Dysadherin Expression is Associated with Advanced Tumorstage while Complexity Index is Related to Poorly Differentiated Tumors in Colon Carcinoma
Received: 08-Apr-2016 / Accepted Date: 21-Apr-2016 / Published Date: 25-Apr-2016 DOI: 10.4172/2476-2024.1000111
Abstract
Dysadherin expressions are associated with the aggressive tumors that show poor prognosis in different types of cancers. Aggressive tumors often presented with infiltrative type of growth pattern in colon cancer and that has been associated with poor prognosis as well. The aim of this was to evaluate Dysadherin expression in paired normal and tumor samples, and tumor growth pattern in the form of complexity index in the same patient colon cancer samples, and finally to correlate with each other and different clinical and pathological parameters. To evaluate Dysadherin expression, we selected 45 patients and performed RT-qPCR on normal and paired tumor samples. To determine growth pattern, we captured pictures from the immunohistochemically stained tumor invasive fronts and then evaluated by using Photoshop CS, Fovea Pro, and Image J computer programs. Paired t test was applied for the Dysadherin expression between normal and paired normal samples while Pearson chi square and Fischer exact test between dichotomized Dysadherin expression, complexity index and different clinicopathological parameters. Mean Dysadherin expression in normal samples was 0.155+0.0167 and in tumor 0.869+0.083 and was significantly different between two groups of samples (P value<0.0001). In dichotomized groups, increased Dysadherin expression was significantly correlated with the lymph node metastasis and advanced Dukes stages. Higher complexity index was significantly associated with low differentiated tumors while non-significantly with other clinicopathological parameters. In conclusion, Dysadherin expression and tumor growth pattern are independent features, where Dysadherin expression is associated with advanced Dukes staged tumors and complexity index with the poorly differentiated tumors.
Keywords: Complexity index; Dysadherin; Clinicopathological parameters; Tumor node metastasis
Introduction
Dysadherin is a cell surface glycoprotein that was identified recently and found to be expressed in diverse types of cancer cells, but only in a small number of normal cells [1]. The human Dysadherin gene sequence is closely related to mouse gene related to ion channels (RIC).
Dysadherin is a member of the single-pass membrane protein family called FXYD protein family. These single-pass proteins are known to interact with and regulate Na+/K+-ATPase activities [2]. Prediction from the Dysadherin/FXYD5 cDNA sequence suggests that it has 178 amino acids arranged into signal peptide and extracellular, transmembrane and cytoplasmic tail domains [1,3]. In tumor cells, Dysadherin extracellular domain has a larger number of attached glycosides relative to the normal cells, which provide stability to the protein [4].
Increased Dysadherin expression has been reported in colon, pancreatic, stomach and breast cancer cells [1]. Dysadherin overexpression has also been reported in several other cancers such as gastrointestinal carcinoma and has been associated with poor prognosis and tumor progression [5-12]. Previous studies has reported that Dysadherin disturb the cell-cell adhesion by post-transcriptional down regulation of E-cadherin (cellular adhesion protein), thus enhancing formation of metastasis. In some of the clinical studies such as head-and-neck carcinoma [13,14], pancreatic ductal adenocarcinoma [15], tongue carcinoma [12] and thyroid carcinoma [16], a significant inverse relation between Dysadherin and E-cadherin was observed at the epithelial tight junctions. In other cancers such as lung cancer [17], non-small lung carcinoma [10,18], epitheloid sarcoma [9], thyroid carcinoma [8] breast cancer [19], colorectal carcinoma [11] and malignant melanoma [20], no inverse correlation between Dysadherin and E-cadherin was observed. This suggests that Dysadherin potentially has some unknown mechanisms by which it can effect tumor growth and progression. Additionally, overexpression of Dysadherin was recently reported to confer stem cell and tumorigenic potential of and and enhanced tumourigenic potential of hepatocellular carcinoma cells [21,22].
Colorectal cancer (CRC) is one of the most common types of cancer in high income countries. According to World Health Organization in 2012, CRC was ranked second and third most common cancer in women and men respectively, and was placed the second leading cause of cancer associated deaths in men and women (http://globocan.iarc.fr/Pages/fact_sheets_population.aspx).
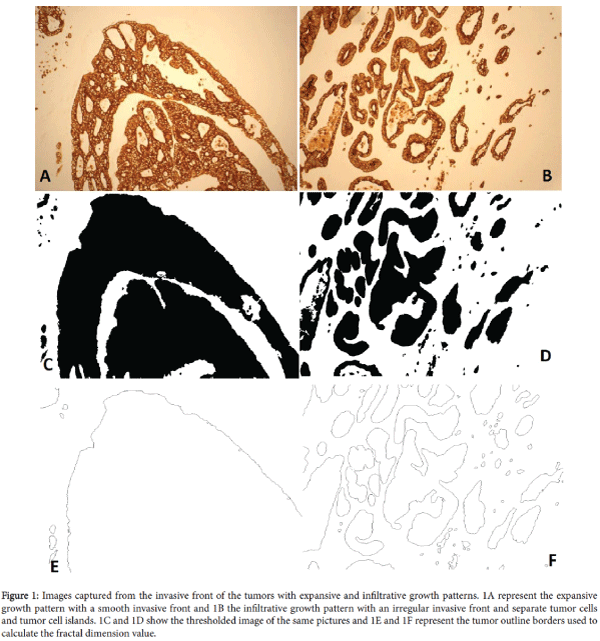
CRC initiates locally, develops through the intestinal wall and metastasizes via blood and lymph vessels to lymph nodes and distant organs. Tumor growth pattern is classified as invasive and infiltrative (Figure 1). However, such classification is based on the semi quantitative evaluation by pathologists therefore results are prone to variation among the pathologists [23]. Previously, Franzén and Hahn- Strömberg [24] presented a computer based method for the quantitative determination of colon cancer growth pattern by using computer software. This method provides quantitative and reproducible data with a range of 1-5 for the tumor growth pattern that was named complexity index.
Figure 1: Images captured from the invasive front of the tumors with expansive and infiltrative growth patterns. 1A represent the expansive growth pattern with a smooth invasive front and 1B the infiltrative growth pattern with an irregular invasive front and separate tumor cells and tumor cell islands. 1C and 1D show the thresholded image of the same pictures and 1E and 1F represent the tumor outline borders used to calculate the fractal dimension value.
Though studies have reported increased Dysadherin expression in different tumors including colorectal carcinoma, none of the previous studies have reported a quantitative expression of Dysadherin in colon cancer patients. Such a study will help to understand if increased expression is due to post-translation modification or some other mechanisms may also involve in increased Dysadherin expression. Additionally, as mentioned earlier, Dysadherin has been associated with tumor progression by decreasing cellular adhesion, and therefore can affect tumor growth pattern. Recently, we reported a significant association between growth pattern and tumor wall penetration [25]. Therefore, in this study, we aim to determine the expression of Dysadherin in colon cancer tissue samples and to correlate with the tumor growth pattern in terms of complexity index, and also with other clinical and pathological parameters. Our hypothesis is that increased Dysadherin expression is seen in higher complexity index tumors resulting in poor prognosis.
Material And Methods
Patient samples
A total of 45 formalin fixed and paraffin embedded colon cancer and paired normal patient samples were selected from the Örebro University Hospital tissue archives. Patients were diagnosed with colon carcinoma in 2002-2006. Samples were collected randomly both from men and women, unidentified and assigned numbers from 1-45. Rectal carcinomas were excluded in our study as most of the patients receive local radiations therapy prior to surgery thereby altering the tumor growth pattern. Ethical permission was approved by the Ethical committee at Örebro University Hospital, Örebro Sweden.
RNA extraction and RT-qPCR
For RNA extraction paired tumor and normal colon samples were taken from each patient sample. Tumor cells rich area and normal colon cells on the FFPE block were outlined by the pathologist. A punch of 1 mm diameter was taken from the demarcated area and subjected to total RNA extraction by using RNeasy FFPE Kit as per instructions provided by the manufacturer (Qiagen Sample and Assay Technologies, Hilden Germany).
Total one microgram RNA in a 20 μl reaction mixture was subjected to the reverse transcript polymerase chain reaction (RT-PCR) by using High-Capacity Reverse Transcription Kit in accordance with the manufacturer’s instructions (Applied Biosystems, Foster City, CA USA). RT-PCR was carried in LifePro Thermal Cycler (Bulldog Bio, Inc. USA) with the following PCR conditions: at 25°C for 10 min, 37°C for 120 min and 85°C for 5 min. Synthesized cDNA was stored at -75°C prior to further use.
In this study beta-2-microglobulin (B2M) gene expression was taken as an endogenous control. Dysadherin gene expression assay and B2M gene expression assays were purchased from the Life Technologies (Applied Biosystems, Foster City, CA USA). Quantitative PCR reactions were performed in triplicate with each reaction was performed in a total volume of 10 μl. Each reaction contained 20X TaqMan® Gene Expression Assay 0.5 μl, 2X TaqMan® Gene Expression Master Mix 5 μl (0.05 U), 2 μl of 10X diluted cDNA and 2.5 μl RNasefree water. As a negative control, cDNA was replaced with the RNasefree water. PCR reactions were performed in Applied Biosystems 7500 Fast real time PCR (Applied Biosystems, Foster City, CA USA).
Immunohistochemistry and Computer image analysis
FFPE blocks from archived samples containing colon cancer were prepared. Envision technique was used for immunohistochemical staining. Sections of four micron were sectioned from FFPE cancer tissue blocks and mounted on (poly-lysine coated) glass slides. Mounted sections were deparaffinised in xylene, hydrated in descending concentrations of 99%, 96% and 70% ethanol, and washed in distilled water. Samples were preheated in Tris EDTA buffer PH 9.0 for half an hour in a microwave oven (680 W), followed by washing with the distilled water. Anti-cytokeratin (CK 8) from Bioscience (San José, USA), was used as a primary antibody at a dilution rate of 1:25. Prior to mounting, sections were transferred through a series of increasing concentrations of ethanol and xylene.
Tumor growth pattern was determined according to the method described by [24]. Images from the invasive front of immunohistochemically CK 8 stained tumor slides were captured by using Olympus ALTRA20 digital camera mounted on an Olympus BX40 microscope by using 10X objective lens (Olympus Soft Imaging System, Beijing China). Captured images stored in TIF format, and were processed and thresholded to get tumor cell clusters and tumor outlines (Figure 1). Tumor outlines were used to estimate fractal dimension. The values of tumor cell clusters and fractal dimensions were used to grade tumor complexity from 1 to 5. Where, 1 represents a smooth bordered tumor with no tumor cluster and 5 a highly irregular tumor border with multiple numbers of tumor islands. For each tumor specimen, depending upon the length of the invasive front of the tumor, on an average, 8 images with a range of 4-10 images were captured and analysed per sample. A total of 372 images were used to calculate the Complexity index (CI) of each tumor by evaluating the mean values of tumor cell clusters and fractal dimensions from each tumor image.
Statistical analysis
Mean and standard error of mean was calculated for the continuous variables while frequencies and percentages were determined for the categorical variables. We applied paired t test between normal and paired tumor tissue samples to evaluate differences in Dysadherin mRNA expression. To ascertain statistical correlation between dependent and explanatory variables, Pearson Chi square test was used between the groups provided that frequencies in all sub-groups are more than 5, otherwise Fischer exact test was applied. A P value of less than 0.05 was considered statistically significance. Figures of Dysadherin expression were constructed by using micro soft excel (MS excel 2010) while all statistical analyses were performed by using PSS version 20 software (SPSS, Inc, Chicago, IL, USA).
Results
In this study we randomly selected 45 FFPE colon cancer tissue samples that were collected from 21(46.7) males and 24 (53.3) females patients with an average age and standard deviation of 74 and 10.19 respectively. About 75% (34) of the patient tumors were localized in the right colon and 51% (23) were low differentiated tumors. Tumor node metastasis (TNM) values showed that 80% of the tumors were at T3 stage, 44.4% of the tumor metastasized into local lymph nodes, but none of the patients had tumor metastasis into distant organs. Similarly 55.6% tumors were at Dukes stages of A and B. Detailed information on patient tumor clinical and pathological parameters is provided in table (Table 1).
| Clinicopathological Parameters | Number (%) |
|---|---|
| Gender | |
| Male | 21 (46.7) |
| Female | 24 (53.3) |
| Age | |
| 65≤ | 8 (17.8) |
| >65 | 37 (82.2) |
| Localisation | |
| Right | 34 (75.6) |
| Left | 11 (24.4) |
| Differentiation | |
| Low | 23 (51.1) |
| High | 22 (48.9) |
| Tumor penetration (T) | |
| T2 | 6 (13.3) |
| T3 | 36 (80) |
| T4 | 3 (6.7) |
| lymph node (N) | |
| N0 | 25 (55.6) |
| N1 | 8 (17.8) |
| N2 | 12 (26.7) |
| M* | |
| 1 | 0 |
| 0 | 45 (100) |
| Dukes staging | |
| A | 4 (8.9) |
| B | 21 (46.7) |
| C | 20 (44.4) |
| M-Distant metastasis | |
Table 1: Clinicopathological parameters of patient samples.
Dysadherin expression
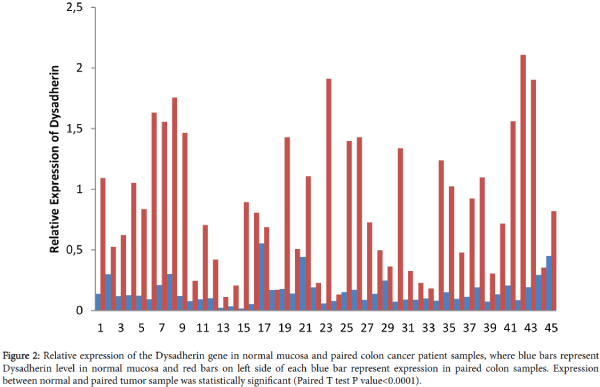
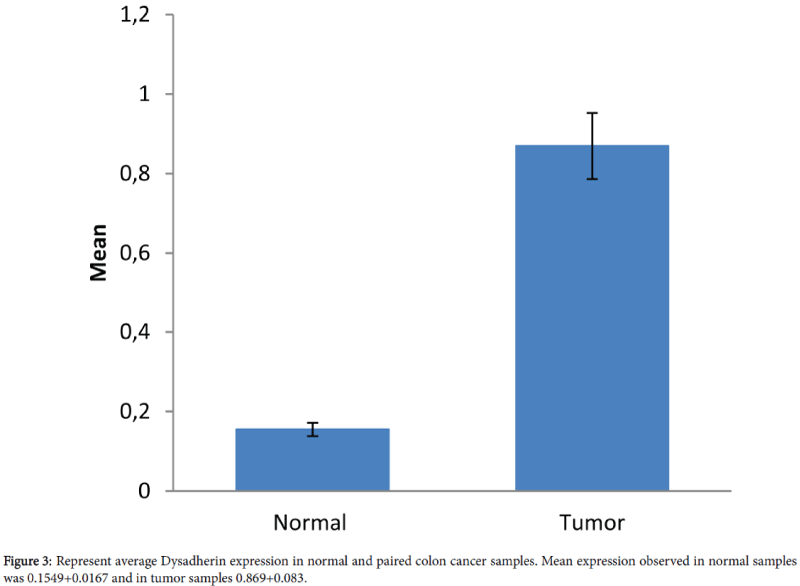
We evaluated Dysadherin expression in 90 paired colon tissue samples that were obtained from 45 patients. Relative expression of Dysadherin in tumor and paired normal and fold changes in Dysadherin in tumor compared to paired normal samples were calculated. Compared to normal, all tumor samples were presented with an increase in Dysadherin expression with mean value of 0.155+0.0167 and 0.87+0.083 in normal and tumors respectively (Figures 2 and 3). Relative Dysadherin expression in normal and tumor samples was statistically significantly different between paired samples (P value<0.0001). There was an average increase of 5.6 fold in Dysadherin expression in tumor samples compared to paired normal tissues.
Figure 2: Relative expression of the Dysadherin gene in normal mucosa and paired colon cancer patient samples, where blue bars represent Dysadherin level in normal mucosa and red bars on left side of each blue bar represent expression in paired colon samples. Expression between normal and paired tumor sample was statistically significant (Paired T test P value<0.0001).
Complexity index
Tumor growth pattern as tumor complexity index was determined in all 45 patient tumor samples. Results of tumor growth pattern showed that most of the patients were having values of extreme complexity index. Of total, 28 tumor complexity index was 1, 8 tumor presented with 2, one with 3 and 8 with complexity index of 5. However, none of the tumors had a complexity index of 4.
Correlation of Dysadherin expression and complexity index with clinicopathological parameters
To correlate Dysadherin expression further with different clinical and pathological parameters, we divided patients into two groups depending on the fold changes in Dysadherin expression. The low Dysadherin expression group included patients with less than 5 fold increase in Dysadherin mRNA level in their tumor compared with the normal and high Dysadherin expression included all samples with equal to and or more than 5 fold increase.
Dysadherin expression was significantly higher in tumors with lymph node metastasis (P value=0.008) and advanced Dukes stages (P value=0.015). Results were non-significant with other clinicopathological parameters (Table 2). Similarly no correlation was observed between tumor growth patter (Complexity index) and Dysadherin expression (Table 2).
| Clinicopathological Parameters | Dysadherin expression | *P value | Complexity Index | *P value | |||
|---|---|---|---|---|---|---|---|
| Low | High | Low | High | ||||
| N (%) | N (%) | N (%) | N (%) | ||||
| Gender | 0.493 | 0.47 | |||||
| Male | 10 (52.6) | 11 (42.3) | 18 (50) | 3 (33.3) | |||
| Female | 9 (47.4) | 15 (57.7) | 18 (50) | 6 (66.7) | |||
| Age | 0.435 | 0.651 | |||||
| 65 ≤ | 2 (10.5) | 6 (23.1) | 6 (16.7) | 2 (22.2) | |||
| >65 | 17 (89.5) | 20 (76.9) | 30 (83.3) | 7 (77.8) | |||
| Localisation | 0.485 | 0.087 | |||||
| Right | 13 (68.4) | 21 (80.8) | 25 (69.4) | 9 (100) | |||
| Left | 6 (31.6) | 5 (19.2) | 11 (30.6) | 0 (0) | |||
| Differentiation | 0.767 | 0.001† | |||||
| Low | 9 (47.4) | 14 (53.8) | 14 | 9 | |||
| High | 10 (52.6) | 12(46.9) | 22 | 0 | |||
| Tumor penetration (T) | 0.532 | 0.38 | |||||
| T2 | 4 (21.1) | 2 (7.7) | 6 (16.7) | 0 (0) | |||
| T3 | 14 (73.7) | 22 (84.6) | 28 (77.8) | 8 (88.9) | |||
| T4 | 1 (5.3) | 2 (7.7) | 2 (5.6) | 1 (11.1) | |||
| lymph node (N) | 0.008† | 0.46 | |||||
| N0 | 15 (78.9) | 10 (38.5) | 21 (58.3) | 4 (44.4) | |||
| N1 | 3 (15.8) | 5 (19.2) | 5 (13.9) | 3 (33.3) | |||
| N2 | 1 (5.3) | 11 (42.3) | 10 (27.8) | 2 (22.2) | |||
| Dukes staging | 0.015† | 0.75 | |||||
| A | 3 (15.8) | 1 (3.8) | 4 (11.1) | 0 (0) | |||
| B | 12 (63.9) | 9 (34.6) | 17 (47.2) | 4 (44.4) | |||
| C | 4 (21.1) | 16 (61.5) | 15 (41.7) | 5 (55.6) | |||
| Complexity Index | 1 | NA | |||||
| Low | 15 (78.9) | 21 (80.8) | NA | NA | |||
| High | 4 (21.1) | 5 (19.2) | NA | NA | |||
| *P-value was calculated by Pearson chi square (if number of samples >5 sample in each group) and Fischer exact test (if number of samples). †- Results are significant, NA:Not Applicable | |||||||
Table 2: Correlation between Dysadherin and complexity index and different clinical and pathological parameter.
Similar to Dysadherin grouping, for further analysis, we also dichotomized patient tumors into low and high complexity index groups. Tumors with complexity index value of 1-2 were placed into low and with 3-5 into high complexity group.
Statistical correlation revealed that tumors with low differentiation were significantly associated with higher complexity index. Though all tumors with higher complexity index were from the right sided colon, the results were statistically non-significant (P value=0.087). We found non-significant correlation between complexity index and all other clinical and pathological parameters (Table 2).
Discussion
Reduced cellular binding is one of the primary steps in the metastasis of all types of cancers. Dysadherin is a relatively new tumor marker; its increased expression has been associated with the progressive tumors and poor patient prognosis in various types of carcinoma [1,8,11,26]. It is known that Dysadherin decrease cellular adhesion by down regulation of the E-cadherin, a cell-cell binding protein [1]. Although many studies have been reported an increased Dysadherin protein expression, but none of the conducted studies have evaluated the quantitative change in Dysadherin mRNA level [5-8,10,12,27] Furthermore, due to its effect on lowering cellular adhesion, increased Dysadherin expression may alter the growth pattern of the tumor. Therefore, in this study we evaluated the expression of Dysadherin in paired normal and tumor colon cancer samples and also estimated complexity index of the tumor.
We observed increased levels of Dysadherin mRNA expression in all tumor samples compared to the paired normal samples (Figure 2). Mean value of Dysadherin mRNA levels in tumor and paired normal tissue samples was 0.867+0.0829 and 0.155+0.0167 (P<0.0001), respectively (Figure 3). Our data is consistent with the earlier observation made in different studies in various carcinomas including colon cancer that the Dysadherin expression is variable in different tumor tissues [5,7,10,11]. It been reported that Dysadherin normally is not expressed in normal gastrointestinal cells including the large intestine [1,11,27]. However, in current study we showed that very low levels of the Dysadherin mRNA (0.155+0.0167) are present in the normal mucosa. This may be partially true considering Dysadherin ability in the regulation of the Na+/K+-ATPase pump which are present in the large intestine [2,28]. Alternatively, in the normal large intestine mucosa, the lower level of Dysadherin may be degraded quickly by micro RNAs before their translation into protein. Additionally, normal samples were taken from the adjacent tumor tissues, so tumor microenvironment may have some impact on the adjacent normal tissues.
After dichotomizing fold changes (5< and >5 fold) in tumor Dysadherin mRNA level, we correlated Dysadherin expression with different clinical and pathological parameters. Consistent with the earlier study result, increased Dysadherin expression in the tumors was associated with the lymph node metastasis (P value=0.008) [11]. Increased expression was also associated with advanced Dukes stages (P value=0.015) that further support the existence notion that increased Dysadherin is associated with advanced tumors. Similar results have been reported in earlier studies in different carcinomas including CRC where increased Dysadherin expression was associated with metastasis and aggressive tumors. This may be due to Dysadherin ´s role in downregulation of E-cadherin [11-16] or due to Dysadherin ´s effect on increasing CXCR2 and CCL2 mediating tumor survival and metastasis and or both mechanisms may be involved [29]. Other than tumour progression, higher Dysadherin expression has also been reported to enhance tumor stem cell like properties. Another mechanism by which Dysadherin can effect tumor survival is AKT mediated multi-drug resistance gene upregulation [30]. Taken together, all before mentioned mechanisms add into tumor progression and cancer cell survival against therapy.
Other clinical and pathological parameters were un-associated with Dysadherin expression which is in agreement with a previous study in CRC [11]. We also correlated to tumor growth pattern to determine if increased Dysadherin expression is associated with tumor complexity index, but results were statistically insignificance. This may be because those multiple factors are involved in determining the overall growth pattern of the tumor.
We further correlated complexity index with different clinical and pathological parameters (Table 2). Low differentiated tumors were significantly associated with higher complexity index. This may be due that poorly differentiated tumors are aggressive in nature and are associated with poor prognosis [31]. However results of this study are in divergence with the observation made in an earlier study of colon carcinoma [25]. This discrepancy may be due to not dichotomized results of complexity index and also comparatively lower numbers of low graded and highly graded tumors used in the previous study. Increasing trend of high complexity index was observed for right sided colon cancers, but the results were statistically non-significant (P value=0.087). All other clinical and pathological parameters were not statistically associated with the colon carcinoma. Current results on tumor wall penetration are also divergent to our previous observation in colon cancer [25]. As shown in Table 2, all tumors with higher complexity index are at T3 and T4 stages which show a trend of higher complexity index with high penetrating tumors, therefore insignificant results in this study may be due to lower number of patients.
In conclusion, we showed that Dysadherin expression increase significantly in tumor samples and higher expressions were associated with lymph node metastasis and advanced Dukes stages.
No correlation was observed between tumor growth pattern and Dysadherin mRNA expression level. Complexity index was significantly associated with tumor differentiation. This may suggest that Dysadherin and complexity indexes are independent factors associated with the aggressiveness of colon carcinoma and could be used as prognostic markers.
References
- Ino Y, Gotoh M, Sakamoto M, Tsukagoshi K, Hirohashi S (2002) Dysadherin, a cancer-associated cell membrane glycoprotein, down-regulates E-cadherin and promotes metastasis. Proc Natl Acad Sci USA 99: 365-370.
- Lubarski I, Pihakaski-Maunsbach K, Karlish SJ, Maunsbach AB, Garty H (2005) Interaction with the Na,K-ATPase and tissue distribution of FXYD5 (related to ion channel). J Biol Chem 280: 37717-37724.
- Geering K (2006) FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Renal Physiol 290: F241-250.
- Tsuiji H, Takasaki S, Sakamoto M, Irimura T, Hirohashi S (2003) Aberrant O-glycosylation inhibits stable expression of dysadherin, a carcinoma-associated antigen, and facilitates cell-cell adhesion. Glycobiology 13: 521-527.
- Liang JF, Zheng HX, Xiao H, Li N, Cheng CX, et al. (2009) Dysadherin expression in gastrointestinal stromal tumors (GISTs). Pathol Res Pract 205: 445-450.
- Batistatou A, Scopa CD, Ravazoula P, Nakanishi Y, Peschos D, et al. (2005) Involvement of dysadherin and E-cadherin in the development of testicular tumours. Br J Cancer 93: 1382-1387.
- Batistatou A, Charalabopoulos AK, Scopa CD, Nakanishi Y, Kappas A, et al. (2006) Expression patterns of dysadherin and E-cadherin in lymph node metastases of colorectal carcinoma. Virchows Arch 448: 763-767.
- Sato H, Ino Y, Miura A, Abe Y, Sakai H, et al. (2003) Dysadherin: Expression and clinical significance in thyroid carcinoma. J ClinEndocrinolMetab 88: 4407-4412
- Izumi T, Oda Y, Hasegawa T, Nakanishi Y, Iwasaki H, et al. (2006) Prognostic significance of dysadherin expression in epithelioid sarcoma and its diagnostic utility in distinguishing epithelioid sarcoma from malignant rhabdoid tumor. Modern Pathology 19: 820-831.
- Tamura M, Ohta Y, Tsunezuka Y, Matsumoto I, Kawakami K, et al. (2005) Prognostic significance of dysadherin expression in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 130: 740-745.
- Aoki S, Shimamura T, Shibata T, Nakanishi Y, Moriya Y, et al. (2003) Prognostic significance of dysadherin expression in advanced colorectal carcinoma. Br J Cancer 88: 726-732.
- Nakanishi Y, Akimoto S, Sato Y, Kanai Y, Sakamoto M, et al. (2004) Prognostic significance of dysadherin expression in tongue cancer: immunohistochemical analysis of 91 cases. Appl ImmunohistochemMol Morphol 12: 323-328.
- Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ, Nakanishi Y, et al. (2006) Dysadherin expression in head and neck squamous cell carcinoma: association with lymphangiogenesis and prognostic significance. Am J Surg Pathol 30: 185-193.
- Muramatsu H, Akimoto T, Maebayashi K, Kita M, Mitsuhashi N (2008) Prognostic significance of dysadherin and E-cadherin expression in patients with head and neck cancer treated by radiation therapy. Anticancer Res 28: 3859-3864.
- Shimamura T, Sakamoto M, Ino Y, Sato Y, Shimada K, et al. (2003) Dysadherin overexpression in pancreatic ductal adenocarcinoma reflects tumor aggressiveness: relationship to e-cadherin expression. J Clin Oncol 21: 659-667.
- Batistatou A, Charalabopoulos K, Nakanishi Y, Vagianos C, Hirohashi S, et al. (2008) Differential expression of dysadherin in papillary thyroid carcinoma and microcarcinoma: correlation with E-cadherin. Endocr Pathol 19: 197-202.
- Mitselou A, Batistatou A, Nakanishi Y, Hirohashi S, Vougiouklakis T, et al. (2010) Comparison of the dysadherin and E-cadherin expression in primary lung cancer and metastatic sites. Histol Histopathol 25: 1257-1267.
- Ono K, Uramoto H, Hanagiri T (2010) Expression of dysadherin and cytokeratin as prognostic indicators of disease-free survival in patients with stage I NSCLC. Anticancer Res 30: 3273-3278.
- Batistatou A, Peschos D, Tsanou H, Charalabopoulos A, Nakanishi Y, et al. (2007) In breast carcinoma dysadherin expression is correlated with invasiveness but not with E-cadherin. Br J Cancer 96: 1404-1408.
- Nishizawa A, Nakanishi Y, Yoshimura K, Sasajima Y, Yamazaki N, et al. (2005) Clinicopathologic significance of dysadherin expression in cutaneous malignant melanoma: immunohistochemical analysis of 115 patients. Cancer 103:1693-1700.
- Park JR, Kim RJ, Lee YK, Kim SR, Roh KJ, et al. (2011) Dysadherin can enhance tumorigenesis by conferring properties of stem-like cells to hepatocellular carcinoma cells. J Hepatol 54: 122-131.
- Jiang N, Li Y, Ruan D (2015) Effect of aberrantly regulated dysadherin and Bcl2/Bax2 on tumorigenesis and DNA targeting drugresistance of liver cancer stem cells. American Society of Clinical Oncology Annual Meeting. J Clin Oncol 33: e15167.
- Jass JR, Atkin WS, Cuzick J, Bussey HJ, Morson BC, et al.(2002) The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology 41:59-81.
- Franzén LE, Hahn-Strömberg V, Edvardsson H, Bodin L (2008) Characterization of colon carcinoma growth pattern by computerized morphometry: definition of a complexity index. Int J Mol Med 22: 465-472.
- Mannan A, Hahn-Strömberg V (2012) K-ras mutations are correlated to lymph node metastasis and tumor stage, but not to the growth pattern of colon carcinoma. APMIS 120: 459-468.
- Nam JS, Hirohashi S, Wakefield LM (2007) Dysadherin: a new player in cancer progression. Cancer Lett 255: 161-169.
- Maehata Y, Hirahashi M, Aishima S, Kishimoto J, Hirohashi S, et al. (2011) Significance of dysadherin and E-cadherin expression in differentiated-type gastric carcinoma with submucosal invasion. Hum Pathol 42: 558-567.
- Lückhoff A, Horster M (1984) Hormonal regulation of electrolyte and water transport in the colon. Klin Wochenschr 62: 555-563.
- Schüler Y, Lee-Thedieck C, Geiger K, Kaiser T, Ino Y, et al. (2012) Osteoblast-secreted factors enhance the expression of dysadherin and CCL2-dependent migration of renal carcinoma cells. Int J Cancer 130: 288-299.
- Lee YK, Lee SY, Park JR, Kim RJ, Kim SR, et al. (2012) Dysadherin expression promotes the motility and survival of human breast cancer cells by AKT activation. Cancer Sci 103: 1280-1289.
- Laohavinij S, Maneechavakajorn J, Techatanol P (2010) Prognostic factors for survival in colorectal cancer patients. J Med Assoc Thai 93: 1156-1166.
Citation: Mannan A, Hahn-Stromberg V (2016) Dysadherin Expression is Associated with Advanced Tumorstage while Complexity Index is Related to Poorly Differentiated Tumors in Colon Carcinoma. Diagn Pathol Open 1:111. Doi: 10.4172/2476-2024.1000111
Copyright: © 2016 Mannan A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 10630
- [From(publication date): 5-2016 - Apr 27, 2024]
- Breakdown by view type
- HTML page views: 9944
- PDF downloads: 686