scored as a percentage using the Braun-Blanquet scale on a 0-100%. Termite damage was estimated as a percentage cover on stems and twigs of termite mud chambers. Distance to water (m) was estimated using 1:250 000 Surveyor General Maps from the center of the study plots. Other observations were recorded of fungal attacks and drought attack. Inference on woody plant s competition was noted by enlisting the Shannon diversity indices (H) and sizes. Interesting ecological notes were recorded while walking in the study plots. Plot locations were determined using a Garmin GPS.
Results
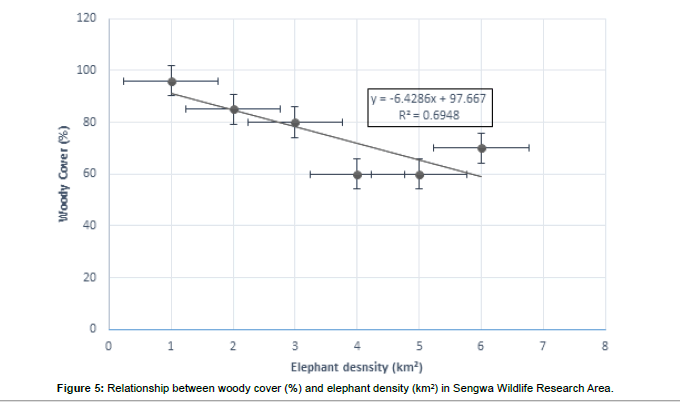
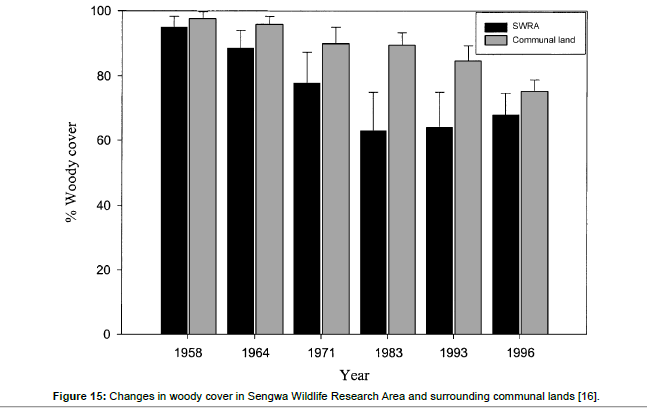
Significant differences (p<0.05) in elephant densities (no/km2 ) were recorded in SWRA between 1958 and 1996, 1958 and 1993 suggesting that increasing elephant densities were negatively impacting on woody cover (%) (Figure 5). Woody cover dramatically from 96% in 1958 to 70% in 1996, 96% in 1958 to 60% in 1983 and 1993 (Figure 5).
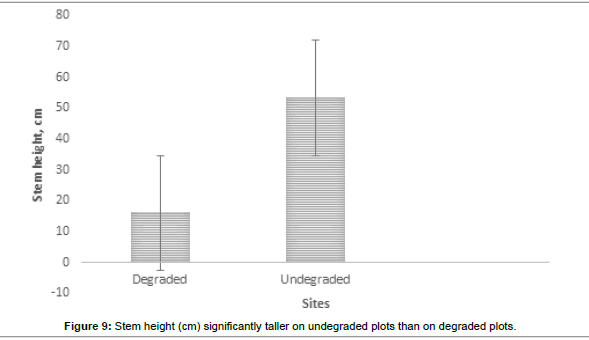
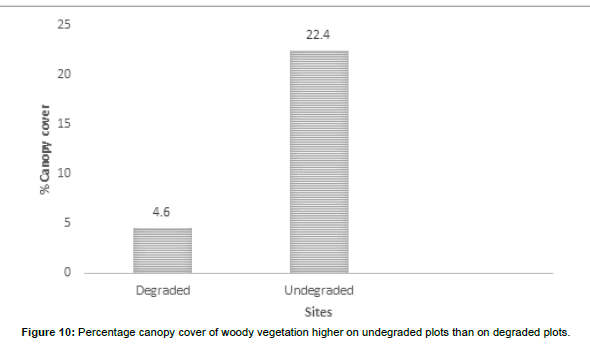
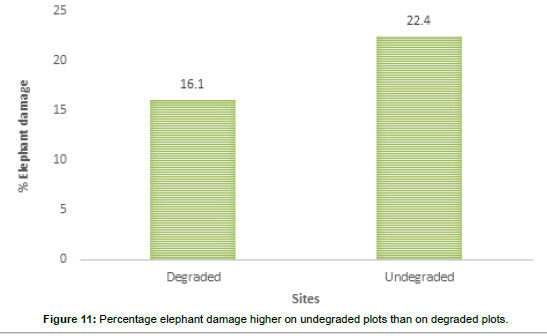
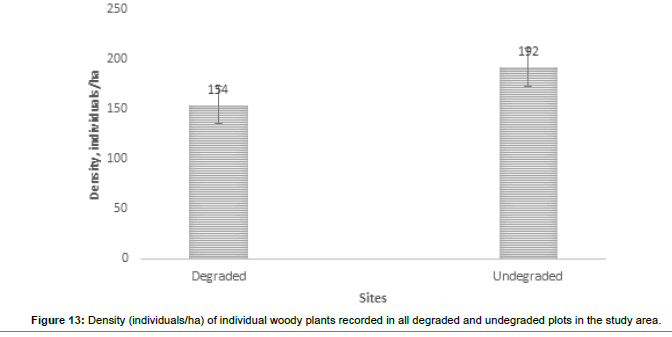
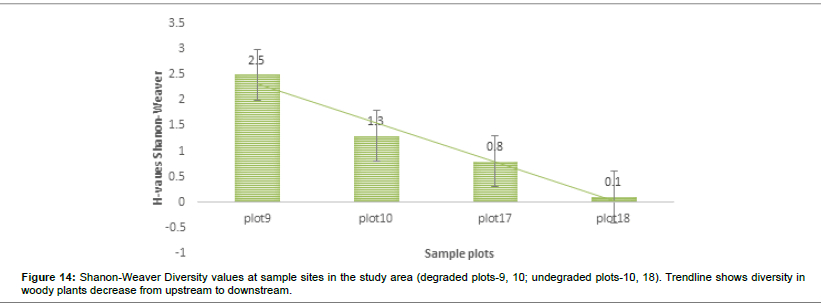
The visual observations at each study plot were an important force in identifying the underlying ecological conditions prevailing at the visited plots. From the previous vegetation descriptions of the SWRA the vegetation was much diverse but subjected to increasing mega herbivory pressures chief among them the elephant wasteful feeding behaviour. The vegetation condition may be described as different now. What are the drivers of vegetation condition now and possibly the future? Visual observations identified some easily overlooked factors measured in the study plots. Figures 6-17 suggested the need to carefully consider the factors modifying the natural vegetation. Termite attack, fungal attack, fire attack and feeding herbivorys were identified as important (Figures 5-6). Figures 12-17 show woody species characteristics measured or estimated on woody species in the study plots. The Shannon-Weaver diversity indices (H) were calculated and compared between the studies (Figure 13). The study showed that noxious invasive weeds LC were establishing in gaps on some plots to the near exclusion of other native plant species. Trend line shows decreasing diversity of woody plants from upstream to downstream (Figure 13). LC colonies were established on poor weathering sandstone Sengwa riverbanks in the upstream but bloomed vigorously on fertile old dune terrace formations in the downstream (Figure 17). The forest floor is characterized by a fire hazard accumulating as brushwood debris in the study plots. Figure 17 shows the shifts in rangeland condition that obtain when environmental agents facilitate change.
Discussion
The combination of growing aridity, termite attack, fungal attack, fire attack and feeding herbivorys have an important modifying force in the study plots. The exogenous factors may keep the woody plants oscillating in the series of succession and delay the processes towards reaching a climax that is determined by the potential of climate and soil factors. The woody plants encountered in the study plots showed damage features that included broken stems and broken branches, burnt stumps, coppice stems and varied growth forms that included a prevalence of thin stems. SWRA vegetation may be on a path of regeneration from previous recorded extensive damage between 1960 and 1989. Mapaure et al. [16] showed that miombo (Brachystegia spp associes) woodland declined in canopy cover from 95.2% in 1958 to 62.9% in 1996 and attributed the impacts to increasing elephant densities. This study showed a perceptible shift in elephant feeding behaviour towards the dense younger regrowth in the undegraded plots made possible by coppice regrowth.
Elephants alone can degrade and maintain semi-arid miombo woodland into coppice, largely due to their damaging impacts on mature canopy trees and fire acts to speed up the process by suppression of an already low recruitment [1]. The probability of tree death on elephant impact has been found to be related to aridity, soil type, and size of tree, nutrient status and the response of woody species to elephant damage. Fire alone has a lesser influence on woodland structure than elephants probably because of low fuel loads due to heavy grazing and low grass production as a result of low rainfall and inherently poor soils in the area [1,5]. A reduction in elephant population should decrease local elephant density and attendant density-dependent effects of decreased foraging impacts, and rangeland degradation. But it remains clear that the effects of fire are dependent on timing, frequency, duration and area of burn [2,31]. Brachystegia, Julbernadia, Isoberlinia, Colophospermum, some Combretum, Terminalia, and a range of other shrub species have a higher probability of coppicing [14].
Figure 16:Variations in elephant densities in SWRA between1965 and1998 (No data were available between1976 and 1979 due to independence war disturbances, and no survey was done in 1990. Plotted values for 1976^1979 were obtained by calculation using a mean growth rate of 4.18% per annum between 1965 and 1979. The value for 1990 was calculated using a mean rate of growth for the previous 3 years) [16].
The ecological effects of burning may include burns in subsequent years, as dead trees topple to the ground, opening up the habitats to drying by sunlight, and building up the fuel load with an increase in fire-prone species. Other negative impacts can include promotion of invasive species, loss of soil nutrients, and changes in species composition particularly of plants.
Termites (Hodotermes mossambicensis) were found reworking organic material to construct mounds, chambers and tunnels on a variety of soil types and on woody debris including live standing woody individuals a phenomenon also recorded by Amanda et al. [32]. The role of termites as eco engineers is well documented in literature [33] but in SWRA increased termite attack is a phenomenon recently observed but not understood. Ahmed et al. [34] cite farmers in Uganda and Zambia as having more termite problems now than in the past. Damage by termites is greater during dry periods or droughts than periods of regular rainfall. The increases in termite damage could also be associated with climate change induced drought. In recent decades, drought linked to El Niño episodes has become more intense and widespread in southern Africa [34]. Environmental stresses such as extremes in temperature or moisture, site problems such as soil compaction, extremes in soil pH, poor soil texture for plant requirements, mechanical injuries to trunk or roots, insect infestations and diseases such as defoliating leaf diseases, root rots and wilt diseases. The action variables limiting state in the transition of woody vegetation towards a climax probably include termite activity, disease, herbivory and drought among other factors. While each of these factors alone may not be that detrimental to the plant, in combination they may cause the decline and death of the plant. The plant, in order to compensate for this stress, will decrease the amount of top growth to restore the delicate balance between the branches and roots. A declining plant will also be more susceptible to other disease and insect problems.
Herbivory has been underscored in the present study as initiating tree damage with drought, termite and fungi settling in to complete tree loss events that maybe accelerated by fire to cause radical changes to structure and composition of the forests. In the foregoing, tree damage and loss may be a composite phenomenon that sets in forest gaps that may be colonised by noxious invasive species (e.g. Lantana camara hereafter LC). Nhokovedzo [35] noted that species diversity was significantly high in the free areas than in LC invaded areas. LC was heavily distributed in stream banks [35], a phenomenon recorded in this study. LC favoured sites with high soil pH, soil organic carbon and sodium. An auto ecological approach may hold clues to the phenomenon of increased termite activity on some plant species.
In SWRA the effect of fungal attack may easily be overlooked particularly as it affects the inside of stems and branches a phenomenon not easily visible to a wildlife manager. Fungal attack on trees may be confounded by elephant damage and drought. Fungal attack has been reported on mukwa Pterocarpus angolensis trees in northwest Zimbabwe [14]. Vascular wilt disease of P. angolensis caused by the soil borne fungus Fusarium oxysporium. F. oxysporium is a contributing factor in stand dieback and that drought, frost, fire, insects and infections by mistletoe Loranthus spp were seen as predisposing or inciting factors. Mycorrhizal fungi leads to tree population decline in mukwa [36,37]. Epidemic dieback is not a common feature in the study area.
The SWRA may have cyclic changes that prevent woody vegetation transformation towards a climax but a combination of stocking densities and elaborate fire management may be desirable to reduce rates of woodland conversion. Later developments in elephant management have included organized safari hunting and a proactive fire management. The direction of future vegetation change remains hinged largely on amounts of precipitation and extent of human encroachment on the hard edges. Dye et al. [38] described the various states and agents of change in the transition of Savana vegetation to a climax and indicated when and what stages may be achieved if combinations of drought, fire, herbivory, diseases and edaphic factors intervened episodically. Increases in the frequency, duration, and/or severity of drought and heat stress associated with climate change could fundamentally alter the composition, structure, and biogeography of forests in many regions [39]. Of particular concern are potential increases in tree mortality associated with climate-induced physiological stress and interactions with other climate-mediated processes such as insect outbreaks and wildfire [39].
As the study tends to show a physiognomically complex and flororistically complex vegetation is not protected by resilience but by exogenous factors that have an overarching influence on multiple states in the series of succession towards a climax. A regeneration and recruitment in the plots suggest that equilibrium in megaherbivory populations and food availability may have been reached but exogenous factors remain an important force in the struggle towards a climax. The study of low elephant browsing impact on natural systems recorded here after a long period of purposeful culling is in agreement with observations recorded in Tsavo National Park, Kenya, where elephant deaths resulted in the recovery of woodlands typical of that region [40] but with later events dominated by environmental agents such as drought, fire, diseases and termite attacks. For some time to come the vegetation in SWRA will continue to be a flux of change cycling between understorey seedlings, shrubs and canopy trees.
Conclusion
The SWRA presents interesting Savana features and management styles available in the menu to rangeland ecologists. But the scale and intensity of disturbances in open rangelands need exhaustion after longterm studies in the varied habitats created by mega herbivorys and antecedent environmental factors. It makes sense to consider de-novo the top down and bottom up controls on the vegetation in the light of termite activity, fungal attack, LC invasions and the drought polycycle’s in the prognosis of IPCC [41] for Southern Africa [42-47].
Acknowledgements
Grateful thanks are extended to many individuals who are too numerous to mention in the execution of the research. The National Botanic Gardens assisted with plant specimen identification and are gratefully acknowledged.
References
- Mapaure I (2013) Short-term responses of shrub layer communities to dry season fires and tree thinning in semi-arid miombo woodlands of north-western Zimbabwe. Afr J Plant Sci 7: 414-425.
- Tafangenyasha C (2001) Decline of Brachystegiaglaucescens in the Gonarezhou National Park, southeast Zimbabwe. Journal of Environmental Management 63: 37-50.
- Valeix M, Fritz H, Sabatier R, Murindagomo F, Cumming D, et al. (2011) Elephant-induced structural changes in the vegetation and habitat selection by large herbivores in an African savanna. Biological Conservation 144: 902-912.
- Mapaure I, Mhlanga L (1998) Elephants and woodlands: The impact of elephant damage on Colophospermummopane on Namembere Island, Lake Kariba, Zimbabwe. ZimbSci News 32: 15-19.
- Mapaure I (2001) Small-scale variations in species composition of miombo woodland in Sengwa, Zimbabwe: the influence of edaphic factors, fire and elephant herbivory. Systematics and Geography of Plants 71: 935-947.
- Mukwashi K, Gandiwa E, Kativu S (2012) Impacts of African elephants on Baikiaeaplurijuga woodland around natural and watering points in Hwange National Park, Zimbabwe. International Journal Environmental Sciences 2: 1355-1368.
- Gandiwa E, Kativu S (2009) Influence of fire frequency on Colophospermummopane and Combretumapiculatum woodland structure and composition in northern Gonarezhou National Park, Zimbabwe. Koedoe 51: 13.
- Zisadza-Gandiwa P, Gandiwa E, Makotwe TB, Gwazani R, Mashapa C, et al. (2014) Preliminary assessment of vegetation fires and their impact in Nyanga National Park. Greener Journal of Biological Sciences 4: 009-017.
- Furley PA, Rees RM, Ryan CM, Saiz G (2008) Savanna burning and the assessment of long-term fire experiments with particular reference to Zimbabwe. Progress in Physical Geography 32: 611-634.
- Dublin HT, Sinclair ARE, McGlade J (1990) Elephants and fire as causes of multiple stable states in the Serengeti-Mara woodlands. Journal of Animal Ecology 59: 1147-1164.
- Frost PGH, Robertson F (1987) Theecological effects of fire in Savannas. In: Walker BH (ed.) Determinants of Tropical Savannas. IRL Press, Paris. pp: 93-140.
- Scholes RJ, Walker BH (1993) An African Savanna: synthesis of the Nylsvley study. Cambridge University Press, Cambridge.
- Anderson GD, Walker BH (1974) Vegetation composition and elephant damage in the SWRA. Rhodesian Journal Southern Africa Wildlife Management Association 4: 1-14.
- Campbell BM, Swift MJ, Hatton J, Frost PGH (1988) Small-scale vegetatio pattern and nutrient cycling in Miombo woodland. In: Verboeven JTA, Heil GW, Werger MJA, Chafota J, Owen-Smith N (eds.). Vegetative structure in relation to carbon and nutrient economy. SPB Academic Publishing, The Hague, Netherlands. pp: 69-85.
- Mapaure I, Campbell BM, Gambiza J (2009) Evaluation of the effectiveness of an early peripheral burning strategy in controlling wild fires in north-western Zimbabwe. Afr J Ecol 47: 518-527.
- Mapaure I, Campbell BM (2002) Changes in miombo woodland cover in and around Sengwa Wildlife Research Area, Zimbabwe, in relation to elephants and fire. African Journal Ecology 40: 212-219.
- Mapaure I, Moe SR (2009) Changes in the structure and composition of miombo woodlands mediated by elephants and fire over a 26-year period in north-western Zimbabwe. Afr J Ecol 47: 175-183.
- Cumming DHM (1981) The management of elephant and other large mammals in Zimbabwe. In: Jewel PA, Holt S (eds). Problems in managing locally abundant wild animals, Academic Press, New York. pp: 91-118.
- Cumming DHM (1983) TheSengwa Wildlife Research Area and Institute. ZimSci News 17: 32-37.
- Cumming DHM, Fenton MB, Rautenbach IL, Taylor RD, Cumming GS, et al. (1997) Elephants, wood-lands and biodiversity in southern Africa. S Afr J Sci 93: 231-236.
- Guy PR (1976) The feeding behavior of elephant (Loxodonta Africana) in the Sengwa area, Rhodesia. South African Journal of Wildlife Research 6: 55-63.
- Guy PR (1989) The influence of elephant and fire on the Brachystegiajulbernadia woodland in Zimbabwe. Journal of Tropical Ecology 5: 215-225.
- Tafangenyasha C (1997) Should Benji Dam be dredged? A preliminary impact assessment to dredging a water reservoir in an African national park. The Environmentalist 17: 191-195.
- Craig GC (1983) Vegetation survey of Sengwa. Bothalia African Biodiversity and Conservation 14: 759-763.
- Mazvimavi D (1998) Water availability and utilization in Zimbabwe. Geographical Journal of Zimbabwe 29: 23-36.
- Mazvimavi D (2003) Estimation of flow characteristics of ungauged catchments: Case study in Zimbabwe. PhD Thesis, Wageningen University, Netherlands. pp: 176.
- Mazvimavi D (2010) Investigating changes over time of annual rainfall in Zimbabwe. Hydrol Earth SystSci 14: 2671-2679.
- Wild H, Barbosa G, Fernandez A (1967) Vegetation map of the flora Zambesiaca area. Salisbury, Rhodesia: M. O. Collins (Pvt.) Ltd, South Africa. pp: 71.
- Thomson PJ (1975) The role of elephants, fire and other agents in the decline of a Brachystegiaboehmii woodland. Journal Southern African Wildlife Management Association 5: 11-18.
- Brunt MA, Clarke VJ, Greling C (1986) Tsetse area development and land use planning in the Zambezi Valley, Zimbabwe. FAO Consultant Report.
- Tafangenyasha C (1998) Tree loss in Gonarezhou National Park (Zimbabwe) between 1970 and 1983. Journal of Environmental Management 49: 355-366.
- Vambe A, Tarakini T, Gandiwa E, Tafangenyasha C (2016) Vegetation structure and composition on termitaria and non-termitaria areas in Lake Chivero Recreational Park, Zimbabwe. Journal of Agricultural Extension and Rural Development 4: 400-405.
- Dangerfield JM, Mccarthy TS, Ellery WN (1998) The mound building termite Macrotermesmichaelseni as an ecosystem engineer. Journal of Tropical Ecology 14: 507-520.
- Ahmed BM, Nkunika PO, Sileshi GN, French JRJ, Nyeko P, et al. (2011) Potential impact of climate change on termite distribution in Africa. British Journal Environment and Climate change 1: 172-189.
- Nhokovedzo H (2013) Distribution of invasive Lantana camara (L). and its influence on soil chemical properties and herbaceous species diversity in Chibi farm, Chivi, Zimbabwe. Bindura University Science Education. Zimbabwe. pp: 63.
- Mehl JWM, Slippers B, Roux J, Wingfield MJ (2010) Die-back kiaatPterocarpusangolensis in Southern Africa: A cause for concern? Southern forests. Journal of Forest Science 72: 121-132.
- Mehl JWM, Slippers B, Roux J, Wingfield MJ (2011) Botryoshaericeae associated with Pterocarpusangolensis in South Africa. Mycologia 103: 534-553.
- Dye PJ, Spear PT (1982) The effect of bush clearing and rainfall variability on grass yield and composition in southwest Zimbabwe. Zimb J Agric Res 20: 103-117.
- Craig DA, Alison KM, Haroun C, Dominique B, Nate M, et al, (2009) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest ecology and management 259: 660-684.
- Leuthold W (1996) Recovery of woody vegetation in Tsavo National Park, Kenya, 1970��?1994. African Journal of Ecology 34: 101-112.
- IPCC (Intergovernmental Panel on Climate Change) (2007) Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK.
- ChitangaP (2007) Impact of the invasive alien species, LantanaCamara (L). On native vegetation in northern Gonarezhou National Park, Zimbabwe. MSc Dissertation. University of Zimbabwe. pp: 92.
- GandiwaE (2013) Vegetation factors influencing density and distribution of wild large herbivores in a southern African savannah. African Journal of Ecology 52: 274-283.
- HananNP,SeaWB,DangelmayrG, GovenderN(2008)Do fires in savannasconsume woody biomass? A comment on approaches to modelingsavanna dynamics. The American Naturalist 171: 851-856.
- ShaankerUR,JosephG,AravindNA,KannanR,GaneshaiahKN (2010) Invasive plants in tropical human-dominated landscapes: Need for an inclusive management strategy. In: Perrings C, Mooney H, Williamson M (eds.) Bioinvasions and globalization: ecology, economics, management and policy. Oxford: Oxford University Press, USA.pp: 202-219.
- SkarpeC (1992) Dynamics of savanna ecosystems. Journal of Vegetation Science3: 293-300.
- TimberlakeJ, NobandaN,MapaureI (2004) The communal lands vegetation survey. The Biodiversity Foundation and the Zambezi Society. Bulawayo.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 11682
- [From(publication date):
June-2016 - Sep 25, 2024] - Breakdown by view type
- HTML page views : 10822
- PDF downloads : 860