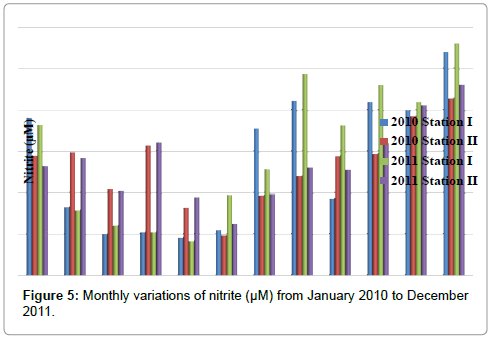Physico-Chemical Characteristics of Kottakudi and Nari Backwaters, South East of Tamil Nadu
Received: 20-Mar-2017 / Accepted Date: 15-Apr-2014 / Published Date: 17-Apr-2014 DOI: 10.4172/2157-7617.1000199
Abstract
The study of physico-chemical properties of coastal environments is important, because the variations in the physicochemical properties were influence on the floral and faunal production. During the study period, the maximum and minimum values of rainfall, water temperatures, salinity and dissolved oxygen recorded were 16.76mm to 745.76mm, 24 to 33°C, 24 to 37% and 3.8 to 4.6 ml/l for both the stations. And the dissolved nutrients of nitrite, nitrate, ammonia, inorganic phosphate and reactive silicate recorded were 0.83 to 5.62 μM, 10.3 to 25.6 μM, 0.11 to 5.62 μM, 0.52 to 3.76 μM and 30 to 123 μM for both the stations respectively. The variable of physico-chemical parameters is mainly affect the species diversity, pattern of diversity, reproduction, spawning, survival and other activities. To maintain optimum level of water quality parameters this is better to species survival and healthy ecosystem.
Keywords: Physico-chemical parameters; Affects; Coastal water; Monitoring
Introduction
The total life of the world depends on water and hence the hydrological study is very much essential to understand the relationship between its different trophic levels and food webs. The environmental conditions such as topography, water movement and stratification, salinity, oxygen, temperature and nutrients are characterizing particular water mass also determining the composition of its biota [1]. Usually in the near shore waters and estuaries, they exhibit considerable seasonal variations depending on the local conditions of rainfall, tidal incursions, various abiotic and biotic processes, quantum of freshwater inflow affecting the nutrient cycle of different coastal environments [2]. The water quality, comprising the environmental master factors such as temperature, salinity, oxygen, besides organic matter and nutrients forms the basis for an ecosystem. The interactive physical, chemical and biological processes operation in the coastal ecosystems sustain higher level of productivity, as reflected in a wide spectrum of flora and fauna, leading to richness in biodiversity [3-5]. The anthropogenic pressures are the main modifying factor that is responsible directly or indirectly for the biodiversity loss [6,7]. Therefore, it is of great importance and significance to know about the ecological parameters and their influences on the ecologically sensitive and fragile, but productive coastal ecosystems. The present investigation deals with the fluctuations in physico-chemical parameters of two different (Kottakudi and Nari backwaters) stations.
Materials And Methods
Surface water samples were collected in monthly interval from the stations I (Kottakudi) and station II (Nari backwaters) for a period of two years from January 2010 to December 2011 to estimate the physicochemical and biological parameters. The water samples were collected in new polythene container and preserved by small pinch of mercury chloride for nutrient analysis at the early morning of every month. The rainfall data were collected from the meteorological unit. The water temperature was measured using a digital centigrade thermometer. Salinity was estimated with the help of a hand refractrometer (ERMA, Japan) and pH was measured using an ELICO Grip pH meter. Dissolved oxygen was estimated by the modified Winkler's method [8]. For the analysis of nutrients, surface water samples which collected in clean polyethylene container were filtered by using a Millipore filtering unit and analyzed for dissolved inorganic nitrate, nitrite, ammonia, inorganic phosphate and reactive silicate adopting the standard procedures described by [8].
Statistical analysis
Pearson - correlation coefficient analysis was performed between physico-chemical parameters of both the stations.
Results
For convenient, the study period was divided into 4 seasons viz., summer (April, May and June), premonsoon (July, August and September), monsoon (October, November and December) and post monsoon (January, February and March).
Rainfall
The rainfall was ranged from 16.76 mm to 745.76 mm for both the stations. No rainfall was recorded during the months of January 2010 and March 2011. The maximum rainfall of 745.76 mm was recorded during the monsoon season (November, 2010) and minimum of 16.76mm during summer season (June, 2011).
Water temperature
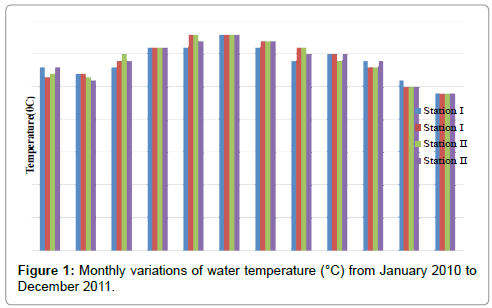
The water temperatures were ranged from 24 to 33°C for both the stations. Minimum water temperature was recorded in the month of December (2011 & 2011) and maximum in the months of May (2011) and June (2010 & 2011) in station I. In station II, minimum water temperature was recorded in the month of December (2010 & 2011) and maximum in the months of May (2011) and June (2010 & 2011) (Table 1 and Figure 1). In station I, temperature is positively correlated with salinity, pH and ammonia and negatively correlated with dissolved oxygen, nitrate, nitrite and reactive silicate. However, temperature is positively correlated with salinity and pH in station II (Tables 2 and 3).
| Seasons | Months | 2010 | 2011 | ||
|---|---|---|---|---|---|
| Station I | Station II | Station I | Station II | ||
| Post monsoon | January | 28 | 28 | 26.5 | 27 |
| February | 27 | 26 | 27 | 26.5 | |
| March | 28 | 29 | 29 | 30 | |
| Summer | April | 31 | 31 | 31 | 31 |
| May | 31 | 32 | 33 | 33 | |
| June | 33 | 33 | 33 | 33 | |
| Pre monsoon | July | 31 | 32 | 32 | 32 |
| August | 29 | 30 | 31 | 31 | |
| September | 30 | 30 | 30 | 29 | |
| Monsoon | October | 29 | 29 | 28 | 28 |
| November | 26 | 25 | 25 | 25 | |
| December | 24 | 24 | 24 | 24 | |
Table 1: Monthly variations of water temperature (°C) from January 2010 to December 2011.
| Parameters | Temp | Salinity | pH | DO | NO3 | NO2 | NH4 | IP | SiO3 |
|---|---|---|---|---|---|---|---|---|---|
| Temp | 1 | ||||||||
| Salinity | 0.8668** | 1 | |||||||
| pH | 0.8784** | 0.8163** | 1 | ||||||
| DO | -0.6106** | -0.744** | -0.6749** | 1 | |||||
| NO3 | -0.563* | -0.6866 | -0.5163* | 0.7155** | 1 | ||||
| NO2 | -0.5252* | -0.6493** | -0.4895 | 0.5959** | 0.8120** | 1 | |||
| NH4 | 0.7914** | 0.7258** | 0.6070** | -0.7096** | -0.7308** | -0.5265 | 1 | ||
| IP | 0.2247 | 0.2189 | 0.342 | -0.1846 | 0.2669 | 0.5107* | 0.1446 | 1 | |
| SiO3 | -0.6538** | -0.6125** | -0.5276* | 0.5971** | 0.6286** | 0.6198** | -0.6188** | 0.6543** | 1 |
*Correlation is significant at the 5% level (P<0.05)
**Correlation is significant at the 1% level (P<0.01).
Table 2: Correlation (r) values between physico-chemical parameters for station-I.
| Parameters | T | Salinity | pH | DO | NO3 | NO2 | NH4 | IP | SiO3 |
|---|---|---|---|---|---|---|---|---|---|
| T | 1 | ||||||||
| Salinity | 0.8265** | 1 | |||||||
| pH | 0.9354** | 0.7337** | 1 | ||||||
| DO | -0.5268 | -0.6936 | -0.4218 | 0.3471 | |||||
| NO3 | -0.8322 | -0.7388 | -0.7628 | 0.1747 | 1 | ||||
| NO2 | -0.8302 | -0.8608 | -0.6759 | -0.6507 | 0.8657** | 1 | |||
| NH4 | 0.3032 | 0.3806 | 0.4566* | -0.6439 | -0.2463 | -0.0542 | 1 | ||
| IP | 0.0907 | 0.2561 | 0.0642 | 0.4672* | 0.3805 | 0.0241 | 0.2025 | 1 | |
| SiO3 | -0.4772 | -0.3626 | -0.5237 | 0.086 | 0.7668** | 0.5431** | -0.2832 | 0.5590** | 1 |
*Correlation is significant at the 5% level (P<0.05).
**Correlation is significant at the 1% level (P<0.01).
Table 3: Correlation (r) values between physico-chemical parameters for station-II.
Salinity
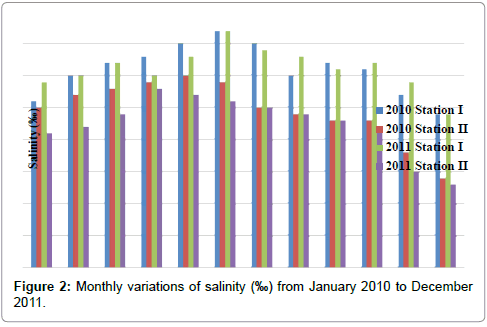
The salinity was ranged from 24 to 37‰ in station I and 13 to 30‰ in station II. Minimum salinity was recorded in the month of December (2010 & 2011) and maximum in the month of June (2010 & 2011) in station I. In station II, minimum salinity was recorded in the month of December (2011) and maximum in the month of May (2010) (Table 4 and Figure 2). In station I, salinity is positively correlated with pH and ammonia and negatively correlated with dissolved oxygen, nitrate and reactive silicate. In station II, salinity is positively correlated with pH (Tables 2 and 3).
| Seasons | Months | 2010 | 2011 | ||
|---|---|---|---|---|---|
| Station I | Station II | Station I | Station II | ||
| Post monsoon | January | 26 | 25 | 29 | 21 |
| February | 30 | 27 | 30 | 22 | |
| March | 32 | 28 | 32 | 24 | |
| Summer | April | 33 | 29 | 30 | 28 |
| May | 35 | 30 | 33 | 27 | |
| June | 37 | 29 | 37 | 26 | |
| Pre monsoon | July | 35 | 25 | 34 | 25 |
| August | 30 | 24 | 33 | 24 | |
| September | 32 | 23 | 31 | 23 | |
| Monsoon | October | 31 | 23 | 32 | 22 |
| November | 27 | 18 | 29 | 15 | |
| December | 24 | 14 | 24 | 13 | |
Table 4: Monthly variations of salinity (‰) from January 2010 to December 2011.
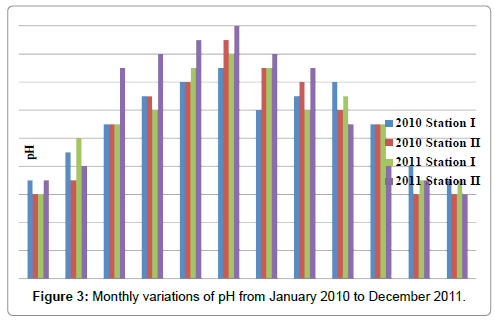
The pH was ranged from 7.4 to 8.4 in station I and 7.4 to 8.6 in station II. Minimum pH was recorded in the month of January (2011) and maximum in the month of June (2011) in station I. In station II, minimum pH was recorded in the months of November (2010), December (2010 & 2011) and January (2010) and maximum in the month of June (2011) (Table 5 and Figure 3). In station I, pH is positively correlated with ammonia and negatively correlated with dissolved oxygen, nitrite, nitrate and reactive silicate. In station II, pH is positively correlated with ammonia (Tables 2 and 3).
| Seasons | Months | 2010 | 2011 | ||
|---|---|---|---|---|---|
| Station I | Station II | Station I | Station II | ||
| Post monsoon | January | 7.5 | 7.4 | 7.4 | 7.5 |
| February | 7.7 | 7.5 | 7.8 | 7.6 | |
| March | 7.9 | 7.9 | 7.9 | 8.3 | |
| Summer | April | 8.1 | 8.1 | 8.0 | 8.4 |
| May | 8.2 | 8.2 | 8.3 | 8.5 | |
| June | 8.3 | 8.5 | 8.4 | 8.6 | |
| Pre monsoon | July | 8.0 | 8.3 | 8.3 | 8.4 |
| August | 8.1 | 8.2 | 8.0 | 8.3 | |
| September | 8.2 | 8.0 | 8.1 | 7.9 | |
| Monsoon | October | 7.9 | 7.9 | 7.9 | 7.6 |
| November | 7.6 | 7.4 | 7.5 | 7.5 | |
| December | 7.5 | 7.4 | 7.5 | 7.4 | |
Table 5: Monthly variations of pH from January 2010 to December 2011.
Dissolved Oxygen
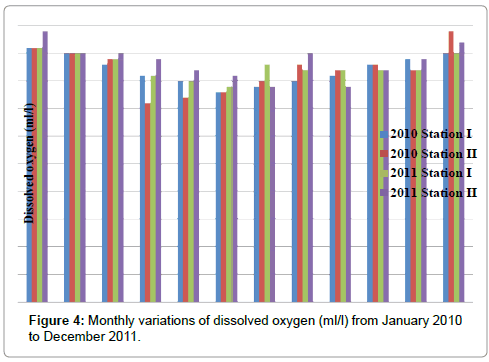
The DO was ranged from 3.8 to 4.6 ml/l in stationI and 3.6 to 4.9 ml/l in station II. The minimum DO was recorded in the month of June (2011) and maximum in the month of January (2010 & 2011) in station I. In station II, minimum DO was recorded in the month of April (2010) and maximum in the months of December (2010) and January (2011) (Table 6 and Figure 4). In station I, DO is positively correlated with nitrate, nitrite and reactive silicate and negatively correlatd with ammonia and inorganic phosphate. In station II, DO is positively correlated with inorganic phosphate (Tables 2 and 3).
| Seasons | Months | 2010 | 2011 | ||
|---|---|---|---|---|---|
| Station I | Station II | Station I | Station II | ||
| Post monsoon | January | 4.6 | 4.6 | 4.6 | 4.9 |
| February | 4.5 | 4.5 | 4.5 | 4.5 | |
| March | 4.3 | 4.4 | 4.4 | 4.5 | |
| Summer | April | 4.1 | 3.6 | 4.1 | 4.4 |
| May | 4.0 | 3.7 | 4.0 | 4.2 | |
| June | 3.8 | 3.8 | 3.9 | 4.1 | |
| Pre monsoon | July | 3.9 | 4.0 | 4.3 | 3.9 |
| August | 4.0 | 4.3 | 4.2 | 4.5 | |
| September | 4.1 | 4.2 | 4.2 | 3.9 | |
| Monsoon | October | 4.3 | 4.3 | 4.2 | 4.2 |
| November | 4.4 | 4.2 | 4.2 | 4.4 | |
| December | 4.5 | 4.9 | 4.5 | 4.7 | |
Table 6: Monthly variations of dissolved oxygen (ml/l) from January 2010 to December 2011.
Nitrite
The nitrite was ranged from 0.83 to 5.62 μM in station I and 0.97 to 4.62 μM in station II. Minimum nitrite was recorded in the month of May (2011) and maximum in the month of December (2011) in station I. In station II, minimum nitrite was recorded in the month of June (2010) and maximum in the month of December (2011) (Table 7 and Figure 5). In station I, nitrite is positively correlated with inorganic phosphate and negatively correlated with ammonia and reactive silicate. In station II, nitrite is positively correlated with inorganic phosphate and negatively correlated with ammonia (Tables 2 and 3).
| Seasons | Months | 2010 | 2011 | ||
|---|---|---|---|---|---|
| Station I | Station II | Station I | Station II | ||
| Post monsoon | January | 3.82 | 2.9 | 3.65 | 2.65 |
| February | 1.66 | 2.98 | 1.58 | 2.85 | |
| March | 1.01 | 2.1 | 1.21 | 2.06 | |
| Summer | April | 1.05 | 3.15 | 1.05 | 3.23 |
| May | 0.92 | 1.64 | 0.83 | 1.89 | |
| June | 1.1 | 0.97 | 1.95 | 1.25 | |
| Pre monsoon | July | 3.56 | 1.94 | 2.58 | 1.97 |
| August | 4.23 | 2.41 | 4.89 | 2.62 | |
| September | 1.86 | 2.89 | 3.64 | 2.57 | |
| Monsoon | October | 4.2 | 2.95 | 4.61 | 3.21 |
| November | 4.01 | 3.86 | 4.2 | 4.13 | |
| December | 5.42 | 4.29 | 5.62 | 4.62 | |
Table 7: Monthly variations of nitrite (μM) from January 2010 to December 2011.
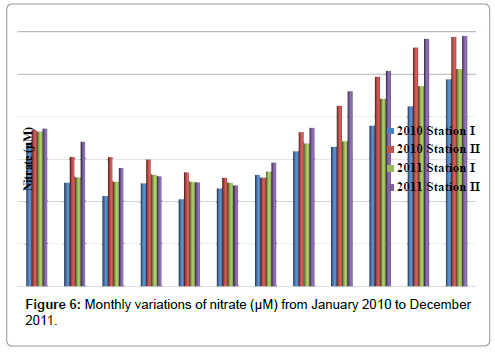
Nitrate
The nitrate was ranged from 10.3 to 25.6 μM in station I and 11.96 to 29.54 μM in station II. Minimum nitrate was recorded in the month of May (2010) and maximum in the month of December (2011) in station I. In station II, minimum nitrate was recorded in the month of June (2011) and maximum in the month of December (2011) (Table 8 and Figure 6). In stations I&II, nitrate is positively correlated with nitrite and reactive silicate (Tables 2 and 3).
| Seasons | Months | 2010 | 2011 | ||
|---|---|---|---|---|---|
| Station I | Station II | Station I | Station II | ||
| Post monsoon | January | 17 | 18.54 | 18.3 | 18.69 |
| February | 12.3 | 15.31 | 12.9 | 17.1 | |
| March | 10.7 | 15.28 | 12.4 | 14 | |
| Summer | April | 12.2 | 14.95 | 13.2 | 13.05 |
| May | 10.3 | 13.5 | 12.4 | 12.34 | |
| June | 11.6 | 12.84 | 12.3 | 11.96 | |
| Pre monsoon | July | 13.2 | 12.85 | 13.6 | 14.65 |
| August | 16.0 | 18.24 | 16.9 | 18.76 | |
| September | 16.5 | 21.36 | 17.2 | 23.0 | |
| Monsoon | October | 19 | 24.73 | 22.1 | 25.42 |
| November | 21.3 | 28.15 | 23.6 | 29.21 | |
| December | 24.4 | 29.41 | 25.6 | 29.54 | |
Table 8: Monthly variations of nitrate (µM) from January 2010 to December 2011.
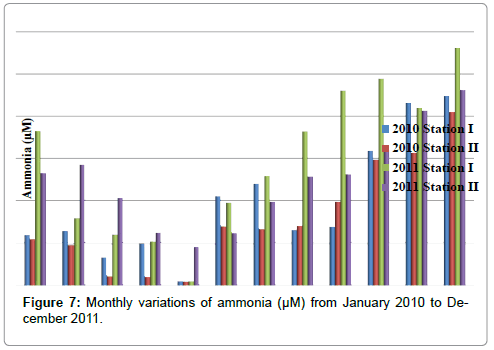
Ammonia
The ammonia was ranged from 0.11 to 5.62 μM in station I and 0.1 to 4.62 μM in station II. The minimum ammonia was recorded in the month of May (2010 & 2011) and maximum in the month of December (2011) in station I. In station II, minimum ammonia was recorded in the month of May (2010) and maximum in the month of December (2011) (Table 9 and Figure 7). In station I, ammonia is negatively correlated with reactive silicate (Table 2).
| k | Months | 2010 | 2011 | ||
|---|---|---|---|---|---|
| Station I | Station II | Station I | Station II | ||
| Post monsoon | January | 1.2 | 1.1 | 3.65 | 2.65 |
| February | 1.3 | 0.96 | 1.58 | 2.85 | |
| March | 0.67 | 0.23 | 1.21 | 2.06 | |
| Summer | April | 1.0 | 0.21 | 1.05 | 1.26 |
| May | 0.11 | 0.1 | 0.11 | 0.92 | |
| June | 2.1 | 1.41 | 1.95 | 1.25 | |
| Pre monsoon | July | 2.4 | 1.34 | 2.58 | 1.97 |
| August | 1.32 | 1.42 | 3.64 | 2.57 | |
| September | 1.4 | 1.97 | 4.61 | 2.62 | |
| Monsoon | October | 3.18 | 2.97 | 4.89 | 3.21 |
| November | 4.32 | 3.13 | 4.2 | 4.13 | |
| December | 4.48 | 4.1 | 5.62 | 4.62 | |
Table 9: Monthly variations of Ammonia (µM) from January 2010 to December 2011.
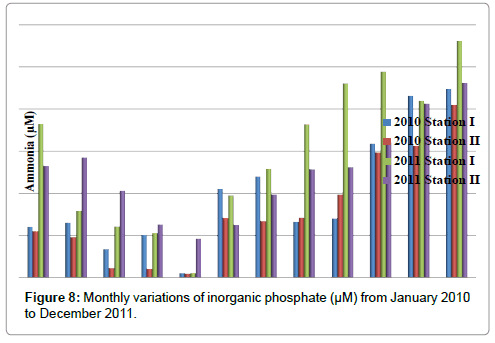
Inorganic Phosphate
The inorganic phosphate was ranged from 0.52 to 3.76 μM in station I and 0.63 to 4.19 μM in station II. Minimum inorganic phosphate was recorded in the months of March (2010) and May (2010) and maximum in the month of December (2011) in station I. In station II, minimum inorganic phosphate was recorded in the months of March (2011) and May (2010) and maximum in the month of December (2011) (Table 10 and Figure 8). In stations I&II, Inorganic phosphate positively correlated with reactive silicate (Tables 2 and 3).
| Seasons | Months | 2010 | 2011 | ||
|---|---|---|---|---|---|
| Station I | Station II | Station I | Station II | ||
| Post monsoon | January | 0.72 | 0.86 | 0.58 | 0.94 |
| February | 0.64 | 0.72 | 0.85 | 0.69 | |
| March | 0.52 | 0.69 | 1.92 | 0.63 | |
| Summer | April | 0.89 | 0.67 | 0.96 | 0.98 |
| May | 0.52 | 0.63 | 0.62 | 0.72 | |
| June | 2.63 | 1.96 | 2.45 | 2.31 | |
| Pre monsoon | July | 2.94 | 2.45 | 2.99 | 2.87 |
| August | 3.2 | 3.4 | 3.18 | 3.28 | |
| September | 2.13 | 2.13 | 2.12 | 1.97 | |
| Monsoon | October | 2.94 | 3.1 | 2.81 | 3.48 |
| November | 3.11 | 3.2 | 3.31 | 2.54 | |
| December | 3.64 | 4.12 | 3.76 | 4.19 | |
Table 10: Monthly variations of inorganic phosphate (μM) from January 2010 to December 2011.
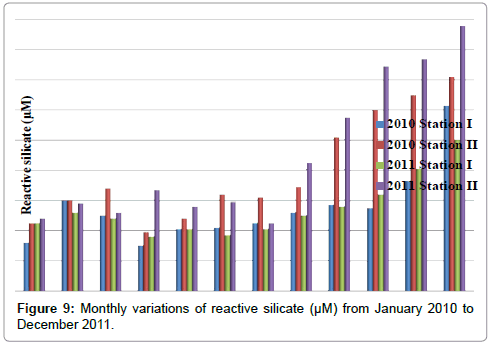
Reactive silicate
The reactive silicate was ranged from 30 to 123 μM in station I and 39 to 176 μM in station II. Minimum reactive silicate was recorded in the month of April (2010) and maximum in the month of December (2010) in station I. In station II, minimum reactive silicate was recorded in the month of April (2010) and maximum in the month of December (2011) (Table 11 and Figure 9).
| Seasons | Months | 2010 | 2011 | ||
|---|---|---|---|---|---|
| Station I | Station II | Station I | Station II | ||
| Post monsoon | January | 32 | 45 | 45 | 48 |
| February | 60 | 60 | 52 | 58 | |
| March | 50 | 68 | 48 | 52 | |
| Summer | April | 30 | 39 | 36 | 67 |
| May | 41 | 48 | 41 | 56 | |
| June | 42 | 64 | 37 | 59 | |
| Pre monsoon | July | 45 | 62 | 41 | 45 |
| August | 52 | 69 | 50 | 85 | |
| September | 57 | 102 | 56 | 115 | |
| Monsoon | October | 55 | 120 | 64 | 149 |
| November | 73 | 130 | 81 | 154 | |
| December | 123 | 142 | 100 | 176 | |
Table 11: Monthly variations of reactive silicate (µM) from January 2010 to December 2011.
Discussion
Rainfall is the most important cyclic phenomenon in tropical countries as it brings important changes in the hydrographical characteristics of the marine and estuarine environments. In the present study, the peak values of rainfall were recorded during the monsoon season of November. The rainfall in India is largely influenced by two monsoons viz., south west monsoon on the west coast, northern and north-eastern India and by the northeast monsoon on the southeast coast [9]. On the other side, the tidal rhythm, water current and evaporation in summer produced only little variation in those parameters or mostly stable in the absence of rainfall. Maruthanayagam [10] have also reported the occurrence of bulk of rainfall during northeast monsoon season along the southeast coast of India.
The temperature variation is one of the factors in the coastal and esturine system, which may influence the physico-chemical characteristics and turn influence the distribution and abundance of flora and fauna. In the present study, it has been observed that high temperature noticed in the months of May and June (summer) for both the stations. Lower temperature was noticed in the the month of December (monsoon). Generally, surface water temperature is influenced by the intensity of solar radiation, evaporation, freshwater influx and cooling and mix up with ebb and flow from adjoining neritic waters. The water temperature during December was low because of high rainfall, strong land sea breeze and precipitation. Similar observations have been already made by many scientists [11-13].
The salinity act as a major ecological factor in the distribution of living organisms and its variation caused by dilution and evaporation is most likely to influence the faunal distribution of the coastal ecosystems [14]. In the present study maximum salinity was recorded in summer especially in the months of May and June and minimum during monsoon particularly in the month of December for both the stations. In general the salinity was influenced by high temperatures of both atmospheric and water. During monsoon season, rainfall and freshwater inflow from the land were moderately reduced the salinity [12,13,15]. Changes in salinity and nutrient concentration play a major role in controlling the distribution of phytoplankton [16].
Hydrogen ion concentration (pH) in surface waters remained alkaline throughout the study period for both the stations with maximum value during the month of June (summer) and minimum during the months of November-January (monsoon and postmonsoon). Higher value of pH during summar was due to uptake of CO2 by photosynthesizing organisms [3,17]. The high summer pH might be due to the influence of sea water penetration as high biological activity [18] and due to influence of high photosynthetic activity [4]. The low pH observed during the months of November-January may be due to the influence of freshwater, dilution of sea water, low temperature and organic matter decomposition as suggested by Ganesan [19]. Smilar trend was already reported by Thangaraj [20] and Srinivasan [21] from Vellar estuarine system, Mathevan [22] from Cuddalore Uppanar waters and Ananthan [3] from Pondicherry coastal waters and Ananthan [13] from Chennai coastal waters.
It is well known that the temperature and salinity affect the dissolution of oxygen [23]. In the present investigation, a higher value of dissolved oxygen was recorded during the month of January (postmonsoon) and minimum during the months of April and June (summer) at both the stations. The higher values of dissolved oxygen might be due to the cumulative effect of higher wind velocity and the resultant freshwater mixing [6,17]. Thirunavukkarasu [12] attributed that seasonal variation of dissolved oxygen is due to freshwater flow and terrigenous input of sediments.
Nutrients are considered as one of the most important parameters in the aquatic environment by influencing the growth, reproduction and metabolic activities of living beings. Nutrients, more importantly the nitrate and silicate have emerged as the main factors controlling the phytoplankton growth [24]. Distribution of nutrients is mainly based on the season, tidal conditions and freshwater flow from land source. The higher nitrite value during monsoon season mainly in the month of December could be due to the increased planktonic excretion, oxidation of ammonia and reduction of nitrate and by recycling of nitrogen and also due to bacterial decomposition of plankton detritus present in the environment [18]. Further, the denitrification and air-sea interaction exchange of chemicals are also responsible for this increased value [2]. The low nitrite value recorded during summer season (May and June) may be due to less freshwater inflow and high salinity [5,13,22].
The highest nitrate value during monsoon season (December) could be mainly due to the organic materials received from the catchments areas during ebb tide [18,25]. The increased nitrate level was due to freshwater inflow and terrestrial run-off during the monsoon season [26]. Another possible way of nitrate entry is through oxidation of ammonia form of nitrogen to nitrite formation [6]. The low values during summer season mainly in the months of May and June could be due to its utilization by phytoplankton as evidenced by high photosynthetic activity and also due to the neritic water dominance, which contained only negligible amount of nitrate [5,13,18].
Higher concentration of ammonia was observed during the monsoon season for both the stations particularly in the month of December. Lower concentration of ammonia was observed during the summer season in both stations mainly in the month of May. The higher concentration could be partially due to the death and subsequent decomposition of phytoplankton and also due to the excretion of ammonia by plankton [3,4,12,27].
The high concentration of inorganic phosphates was recorded during monsoon (December) might possibly be due to intrusion of upwelling seawater, which in turn increased the level of phosphate [28]. Further, regeneration and release of total phosphorus from bottom mud into the water column by turbulence and mixing is also attributed to the higher monsoonal values [29]. Moreover, the weatherings of rock will soluble alkali metal phosphate, the bulk of which are carried into the coastal waters are also responsible for the higher values [30]. The low phosphate value recorded in summer season (May) could be attributed to the high utilization of phosphate by phytoplankton and also the variation may also be due to the processes like adsorption and desorption of phosphate and buffering action of sediment under varying environmental conditions [4].
In general silicate content was higher than other nutrients. Maximum values recorded in monsoon season mainly in the month of December may be due to heavy inflow of monsoonal fresh water derived from land drainage carrying silicate leached out from rocks. Further, due to the turbulent nature of water, the silicate from the bottom sediment might have been exchanged with overlying water [4,31]. The dissolution of particulate silicon carried by the river, the removal of soluble silicates by adsorption and co-precipitation of soluble silicon with humic compounds and iron [4] are some of the processes which might have caused the depletion of silicate during summer season mainly in the month of April. The low summer values could also be attributed to uptake of silicates by phytoplankton for their biological activity [5,12,32]. Hence, in the two different sampling stations is directly or indirectly were affected by sewage and manmade activities. There was a major problem of the species behavior, survival and diversity patterns. Therefore, in the study were indicated that, should be avoid polluted activities from the coastal regions.
References
- Karande AA (1991) Use of epifaunal communities in pollution monitoring. J Environ Biol 12: 191-200.
- Choudhury SB, Panigrahy RC (1991) Seasonal distribution and behavior of nutrients in the creek and coastal waters of Gopalapur, East coast of India. Mahasagar-Bull NatlInstOceanogr 24: 81-88.
- Ananthan G (1995) Plankton ecology and heavy metal studies in the marine environs of Pondicherry, India. Ph.D Thesis, Annamalai University, India.
- Sridhar RT, Thangaradjou T, Kumar SS, Kannan L (2006) Water quality and phytoplankton characteristics in the Palk Bay, southeast coast of India. J Environ Biol 27: 561-566.
- Soundarapandian P, Prem KT, Dinakaran GK (2009) Studies on the physico-chemical Characteristic and Nutrients in the Uppanar Estuary of Cuddalore, South East Cost of India. Curr Res J Biol Sci 1: 102-105.
- Rajasegar M (2003) Physico-chemical characteristics of the Vellar estuary in relation to shrimp farming. J Environ Biol 24: 95-101.
- Kathiresan K, Rajendran N, Thangadurai G (1996) Growth of a mangrove seedling in the intertidal area of Vellar estuary, Southeast coast of India. Indian J Mar Sci 25: 240-243.
- Strickland JD, Parsons TR (1972) A practical hand book of seawater analysis. BullFish Res Bed Canada167: 310.
- Perumal P (1993) The influence of meteorological phenomena on the ecosystems of a tropical region, Southeast coast of India. A case study. Ciencias Marinas 19: 343-351.
- Maruthanayagam C, Subramanian P (1999) Hydrological and zooplankton biomass variation in Palk Bay and Gulf of Mannar along the East coasts of India. J Mar BiolAssIndia41: 7-18.
- Karuppasamy PK, Perumal P (2000) Biodiversity of zooplankton at Pichavaram mangroves, south east coast of India. Adv Biosci 19: 232-32.
- Thirunavukkarasu KB, Gunalan B, Soundarapandian P, Kumaran R, Anand T, et al. (2011) Studies on the physico-chemical characteristic and nutrients in the Kottakudi estuary of Thirupulani, Ramanathapuram-District, South East Coast of India. AquacultAquConser leg Internat J BioflusSoc 4: 313-319.
- Ananthan G, Soundarapandian P, Sankar S (2012) Hydrological and diversity of phytoplankton composition and community structure in Chennai coastal water. Recent advances in Biodiversity of India, Mumbai. Zool Sur India 169-183.Â
- Chandramohan P, Sreenivas N (1998) Diel variation in zooplankton populations in mangrove ecosystem at Gaderu canal, Southeast coast of India. Indian J Mar Sci27: 486-488.
- Balasubramanian B, Kannan L (2005) Physico-chemical characteristics of the coral reef environs of Gulf of Mannar Biosphere Reserve India. Int J Ecol Environ Sci 31: 285-271.
- Paniadima A, Kannapiran E, Vareethiah K (2006) Seasonal variation of phytoplankton in Chinnathurai coast along southwest of India. J Mar Bio Ass India 48: 161-165.
- Paramasivam S, Kannan L (2005) Physico-chemical characteristics of Pichavaram mangrove, south east coast of India. Int J EcolEnv Sci 31: 273-278.
- Govindasamy C, Kannan L, Jayapaul A (2000) Seasonal variation in physico-chemical properties and primary production in the coastal water biotopes of Coromandel coast. Indian J Environ Biol21: 1-7.
- Ganesan MD (1992) Ecobiology of seaweeds of the Gulf of Mannar with special reference to hydrography of heavy metals. Ph.D., Thesis Annamalai University, India.
- Thangaraj GS (1984) Ecology of the marine zone of the Vellar estuary, Ph.D. Thesis, Annamalai University, India. 1-192.
- Srinivasan A, Santhanam R, Krishnamurthi M, Jegadeesan G (1998) Physico-chemical and seasonal distribution of planktonic Tintinnids of Pullavazhi estuary, south east coast of India. Indian J Mar Sci 17: 131-137.
- MathevanP (1994) Hydrological investigations on the inter-tidal diotomsof the Cuddalorre-Uppanar estuary (India).Ph.D, thesis, Annamalai University, India. 1-153.
- Vijayakumar S, Rajan KM, Mridula MR, Hariharan (2000) Seasonal distribution and behaviour of nutrients with reference to tidal rhythm in the Mulki estuary, Southwest coast of India. J Mar Biol Ass India42: 21-23.
- Panigrahy RC, Sangeeta M, GouriS, Mohanty AK (2006) Seasonal distribution of phytoplankton in the surf waters off Gopalpur, East coast of India. J Mar Biol Ass India 48: 156-160.
- Das J, Das SN, Sahoo RK (1997) Semidiurnal variation of some physico-chemical parameters in the Mahanadi estuary, East coast of India. Indian J Mar Sci26: 323-326.
- Santhanam P, Perumal P (2003) Diversity of zooplankton in Parangipettai coastal waters, southeast coast of India. J Mar Biol Ass India 45: 144-151.
- Segar K, Hariharan V (1989) Seasonal distribution of nitrite, ammonia and plankton in effluent discharges area of Mangalore, West coast of India. Indian J Mar Sci18: 170-173.
- Nair NB, Aziz PK, Dharmaraj K, Arunachalam M, Krishnakumar K, et al. (1983) Ecology of Indian estuaries. V. Primary Productivity of the Ashtamudi estuary, Southwest coast of India. Proc Indian Aca Sci93: 9-23.
- Chandran R, Ramamoorthi K (1984) Hydrobiological studies in the gradient zone of the Vellar estuary. I. Physico-chemical parameters. Mahasagar-Bulletin NatlInstOceanog17: 69-78.
- Gowda G, Gupta TR, Rajesh KM, Gowda H, Lingadhal C, et al. (2001) Seasonal distribution of phytoplankton in Nethravathi estuary. Mangalore. J Mar BiolAss India 42: 31-40.
- Govindasamy C, Kannan L (1996) Rotifers of Pichavaram mangroves, Southeast coast of India. Indian Hydrobiol1: 69-76.
- Sujatha Mishra D Panda,Panigraphy RC (1993) Physico-chemical characteristics of the Bahuda estuary (Orissa), East coast of India. Indian J Mar Sci22: 75-77.
Citation: Thirunavukkarasu K, Soundarapandian P, Varadharajan D, Gunalan B (2014) Physico-Chemical Characteristics of Kottakudi and Nari Backwaters, South East of Tamil Nadu. J Earth Sci Clim Change 5: 199. DOI: 10.4172/2157-7617.1000199
Copyright: © 2014 Thirunavukkarasu K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15035
- [From(publication date): 7-2014 - Sep 21, 2024]
- Breakdown by view type
- HTML page views: 10582
- PDF downloads: 4453