Research Article Open Access
Mutagenic Action of Sugarcane Vinasse in the Tradescantia Pallida Test System
Janaína Pedro-Escher, Guilherme Tiago Maziviero and Carmem S. Fontanetti*Department of Biology, Institute of Biosciences-UNESP, São Paulo State University, Av. 24A, n. 1515, 13506-900, Rio Claro, São Paulo, Brazil
- *Corresponding Author:
- Carmem S. Fontanetti
Department of Biology, Institute of Biosciences-UNESP
São Paulo State University, Av. 24A, n. 1515, 13506-900, Rio Claro, São Paulo, Brazil
Tel: 55-19-3526-4139
Fax: 55-19-3526-4136
E-mail: fontanet@rc.unesp.br
Received date: April 21, 2013; Accepted date: May 19, 2014; Published date: May 24, 2014
Citation: Pedro-Escher J, Maziviero GT, Fontanetti CS (2014) Mutagenic Action of Sugarcane Vinasse in the Tradescantia Pallida Test System. J Ecosys Ecograph 4:145. doi:10.4172/2157-7625.1000145
Copyright: © 2014 Fontanetti CS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
The soil is a very complex and dynamic system and its contamination has become one of the main environmental concerns. Sugar-ethanol mills, for example, have a high hazard potential, mainly soil contamination, due to the production of different residues. The use of vinasse, a by-product of ethanol production, as fertilizer is a technically and economically feasible alternative. However, excessive quantities have been used, causing adverse changes in the soil. The present study was aimed at evaluating the toxic potential of vinasse in the Tradescantia pallida test system using the micronucleus test (Trad-MCN). Ten plants were exposed to ultrapure water as the negative control (NC), in MMS as the positive control (PC), raw vinasse (RV), and vinasse diluted to 50% (C1), vinasse diluted to 25% (C2), vinasse diluted to 12.5% (C3). The medium buds of each plant were used to assess the mutagenic effects based on the presence of micronuclei in tetrads. The results revealed that RV and C1 had a mutagenic potential compared to that of NC. The alterations observed suggest damage to the DNA of the test organism, which may not be reintegrated to the cell nucleus. The findings suggest caution in the application of this product to the soil despite this being an economically viable practice.
Keywords
Trad-MCN; Ethanol production; Agroindustrial waste; Fertigation
Introduction
The human exposure to mutagenic agents, effective in causing damage to the genetic material, is currently a great concern worldwide. These agents are closely associated with the development of cancers and can potentially compromise future generations by causing DNA damage to germinative cells, which may be passed on to future generations. The discovery of the vulnerability of the genetic material to environmental stresses has led to a new research field, Toxicological Genetics, in which specialists focus on the study of lesions and alterations induced by chemical compounds and/or physical agents to DNA [1]. Studies in this research area can lead to precise information on the exposure and risks to cell integrity, and thus an effective prevention of health problems.
The agricultural use of organic waste from industrial, urban and agricultural resources is an interesting alternative to disposal, enabling the recycling of nutrients in ecosystems [2]. In this context, it includes the vinasse or stillage, a by-product of the sugarcane industry, residue of ethanol production obtained after distillation of fermented grape, residue known to have high organic content which classifies it as a good fertilizer.
The incentive for the use of ethanol as a fuel, because this has lower pollution potential resulted in a stimulus to the sugarcane agroindustry, gaining great prominence in agricultural activities of socio-economic expression. Consequently, the wastes generated by this activity also increased, in particular the vinasse. For every liter of ethanol are produced 10 to 18 liters of vinasse [3] resulting in a problem for proper disposal.
Species of Tradescantia have been often used to assess the mutagenic potential of various substances in the air, soil, and water capable of inducing chromosome alterations due to the presence by clastogenic agents [4], or chromosome losses as a result of aneugenic activity, thus causing genotoxic damage [5].
The micronucleus test using Tradescantia (TradMCN) is considered a valuable tool by many researchers due to the simplicity of the methodology and sensitivity of the plant exposed to the genotoxic agents [6]. The test was developed by Ma and collaborators in 1976 from the Brookhaven National Laboratory initially using the clone 4430 as test organism and later adapted to the species T. pallida.
Tradescantia pallida, also known as Purple Heart, is native to the Gulf of Mexico. It is easily adaptable to different climate conditions [7] and environments, and exhibits year-round growth in the field and greenhouse in tropical and subtropical regions. The relatively small size of plants and genetic material consisted of six pairs of relatively large and easily observable chromosomes make this plant a suitable organism for cytogenetic studies [4]. Its inflorescences develop sequentially, allowing the exposure to the test substance in all stages of development [8].
Studies conducted by Mielli et al. [9] with young plants of T. pallida and the clone 4430 exposed to water and different concentrations of As2O3 exhibited similar responses. These results support those reported by Suyama et al. [10] with plants exposed to X-rays, suggesting that both are suitable for micronucleus bioassays. The same was not observed in experiments conducted in the field, which showed that T. pallida is more sensitive than clone 4430 [9].
Thus, the present study was aimed at evaluating the toxic potential of vinasse samples from a sugar-ethanol mill using T. pallida as testorganism in mutagenicity tests.
Materials and Methods
Test substance: sugarcane vinasse
The sugarcane vinasse was collected in the Santa Lúcia Mill, located in the city of Araras, São Paulo, Brazil and maintained in a cold storage chamber (4°C) at the Department of Biochemistry and Microbiology of the Institute of Biosciences, UNESP (São Paulo State University), Rio Claro, São Paulo, Brazil, until use. Two vinasse samples were collected from two different crops (crop 1 and crop 2), from different years.
Vinasse samples were sent for analysis to the laboratory TASQA, Paulínia, São Paulo, Brazil, where the following parameters were measured: pH, non-filterable residue, hardness, electric conductivity, nitrate, nitrite, kjeldahl nitrogen, sodium, calcium, potassium, magnesium, sulfate, phosphate, biochemical oxygen demand (BOD), chemical oxygen demand (COD) and metals by atomic absorption spectrophotometry (ICP) (As, Ba, Cd, Cu, Cr, Hg, Mo, Ni, Pb, Se, Zn).
Concentrations of vinasse
Vinasse was diluted in ultrapure water to three concentrations: vinasse diluted to 50% (C1), vinasse diluted to 25% (C2) and vinasse diluted to 12.5% (C3). Raw vinasse (RV) was also tested. These dilutions were tested based on the dose of vinasse applied in the field according to the guideline P4.231 of the Environmental Sanitation Technology Company (CETESB). Two bioassays were carried out with the two samples.
Bioassays
Plants of T. pallida were cultivated in standardized plots and watered weekly until flowering. At that time, plants were cut and brought to the Department of Biology, Institute of Biosciences, UNESP, Rio Claro, São Paulo, Brazil and immediately exposed to different treatments during 24 h.
The positive control (PC) consisted of 0.154 μL methyl methanesulfonate (MMS) in 20 mL of water, and the negative control (NC), ultrapure water. Plants were exposed to different treatments (PC, NC, RV, C1, C2, C3) in a beaker. After exposure, plants were allowed to recover in ultrapure water for 8 h.
Micronucleus test with Tradescantia (Trad-MCN)
For the evaluation of mutagenicity, 10 young inflorescences (all flower buds closed), of approximately 6 to 8 cm were used. After exposure, four clusters were selected. Each cluster consisted of three buds (small, medium, and large). The medium bud (right or left side) was placed on a slide and macerated with a razor blade to remove remnants of inflorescence. The material was then stained with a drop of acetic carmine, cover slipped, and then quickly passed over a flame.
After this procedure, 10 slides per treatment were analyzed under light microscope, totaling 300 tetrads observed per slide and 3000 per treatment.
The statistical analysis was carried out with the non-parametric Mann-Whitney test and p>0.005.
Results
Chemical characterization of raw vinasse samples
The results of the physico-chemical and metal analysis of vinasse are presented in Table 1.
| Parameters | Crop 1 | Crop 2 | Method |
|---|---|---|---|
| Arsenic (mg/L) | <LQ | <LQ | SM21 3120 B |
| Barium (mg/L) | 0.41 | <LQ | SM21 3120 B |
| Cadmium (mg/L) | <LQ | <LQ | SM21 3120 B |
| Lead (mg/L) | <LQ | <LQ | SM21 3120 B |
| Copper (mg/L) | 0.35 | 0.76 | SM21 3120 B |
| Chromium (mg/L) | 0.04 | 3.56 | SM21 3120 B |
| Total sulfur (mg/L) | 1219 | 1681 | SM21 3120 B |
| Mercury (mg/L) | 0.0019 | <LQ | EPA 7470 A |
| Molybdenum (mg/L) | 0,008 | <LQ | SM21 3120 B |
| Nickel (mg/L) | 0.03 | <LQ | SM21 3120 B |
| Selenium (mg/L) | <LQ | <LQ | SM21 3120 B |
| Zinc (mg/L) | 1.66 | <LQ | SM21 3120 B |
| Ammonia NH3 (mg/L) | <LQ | <LQ | USEPA 4405-55-001 |
| Calcium (mg/L) | 719 | 671 | SM21 3120 B |
| Electrical conductivity (µS/cm) | 13530 | 15110 | SM21 2510 B |
| BOD (mg/L) | 5046 | 7941 | SM21 5210 B |
| COD (mg/L) | 13380 | 25,225 | SM21 5220 B |
| Hardness (mg CaCO3/L) | 2493 | 276 | |
| Total phosphate (mg/L) | 1.3 | 207 | SM21 4500-P C |
| Magnesium (mg/L) | 237 | 264 | SM21 3120 B |
| Nitrate (mg/L) | 1.3 | 1.49 | SM21 4500-NO2F |
| Nitrite (mg/L) | 0.008 | 0.033 | SM21 4500-NO2B |
| Ammoniacal nitrogen (mg/Kg) | 276 | - | SM21 4500-H+B |
| Kjeldahl nitrogen (mg/Kg) | 267 | 171 | SM21 3120 B |
| pH (mg/L) | 3.9 | 4.37 | SM21 4500NORG 3 |
| Potassium (mg/L) | 2056 | 3401 | SM21 3120 B |
| Sodium (mg/L) | 50.2 | 114 | SM21 3120 B |
| Sulfate (mg/L) | 710 | 2993 | SM21 4500 SO4 E |
| Non-filterable Residue (mg/L) | 2765 | 1800 | SM21 2540 D |
Table 1: Physico-chemical analysis of raw vinasse.
LQ. limit of quantification; BOD. Biochemical Oxygen Demand; COD. Chemical Oxygen Demand; EPA. Environmental Protection Agency; SM21. Standard methods for the examination of water and wastewater 21:2005; USEPA. United States Environmental Protection Agency.
In general, vinasse had a low pH, and high BOD, COD, and potassium levels. In the crop 1, the concentration of potassium was approximately 12 times higher than that of the crop 2. In the crop 1, levels of calcium, magnesium and sodium were also higher, while in the crop 2, nitrate and kjeldahl nitrogen were higher.
In the crop 2, levels of barium, copper, chrome, nickel, and zinc were also higher.
Mutagenicity analysis
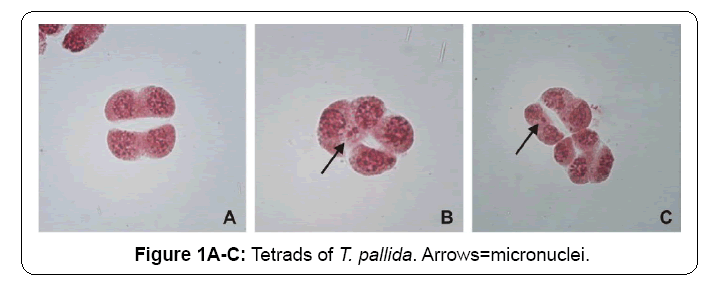
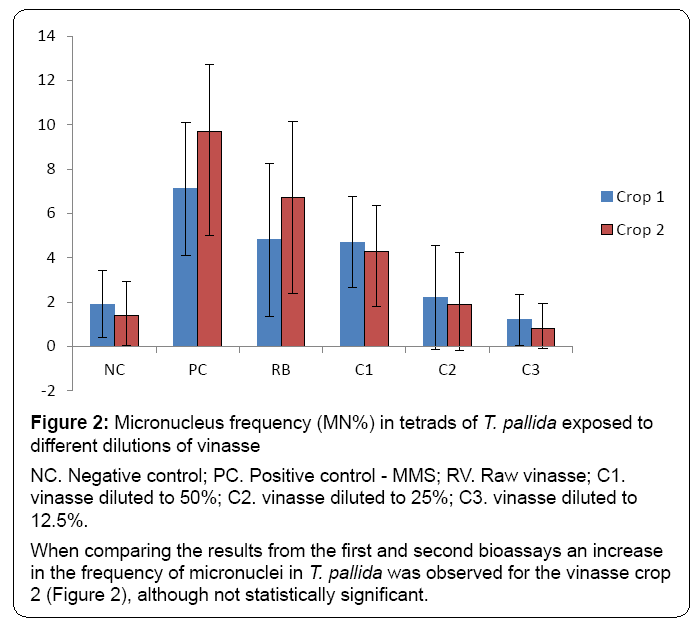
The results found by observing tetrads carrier micronuclei (Figure 1) in the two bioassays were very similar. In the first bioassay (crop 1), a significant micronucleus index was obtained for the RV and C1 treatments when compared to NC, similar to the obtained for the second bioassay (crop 2) (Table 2 and Figure 2).
Figure 2: Micronucleus frequency (MN%) in tetrads of T. pallida exposed to
different dilutions of vinasse
NC. Negative control; PC. Positive control - MMS; RV. Raw vinasse; C1.
vinasse diluted to 50%; C2. vinasse diluted to 25%; C3. vinasse diluted to
12.5%.
When comparing the results from the first and second bioassays an increase
in the frequency of micronuclei in T. pallida was observed for the vinasse crop
2 (Figure 2), although not statistically significant.
| Crop 1 | Crop 2 | |
|---|---|---|
| NC | 1.9 ± 1.52 | 1.4 ± 1.35 |
| PC | 7.1 ± 2.99* | 9.7 ± 4.69* |
| RV | 4.8 ± 3.46* | 6.7 ± 4.32* |
| C1 | 4.7 ± 2.06* | 4.3 ± 2.50* |
| C2 | 2.2 ± 2.35 | 1.9 ± 2.10 |
| C3 | 1.2 ± 1.13 | 0.8 ± 0.92 |
Table 2: Mutagenicity frequency (average and standard deviation) in tetrads of T. pallida after exposure to different dilutions of vinasse. (NC. Negative control; PC. Positive control-MMS; RV. Raw vinasse; C1. vinasse diluted to 50%; C2. vinasse diluted to 25%; C3. vinasse diluted to 12.5%. *Statistically significant).
Discussion
The analysis of micronuclei in different organisms is a widely used method for the assessment of the mutagenic potential. A compound capable of inducing the formation of micronuclei can be considered a clastogenic or aneugenic agent. The clastogenic action of a compound can be demonstrated by the presence of micronuclei resulted from chromosome breaks during cell division. The aneugenic action, on the other hand, occurs when the mitotic fuse is inactivated, resulting in the loss of entire chromosomes, which are absent in the main nucleus of the cell.
Yamamoto and Kikuchi [11] demonstrated that the type of action of the mutagenic agent, clastogenic or aneugenic, could be determined based on micronucleus size. For these authors, clastogenic agents induced small MN, while aneugenic compounds, larger MN. Although the method was satisfactory for several bioassays, it was not reliable in many cases. Combes et al. [12] concluded that micronucleus size was not enough to determine the clastogenic and aneugenic potential of a compound. Fenech [13] reported that because the size of chromosomes in human cells and other cell types are heterogeneous, the correlation between micronucleus size and type of action of the mutagenic agent is unfeasible. For this author, a small micronucleus can be a fragment from a large chromosome or an entire small chromosome.
The results obtained in this study showed that the vinasse samples tested had a mutagenic potential however, it was not possible to discriminate aneugenic and clastogenic modes of action, as MN of various sizes were observed. These observations corroborate the statements of the aforementioned authors [12,13].
The vinasse is a residue produced in many countries from different raw materials such as sugarcane in South America, beets and fruits in Europe, corn in North America [14]. Thus, the chemical properties of the residue depends on the raw material (biomass) used for the production of ethanol [15]. The chemical composition of sugarcane vinasse also varies depending on the each plant used in the production of ethanol as well as the subsequent distillation process. For this feature, two samples of vinasse were used obtained from two different crops. Despite this feature, the results observed by Trad-MN test showed no statistically significant differences in the action of vinasse on the genetic material of the organism used when comparing the two samples. The chemical analysis showed some differences between the samples observed mainly in the quantification of potassium, calcium, magnesium, sodium, nitrate, kjeldahl nitrogen and the metals, barium, copper, chrome, nickel, and zinc. Because it is a complex mixture is not possible to say which of the detected elements were responsible for the induction of micronuclei observed in T. pallida.
In a series of studies on the role of certain nutrients in meiosis, Steffensen [16-18] reported that the presence of micronuclei in the microspores of T. paludosa was considered an indication of chromosome break. According to Xavier et al. [19], large quantities of phosphorus, calcium, and nitrogen present in certain compounds that may promote alterations in the genetic characteristics of plants. One could suggest that a synergistic effect of the elements found in vinasse could cause the observed changes. Also, according to Lerda [20] and Matsumoto et al. [21], this can also be due to the exposure of cells to cadmium, copper, lead, chrome, and nickel, which increase the clastogenic and aneugenic effect on certain plants. The chemical analysis of vinasse samples did not reveal large quantities of these elements. However in the crop 2, large quantities of chrome and phosphate were observed, which could have influenced the results.
Studies conducted by Souza et al. [22] using landfarming soil samples from an oil refinery, rich in PAH and metals, biorremediated with vinasse, demonstrated that the latter potentialized the clastogenic effect of the soil, possibly making metals available.
Genotoxicity studies using A. cepa as test system have demonstrated the genotoxic and mutagenic potential of vinasse combined with the soil. However, a decrease in mutagenic potential was observed after bioprocessing by millipedes [23]. Using the same test system, Pedro- Escher and Fontanetti demonstrated that samples of raw vinasse were mutagenic and genotoxic to the system tested. However, after dilution in water, the mutagenic effect was not observed. The results revealed that T. pallida was more sensitive than A. cepa, supporting the importance of an application of different tests of genotoxicity and mutagenicity to determine the mutagenic action of different agents.
It should be pointed out that the application of this technique reduces the time needed to perform toxicological assays based on conventional chronic exposure and is sensitive to even substances present in low concentrations. Preliminary genotoxicity and mutagenicity studies could be applied to determine the toxicity of different products, as they can identify the effects of excessive use of vinasse, and permanent damage to DNA can be passed on to future generations. This study points to greater care in the use of this waste as fertilizer in agricultural fields. Although this practice is economically viable, the indiscriminate use of this residue in the soil can cause damage to DNA from different organisms.
Acknowledgements
The authors thank the São Paulo Research Foundation (FAPESP–Processes 2009/53047-9 and 12/50197-2) for financial support, to Mr. Almir José Christofoletti for the collection of vinasse, to Cintya Aparecida Christofoletti and Rudy Marcel Escher for assistance during soil collection and experiment setup. The authors also thank Dr Dejanira Franceschi de Angelis of the Department of Biochemistry and Microbiology of UNESP-Rio Claro for allowing the storage of residues.
References
- Rabello-Gay MN, Rodrigues MA, La R, MaonteleoneNeto R (1991) Mutagênese, carcinogênese e teratogênese: Métodos e critérios de avaliação. In: Repetto M (Org.) Toxicologíaavanzada. Madrid: Díaz de As.
- Tasso Junior LC (2007) Produtividade e qualidade de cana-de-açúcarcultivadaem solo tratado com lodo de esgoto, vinhaça e adubosminerais. EngenhariaAgrícola 27:276-283.
- Pedrosa EMR, Rolim MM, Albuquerque PHS, Cunha AC (2005) Supressividade de nematóidesemcana-de-açúcarporadição de vinhaçaao solo. Rev Bras EngAgrAmb 9:197-201.
- Carvalho HA (2005) A Tradescantiacomobioindicador vegetal namonitoração dos efeitosclastogênicos das radiaçõesionizantes. RadiologiaBrasileira 38: 459-462.
- Rodrigues GS, Ma TH, Pimentel D, Weinstein LH (1997) Tradescantia bioassays as monitoring systems for environmental mutagenesis: a review. Crit Rev Sci 16: 325-359.
- Batalha JRF (1999) Exploring the clastogenic effects of air pollutions in São Paulo Brazil using the Tradescantia micronuclei assay. Mut Res 426: 229-232.
- Sisenando HÁ, Batistuzzo SEM, Hacon SS (2009) Tradescantiapallida: mais do queumalindaflor, um importantebioindicador da qualidadeambiental. GenéticanaEscola - SociedadeBrasileira de Genética 4: 9-13.
- Savage JRK, Papworth DG (1998) An investigation of LET “finger-prints” in Tradescantia. Mut Res 422: 313-322.
- Mielli AC, Matta MEM, Nersesyan A, Saldiva PHN, Umbuzeiro GA (2009) Evaluation of the genotoxicity of treated urban sludge in the Tradescantia micronuclei assay. Mut Res 672: 51-54.
- Suyama F, Guimarães ET, Lobo DJA, Rodrigues GS, Domingos M, Alves ES, Carvalho HA, Saldiva PHN (2002) Pollen mother cells of Tradescantia clone 4430 and Tradescantiapallida var. Purpurea are equally sensitive to the clastogenic effects of X-rays. Braz J Med Biol Res 35: 127–129.
- Yamamoto KI, Kikuchi Y (1980) A comparison of diameters of micronuclei induced by clastogens and by spindle poisons. Mut Res 71: 127-131.
- Combes RD, Stopper H, Caspary WJ (1995) The use of the L5178Y mouse lymphoma cells to assess mutagenic, clastogenic and aneugenicativity of chemicals. Mutagenesis 10: 403-408.
- Fenech M (2000) The in vitro micronucleus technique. Mut Res 455: 81-95.
- Gianchini CF, Ferraz MV (2009) Benefícios da utilização de vinhaçaemterras de plantio de cana-de-açúcar: Revisão de literatura. Rev CienEletrAgron VII
- España-Gamboa E, Minagos-Cortes J, Barahona-Perez L, Dominguez-Maldonado J, Hernández-Zarate G, Alzate-Gaviria L (2011) Vinasse: characterization and treatments. Waste Manag Res 29:1235-1250.
- Steffensen D (1953) Induction of chromosome breakage at meiosis by a magnesium deficiency in Tradescantia, Proc Nat AcadSci 39: 613-620.
- Steffensen D (1954) Irregularities of chromosome divisions in Tradescantia grown on low sulfate. Exp Cell Res 6: 554-556.
- Steffensen D (1955) Breakage of chromosomes in Tradescantia with calcium deficiency. Proc Nat AcadSci 41: 155-160
- Xavier BO, Passos L, Oliveira RC, Mielli AC (2011) Avaliação do efeitogenotóxico de diferentestipos de solos parabiomonitoramento com Tradescantiapallida. GenéticanaEscola 6: 67-69
- LERDA D (1992) Study of sperm characteristics in persons occupationally exposed to lead. Amer J Ind Med 22: 567-571
- Matsumoto ST, Fonseca I, Marin-Morales MA, Mantovani MS, Malagutti MI, Dias AL ( 2006) Genotoxicity and mutagenicity of water contaminated with tannery effluents, as evaluated by the micronuclei test and comet assay using the fish Oreochromisniloticus and chromosome aberrations en onion root-tips. Genet MolBiol 29: 148-158
- Souza TS, Henckelein FA, Angelis DA, Fontanetti CS (2013) Clastogenicity of landfarming soil treated with sugar cane vinasse. Environ Monit Assess 185: 1627-1636
- Christofoletti CA, Pedro-Escher J, Fontanetti, CS (2013) Assessment of the genotoxicity of two agricultural residues after processing by diplopods using the Allium cepa. Water, Air and Soil Pollution 224: 1523-1536
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 15763
- [From(publication date):
December-2014 - Jul 13, 2025] - Breakdown by view type
- HTML page views : 11161
- PDF downloads : 4602