Research Article Open Access
Shelf Life and Chemical Stability of Calcium Phosphate Coatings Applied to Poly Carbonate Urethane Substrates
Dunstan H Barnes1*, Ravin Jugdaosingh2, Sinan Kiamil3 and Serena M Best11Cambridge Centre for Medical Materials, Department of Materials Science & Metallurgy, University of Cambridge, Cambridge, UK
2MRC Human Nutrition Research, Cambridge, UK
3Ranier Technology Limited, Cambridge, UK
- Corresponding Author:
- Prof. SM Best
Cambridge Centre for Medical Materials
Department of Materials Science & Metallurgy
University of Cambridge, Cambridge, UK
Tel: +44 (0) 1223334300
Fax: +44 (0)1223334567
E-mail: smb51@cam.ac.uk
Received date: Juner 03, 2011; Accepted date: September 26, 2011; Published date: September 28, 2011
Citation: Barnes DH, Jugdaosingh R, Kiamil S, Best SM (2 011) Shelf Life and Chemical Stability of Calcium Phosphate Coatings Applied to Poly Carbonate Urethane Substrates. J Biotechnol Biomaterial 1:112. doi:10.4172/2155-952X.1000112
Copyright: © 2011 Barnes DH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Many modern orthopaedic implants are coated with calcium phosphate to improve the host tissue response to the implants. Despite numerous studies on the dissolution properties of calcium phosphate (CaP) coatings, the coating “shelf life” (i.e. the chemical stability of these coatings prior to implantation), has not been investigated adequately, particularly for CaP coatings applied to polymeric substrates. This paper examines the chemical stability of CaP coatings that were applied to a poly carbonate urethane (PCU) substrate by an aqueous, low temperature technique. Applying a CaP coating to the PCU surface is designed to enhance the fixation between the implant and adjacent vertebrae. High temperature coating techniques are unfeasible for polymeric substrates, like PCU, because the high temperature may critically degrade the substrate. The CaP coating shelf life was assessed by using FTIR-ATR and SEM to analyze changes in CaP-coated PCU discs over time. The expected in vivo chemical stability of the CaP coating was measured by immersing CaP-coated PCU discs in cell culture media under physiological conditions. ICP-OES was used to measure the calcium and phosphorus concentrations of the immersion solution.
Keywords
Chemical stability; Shelf life; Calcium phosphate; CaP coatings; Poly carbonate urethane; PCU; FTIR-ATR; ICP-OES
Introduction
Calcium phosphate (CaP) coatings are applied to the surface of a range of orthopaedic implants to enhance the interfacial bonding between the implant and host tissue. Hydroxyapatite, a type of calcium phosphate that is similar to the mineral component of bone, is the most commercially popular calcium phosphate coating for biomedical implants [1]. Hydroxyapatite and other calcium phosphate coatings have been investigated extensively for clinical applications due to their ability to form a direct bond with living bone, a property often referred to as “bioactivity” [2]. To provide a tangible benefit in vivo, CaP coatings should exhibit sufficient chemical stability to remain adhered to the substrate. Numerous prior studies have investigated the dissolution of CaP coatings, such as hydroxyapatite, in conditions designed to simulate physiological conditions, i.e. typically at pH 7.4 and at ~37 °C [3-7]. However, most prior studies have focused on a narrow time range of one week or less [4-6]. Further, prior studies have generally focused on simulating physiological rather than storage conditions, meaning that the “shelf life” of CaP coatings has not been investigated adequately despite the wide range and large number of CaP-coated orthopaedic implants in existence. The “shelf life” may be considered the period of time that CaP coatings remain sufficiently chemically stable and sufficiently well-adhered to the underlying substrate after coating formation but prior to implantation.
This paper focuses on the chemical stability of CaP coatings applied to poly carbonate urethane substrates. Polyurethanes have been used extensively in biomedical applications [8-12] and make excellent materials for implantable medical devices due to their high tensile strength, lubricity, abrasion resistance, formability, and good biocompatibility [13-15]. Polyurethane elastomers offer enhanced mechanical properties and improved durability compared to silicon elastomers [16]. However, the use of poly ether urethane and poly ester urethane implants has been limited by their long-term instability in biological environments [17,18]. In the early 1990s, poly carbonate urethanes attracted attention for biomaterial applications because they exhibited enhanced oxidative stability and similar mechanical properties compared to poly ester and poly ether urethanes [13,19]. Further, poly carbonate urethanes have demonstrated better biocompatibility than poly ether urethanes during In vitro tests [20].
Polymers used in orthopaedic applications may require bioactive coatings to encourage bonding between the polymer and host tissue. Miyazaki and Ohtsuki et al. [21] found that coating-substrate bonding could be improved by including carboxyl (-COOH) groups in the polyamide substrate, because the carboxyl groups provided heterogeneous nucleation sites for the formation of CaP coatings during immersion in Simulated Body Fluid (SBF) [21,22].
This paper consists of two parts: (1) a 75-day shelf life pilot study designed to examine the influence of moisture and pH on the chemical stability of the CaP coatings in simulated pre-implantation storage conditions and (2) a 14-day In vitro immersion study conducted to assess the chemical stability of the CaP coatings in a simulated in vivo physiological environment.
Materials
The solid substrates used in this paper were poly carbonate ure-thane (PCU) discs of diameter 10.0 mm and thickness 1.1 mm manufactured by Ranier Technology Limited. The particular PCU studied was developed as endplate material for an artificial spinal disc implant. It contains carboxyl (-COOH) groups, which were incorporated in the PCU to enhance the formation of calcium phosphate coatings on the PCU surface [21].
The PCU discs were coated with calcium phosphate (CaP) by immersion in a concentrated coating solution for five hours at 28°C. A low temperature coating technique was used because temperatures above 150°C can degrade PCUs [23], severely compromising their mechanical properties and rendering PCUs unusable as a load-bearing implant. The concentrated coating solution was produced by dissolving reagent grade chemicals (Sigma Aldrich, 99 %+) in deionised water. The solution was adapted from Kokubo’s Simulated Body Fluid (SBF) [24]. To significantly increase the rate of CaP deposition, the calcium and phosphate ionic concentrations of the solution were increased to approximately ten times their typical concentration in Kokubo’s SBF.
Methods
Cap coating analysis
Scanning electron microscopy (SEM) and Fourier Transform Infra- Red–Attenuated Total Reflectance (FTIR-ATR) were used to examine the PCU substrate and CaP coatings.
SEM provided a means to examine the surface microstructure and morphology of the PCU substrate and CaP coatings. A JEOL 5800-LV microscope equipped with INCA software was used to collect micrographs at an accelerating voltage of 10-15 kV. Samples were sputter coated with a thin layer of palladium (Pd) for 90 seconds at 30-40 mA prior to SEM analysis. At least three discs from each sample group were examined, with numerous micrographs collected from different points on the disc surface.
A Perkin Elmer Spectrum One FTIR Spectrometer was used in direct contact mode to analyse the chemical bonds present near the surface of uncoated and CaP-coated PCU disc samples. The transmittance of infra-red waves was measured between 4000 and 510 cm-1 at a resolution of 4 cm-1. Sample and background spectra were a mean of 8 scans. At least three points on the surface were analysed for each sample, and at least two samples were analysed per sample group.
Shelf life study
To determine the shelf life, CaP-coated PCU disc samples were stored in separate wells in 24-well culture plates (Nunc, Thermo Fisher Scientific, UK) at three different temperatures (15, 37 and 75°C) under five different conditions:
• “dry” samples were stored out of solution adjacent to a quantity of silica desiccant;
• “humid” samples were stored out of solution in an atmosphere of approximately 80 % relative humidity (RH);
• in 1 mL potassium hydrogen phthalate (KHC8H4O4) solution at pH 4.0, in a humidified atmosphere (80 % RH);
• in 1 mL potassium dihydrogen phosphate (KH2PO4) and disodium hydrogen phosphate (Na2HPO4) solution at pH 7.0, in a humidified atmosphere (80 % RH);
• in 1 mL sodium bicarbonate (NaHCO3) and sodium carbonate (Na2CO3) solution at pH 10.0, in a humidified atmosphere (80 % RH).
The buffers used for pH 4.0, 7.0 and 10.0 were selected due to their known buffering abilities.
PCU discs stored at 15 and 37°C were removed from the buffers after 700 and 1800 hours (30 and 75 days). PCU discs stored at 75°C were removed from the buffers after 700 hours (30 days). PCU discs removed from the buffers were carefully rinsed in 100 mL deionised water after removal. FTIR-ATR analysis was used in conjunction with SEM to assess whether CaP coatings were present on the surface of the PCU discs.
In vitro immersion study
McCoy’s 5A solution without phenol red (GIBCO, Invitrogen, UK) supplemented with 10 % fetal bovine serum (FBS; GIBCO, Invitrogen, UK), 1 % penicillin-streptomycin-glutamine (PSG; GIBCO, Invitrogen, UK) and 30 μg/mL L-ascorbic acid phosphate Mg salt n-hydrate (Wako Chemicals GmbH, Germany) was used for the In vitro immersion study. This “McCoy’s 5A supplemented media” was sterile-filtered before the start of the study.
PCU discs were immersed in 5 mL McCoy’s 5A supplemented media in 13 mL sealable polypropylene vials (Sarstedt, UK). The vials were maintained in a humidified atmosphere (80 % RH) at 37°C and 5 % CO2 during the study. At approximately 1, 4, 24, 96, 168 and 336 hours, three PCU discs were removed from their immersion solution and transferred to individual wells of a 24-well plate in preparation for FTIR-ATR and SEM analysis. The corresponding immersion solutions were refrigerated at 4°C prior to total elemental analysis.
Total elemental analysis for calcium and phosphorus was carried out on the immersion solutions collected at the different time points (1-336 hours) by inductively coupled plasma - optical emission spectroscopy (ICP-OES) on a Jobin-Yvon JY2000-2 (Instrument S.A., Longjumeau, France) equipped with a concentric nebuliser and cyclonic spray chamber. The sample flow rate was 1 mL/min. The analytical lines used were 315.887 nm for calcium and 214.914 nm for phosphorus. Peak profiles were used, with a window size of 0.08 nm (0.04 nm either side of the peak) with 21 increments per profile and an integration time of 0.5 second per increment. Standard solutions (0-100 ppm) were prepared in McCoy’s 5A supplemented media from standard ICP solutions (1000 ppm; BDH Ltd) and ran alongside the immersion solutions. All the immersion solutions were analysed in a single run, with blank solutions of McCoy’s 5A supplemented media interspersed in-between every six of the immersion solutions. In addition, one of the standard solutions was interspersed in-between every 20 of the immersion solutions to monitor instrument drift during the analysis. pH was measured for each time point using a calibrated pH meter (Hanna Instruments).
Results and Discussion
CaP coating analysis
Figure 1(a) shows a typical FTIR-ATR spectrum from the surface of an uncoated PCU disc sample, with vibration bands that are consistent with poly carbonate urethane [25,26]. When the PCU substrate was coated with calcium phosphate (CaP), the bands associated with PCU were “suppressed” or “masked” by the CaP coating and bands associable with phosphate bonds appeared, as indicated on Figure 1(b). Since the FTIR-ATR bands associated with CaP were nearly identical for different CaP phases (data not shown), FTIR-ATR analysis could not be used to distinguish between different CaP phases; however, FTIR-ATR spectra could be used to assess whether there was a CaP coating present on the surface of PCU disc samples, because CaP-coated PCU substrates exhibited recognisably different FTIR-ATR spectra compared to uncoated PCU substrates.
`Figure 2(a) and 2(b) show SEM micrographs of uncoated poly carbonate urethane (PCU) disc samples. The “bumps” on the surface of uncoated PCU samples in (a) are from the material’s surface microtexture.
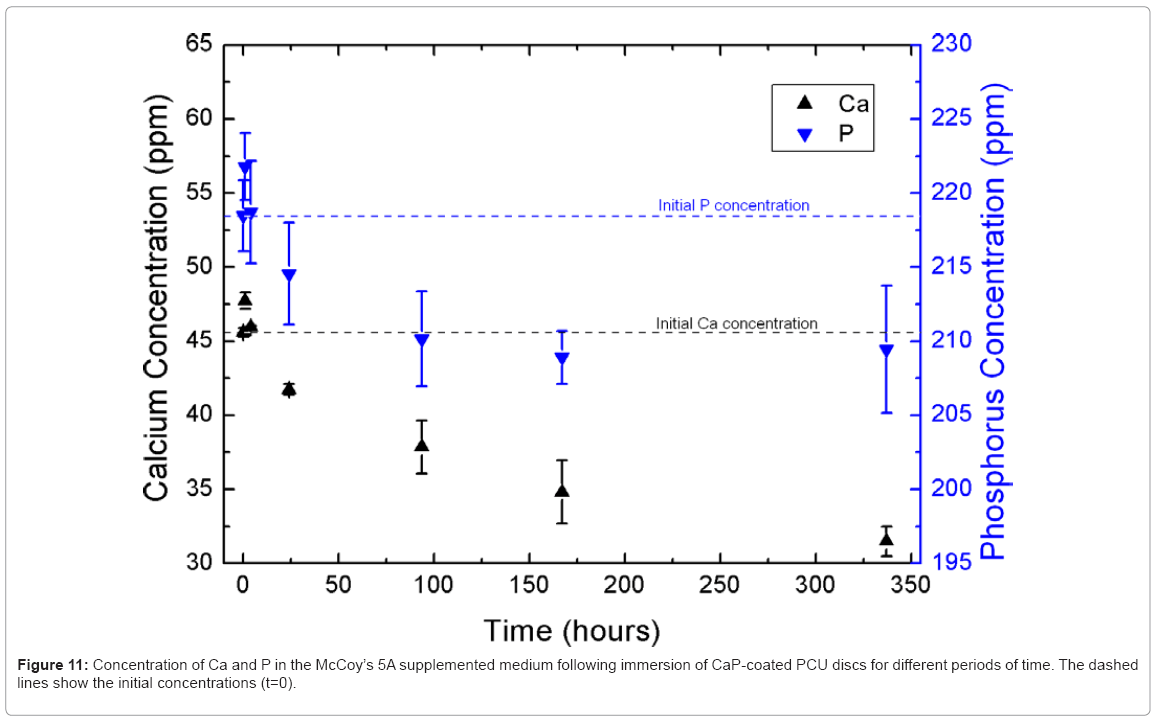
Figure 2: SEM micrographs from the surface of uncoated (a)–(b) and CaP-coated (c)–(e) PCU disc samples at different magnifications. The PCU surface microtexture is visible in (a). The CaP coating exhibited both a fine scale apatite structure and a block-like monetite structure, which were both visible at lower magnification in (c). At higher magnification, the fine scale apatite can be seen in (d), whereas the block-like monetite can be seen in (e).]
SEM micrographs can be used to qualitatively identify CaP phases based upon their morphology. Apatite coatings deposited by an aqueous low-temperature method typically have a fine scale microstructure containing submicron-sized apatite crystals, which are observable in results reported by Liu and Hunziker [27], Kokubo and Takadama [28]. Brushite (DCPD) crystals tend to be plate-like and micron-sized with crystal lengths and widths much greater than their thicknesses [29]. Monetite (DCPA) crystals tend to have a more block-like than platelike morphology when compared to brushite crystals [30,31]. The typical morphologies of brushite and monetite crystals are distinct from the typical morphology of apatite crystals [30,32,33].
CaP-coated PCU disc samples are shown in Figure 2(c)–(e). The CaP coating was composed primarily of two phases (Figure 2(c)), which appeared to be apatite and monetite. A region that exhibited an apatite microstructure is shown in higher magnification in Figure 2(d), and a region that exhibited a block-like monetite structure is shown in higher magnification Figure 2(e).
Shelf life study
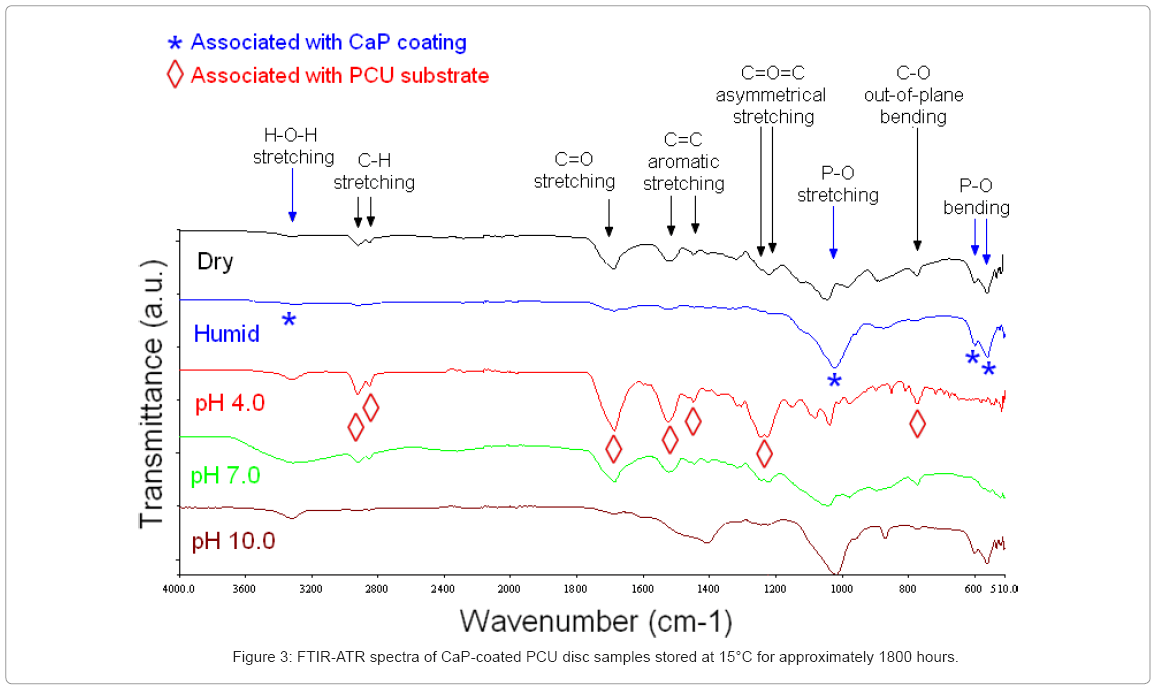
As described in Methods, CaP-coated PCU discs were stored under five different conditions (dry, 80 % RH, in pH 4.0 buffer plus 80 % humidity, in pH 7.0 buffer 80 % RH, and in pH 10.0 buffer 80 % RH) and at three different temperatures (15°C, 37°C, and 75°C) for up to 1800 hours (75 days). Thereafter, samples were rinsed in deionised water and subjected to FTIR-ATR and SEM analysis.
The CaP coatings on PCU disc samples stored at 75°C had significantly degraded after 700 hours, so it was deemed unnecessary to continue the study for these samples for the full 1800 hours.
15°C: Surface FTIR-ATR spectra of the CaP-coated PCU discs stored for 1800 hours at 15°C in pH 4.0 buffer solution exhibited the characteristic bands of the underlying PCU substrate, suggesting that after 1800 hours the CaP coating had dissolved substantially (Figure 3).
CaP-coated PCU discs stored in pH 7.0 buffer exhibited similar FTIR-ATR bands to the discs stored at pH 4.0, except that (1) the bands were less intense, and (2) additional broad bands were observed at 3300 cm-1, associable with adsorbed water, and at 1040 cm-1, associable with asymmetrical P–O stretching. The fact that P–O bending was not observed on these CaP-coated PCU discs suggested that the CaP coatings on these samples were amorphous. Further, the broadening and slight shifting of the bands suggested that the CaP coatings were either poorly crystalline or had poor stoichiometry [26]. Although these FTIR-ATR spectra suggested that the CaP coating was at least partially present after 1800 hours in pH 7.0 buffer, the PCU substrate was also detected.
The CaP-coated PCU discs stored under dry conditions exhibited similar FTIR-ATR bands to the disc samples stored in pH 4.0 buffer, except that the bands were again less intense, and additional bands were seen at 1040 cm-1, associable with asymmetrical P–O stretching and at 600 and 565 cm-1, associable with P–O bending. This suggested that both the CaP coating and the PCU substrate were being detected.
The CaP-coated PCU disc samples stored under a humid atmosphere (80 % relative humidity, RH), or in pH 10.0 buffer, exhibited FTIR-ATR bands consistent with a CaP coating. Bands associated with the PCU substrate were not observed, which suggested that the CaP coating remained intact on the surface of these discs. Discs stored in pH 10.0 buffer also showed a band at 1400 cm-1 with a broad shoulder at approximately 1450 cm-1, attributable to C–O asymmetrical stretching (n3) of carbonate [26]. This suggested that carbonate-substituted CaP had precipitated from the buffer solution, which contained HCO3- and CO32- ions.
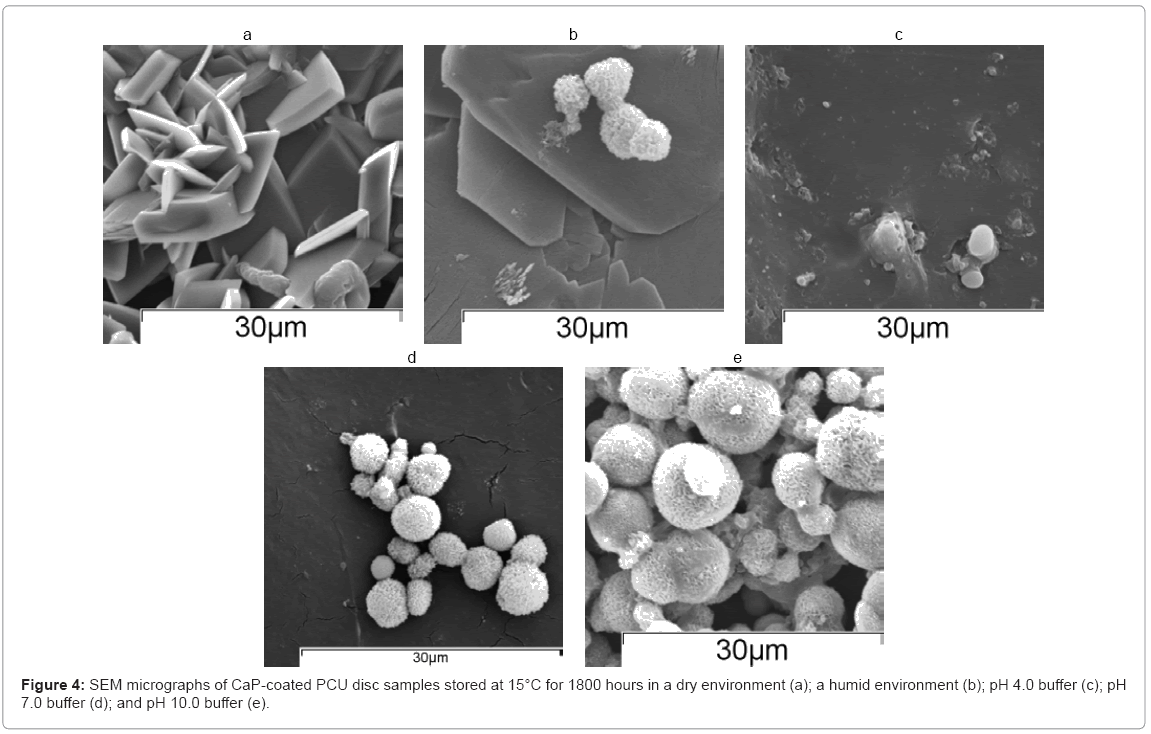
As shown in Figure 4(a), CaP-coated PCU discs stored under dry conditions showed a microstructure representative of monetite. Samples stored in a humidified atmosphere (80 % RH) exhibited small apatite clusters (Figure 4(b)). As shown in Figure 4(c), SEM analysis of CaP-coated PCU discs stored in pH 4.0 buffer suggested that there was no CaP coating present, in agreement with the FTIR-ATR spectra. The samples stored in pH 7.0 buffer showed a typical CaP microstructure, Figure 4(d), but also showed regions with no coating present, which matched with the finding from FTIR-ATR analysis that showed bands associated with both the PCU substrate and the CaP coating. CaP-coated PCU disc samples stored in pH 10.0 buffer showed typical apatite morphology (Figure 4(e)).
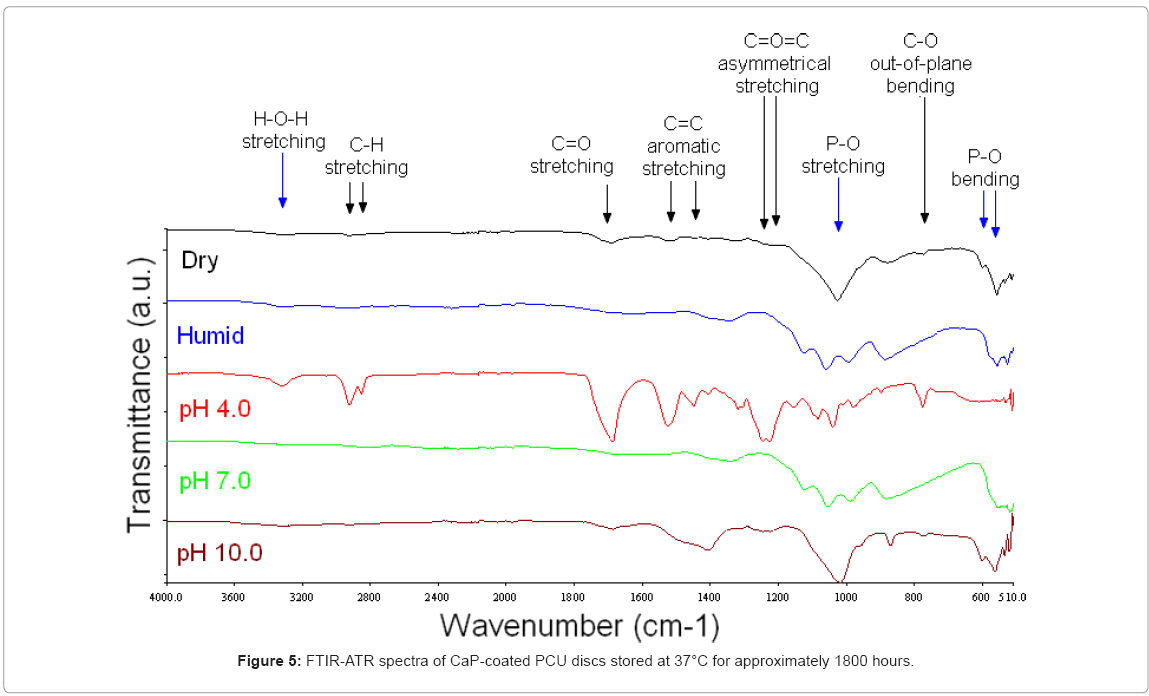
37°C: FTIR-ATR analysis of samples stored at 37°C in pH 4.0 buffer in a humidified atmosphere for 1800 hours suggested that the CaP coating had dissolved substantially. The spectral bands were representative of the underlying substrate, as shown in Figure 5.
In contrast, some of the CaP coating was still present on the PCU disc surface after storage for 1800 hours in pH 7.0 buffer in a humidified atmosphere. As shown in Figure 6, these discs exhibited a band at 1030 cm-1 with a well-defined shoulder at 1110 cm-1, associated with asymmetrical P–O stretching, and bands at 960 cm-1 and 900 cm-1, associated with symmetrical P–O stretching.
Similarly, samples stored under dry conditions at 37°C exhibited FTIR-ATR spectra representative of the CaP coating. The spectra contained a well-defined intense band at 1020 cm-1 with a shoulder from 1100–1020 cm-1 associable with asymmetrical P–O stretching, a band at 960 cm-1 associated with symmetrical P–O stretching and a band at 560 cm-1 with a shoulder at 600 cm-1 associated with asymmetrical P–O bending.
The FTIR-ATR spectra indicated that a CaP coating was present on the PCU discs after 1800 hours at 37°C in pH 10.0 buffer in a humidified atmosphere. There were no peaks attributable to the PCU substrate. An intense band at 1020 cm-1 and a broad shoulder from 1100–1020 cm-1 was associable with asymmetrical P–O stretching. A band at 960 cm-1 was associable with symmetrical P–O stretching. The double band at 600 and 560 cm-1 was associable with asymmetrical P–O bending. A band at 1400 cm-1 with a broad shoulder at approximately 1450 cm-1 was associable with the C–O asymmetrical stretching of carbonate.
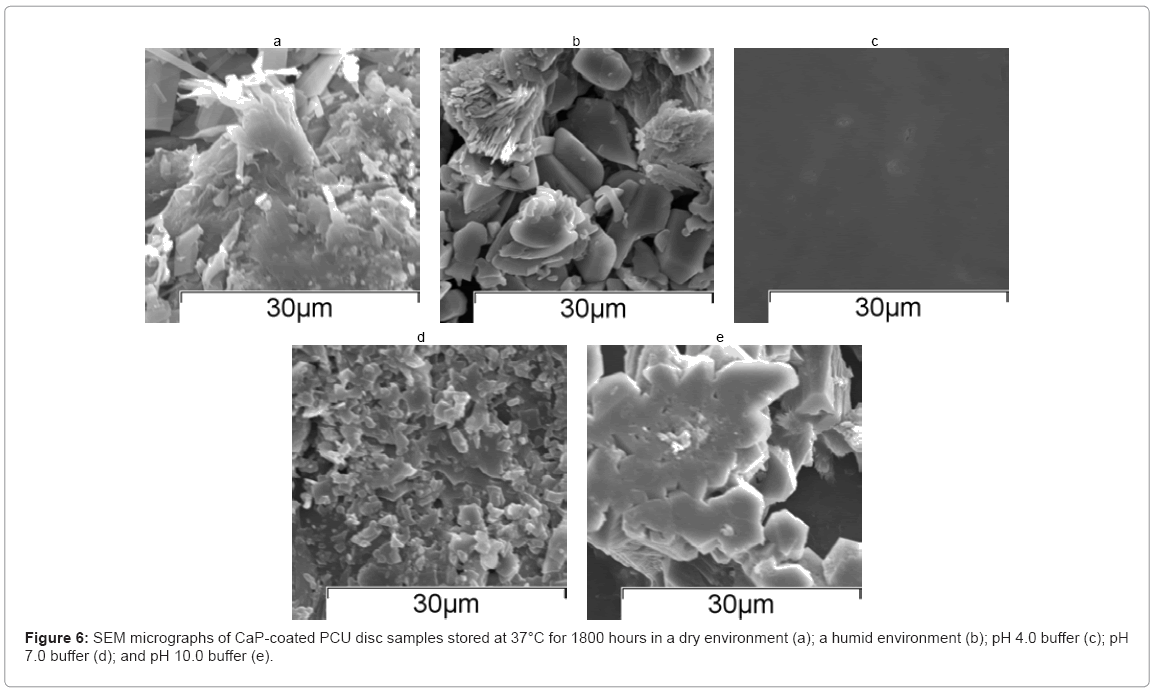
The associated SEM micrographs of discs stored at 37°C for approximately 1800 hours are shown in Figure 6(a)–(e). The samples stored in pH 4.0 buffer showed no evidence of a CaP coating after 1800 hours, suggesting that the coating had fully dissolved. Samples from each of the other four sample groups showed evidence of a CaP coating. The discs stored under dry or humid conditions exhibited a microstructure representative of mixture of monetite and apatite, whereas the discs stored in pH 7.0 buffer or pH 10.0 buffer exhibited a microstructure representative of apatite.
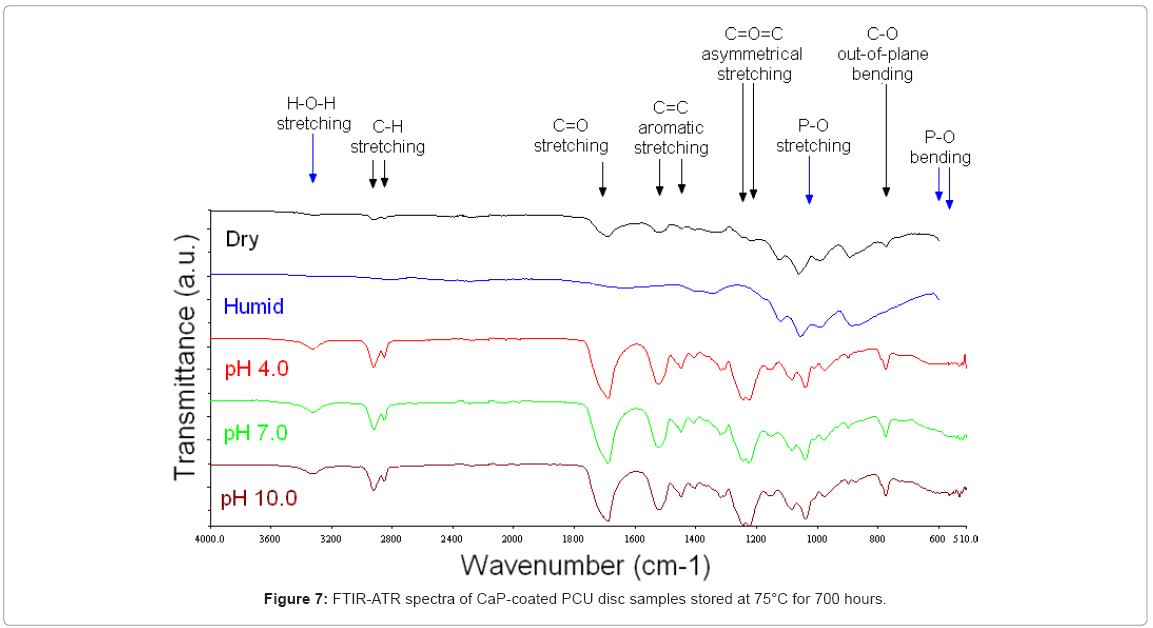
75°C: Figure 7 shows FTIR-ATR spectra from discs stored at 75°C for 700 hours. After 700 hours of storage at 75°C, all the samples stored in the buffers, pH 4.0, pH 7.0 and pH 10.0, exhibited FTIR-ATR spectra associated with the underlying substrate. There were no bands associated with a CaP coating. The FTIR-ATR spectra were representative of the PCU substrate, as shown by the C–H stretching double band at 2910 and 2840 cm-1, the C=O stretching band at 1680 cm-1, the C=C aromatic stretching bands at 1510 and 1440 cm-1, the C=O=C asymmetrical stretching bands at 1230 and 1210 cm-1, and the C–O out-ofplane bending band at 770 cm-1. Samples stored under dry and humid (80 % RH) conditions showed spectra associated with a CaP coating. The band at 1030 cm-1 with a well-defined shoulder at 1110 cm-1 was associated with asymmetrical P–O stretching, and the band at 960 cm-1 was associated with symmetrical P–O stretching. This suggested that a CaP coating was still present on these samples. In addition, under dry conditions, discs exhibited minor bands attributable to the PCU substrate, such as the band at 1680 cm-1 associated with C=O stretching.
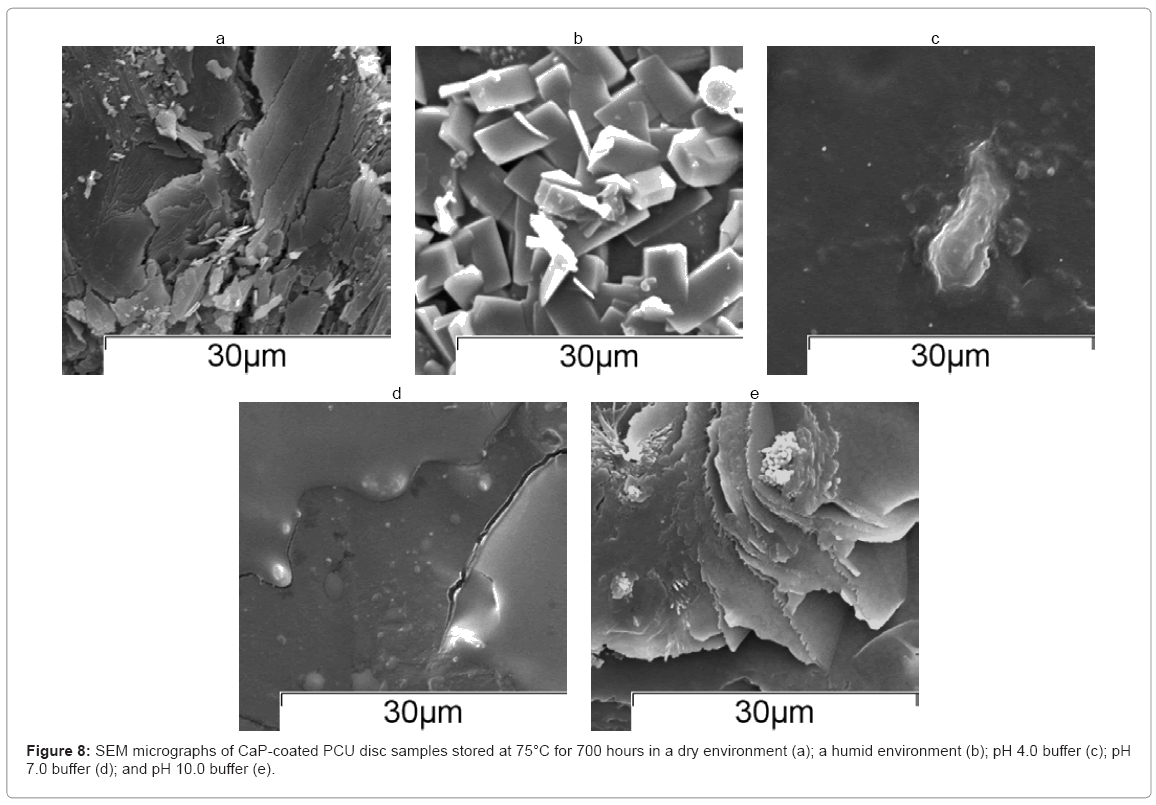
The SEM micrographs of the discs stored at 75°C for approximately 700 hours are shown in Figure 8(a)–(e). A damaged CaP coating was visible on the surface of discs stored under dry conditions, which could explain why minor bands attributable to the PCU substrate were visible by FTIR-ATR analysis. Discs stored under humid conditions (80 % RH) showed a block-like microstructure, representative of monetite. Discs stored in pH 4.0 and pH 7.0 buffer showed no evidence of a CaP coating after 700 hours, which suggested that the CaP coating had fully dissolved, in agreement with FTIR-ATR analysis. Samples stored at pH 10.0 showed a flower-like morphology which could be a phase of CaP, but was certainly not the original CaP coating.
In vitro immersion study
The In vitro immersion study was designed to simulate post-, rather than pre-, implantation conditions, to assess how chemically stable the CaP coatings would be after implantation.
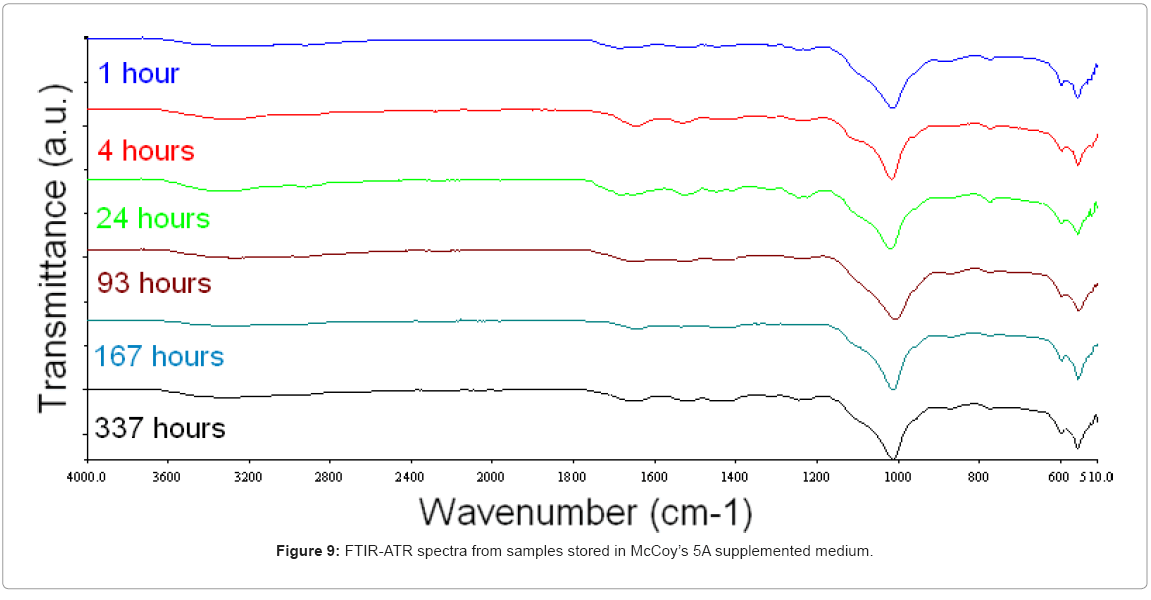
The CaP-coated PCU discs stored in McCoy’s 5A supplemented medium under cell culture conditions (37°C; 5 % CO2) exhibited the same FTIR-ATR bands throughout the period of immersion (0–337 hours), which suggested that the CaP coating remained intact and undissolved (Figure 9). Each FTIR-ATR spectra contained a well-defined intense band at 1020 cm-1 with a shoulder from 1100–1020 cm-1 associable with asymmetrical P–O stretching, and a double band at 600 and 560 cm-1 associated with asymmetrical P–O bending.
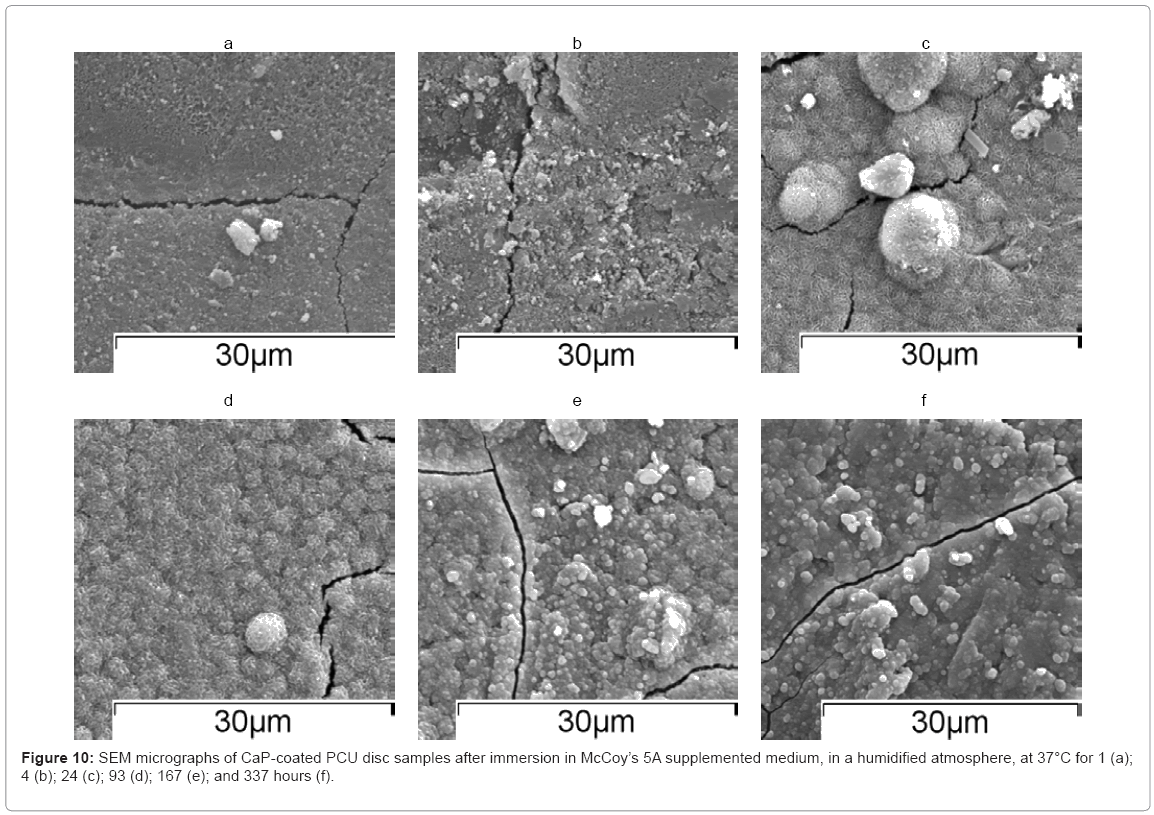
SEM micrographs of discs showed an apatite morphology at each time point (Figure 10(a)–(e)). This confirmed that a CaP coating was present on the surface of the PCU substrates throughout the immersion study. Moreover, there may have been further deposition of CaP from the medium onto the disc surface. Because the microstructure exhibited an apatite structure at each of the time points after immersion (1–337 hours), it appeared that any monetite present at the start of immersion (0 hours) dissolved during the first hour of immersion.
Figure 11 shows the change in calcium and phosphorus ion concentrations in McCoy’s 5A supplemented medium as a function of time, as measured by ICP-OES. Within the first hour of immersion, there was an increase in the concentrations of calcium and phosphorus in the immersion medium, which suggested that calcium and phosphorus had dissolved from the discs. Because monetite is more soluble than apatite, is reasonable to hypothesise that some or all of the monetite present on the surface of the discs dissolved during this period. However, from 4 hours onwards, the calcium and phosphorus concentrations dropped markedly below their initial concentrations in the immersion medium, which suggested that calcium and phosphorus were being lost from the immersion solution. Based on the SEM micrographs, it is likely that calcium and phosphorus were re-precipitating as apatite on the surface of the discs. The CaP coating present on the disc surface may have provided nucleation sites for CaP to deposit onto the disc surface.
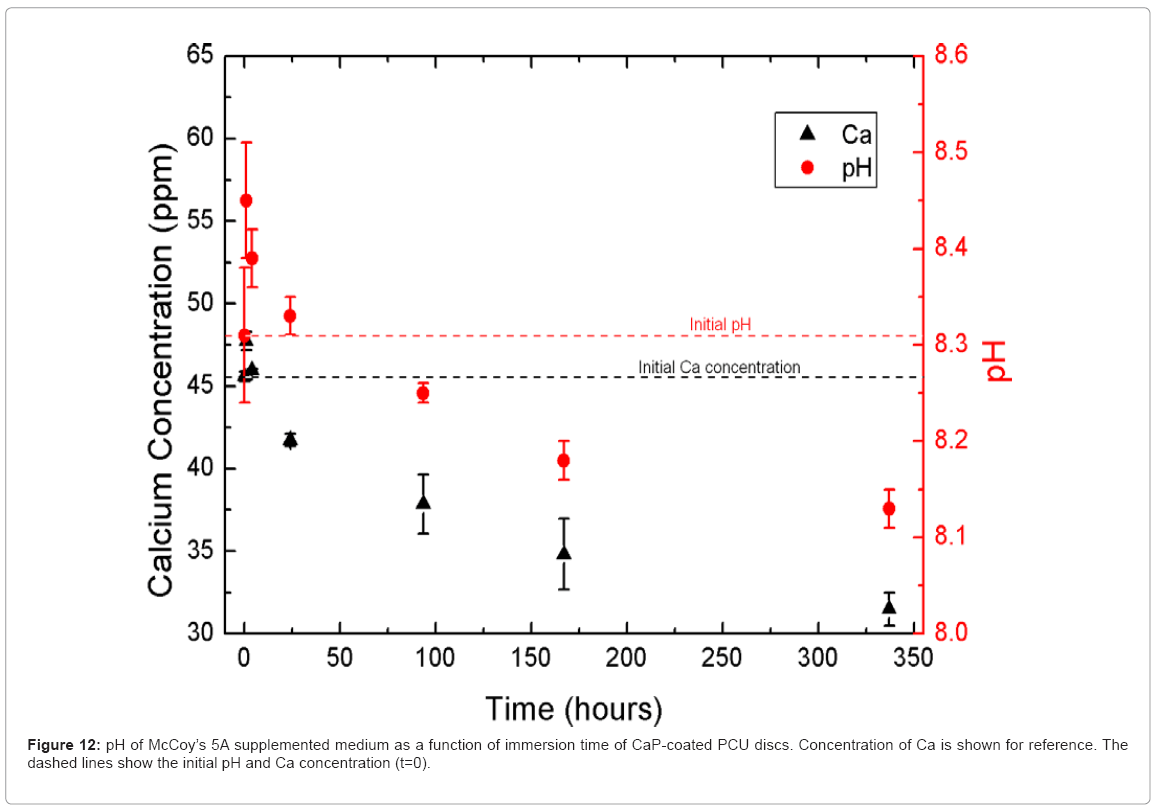
Figure 12 shows the change in pH of the immersion medium as a function of time after incubation of the CaP-coated PCU discs. The pH followed the same trend as the calcium and phosphorus ion concentrations. The release of calcium and phosphorus into the immersion medium caused an increase in the pH of the immersion solution, but then the pH dropped as calcium and phosphorus were lost from solution during re-precipitation of CaP on the surface of the discs.
Conclusions
The results of the shelf life study suggested that calcium phosphate coatings on PCU substrates are most stable prior to implantation when stored under a humid, but not fluid, environment (80 % relative humidity) at 15 or 37°C. The CaP coatings substantially dissolved under acidic conditions (pH 4.0), and did not appear to be stable at 75°C. Based on the results of this study, the shelf life of the CaP-coated PCU discs may be extended by storing the samples at ambient temperatures in a humid or semi-humid environment, but not in an extremely wet (fluid) or dry (desiccated) environment. It was hypothesised that the CaP coatings stored in a dry (desiccated) environment cracked more extensively as they dried out. It is believed that some degree of humidity is required to maintain CaP coatings intact on the surface of PCU discs, although this may require further research
Immersion of the discs in McCoy’s 5A supplemented medium in cell culture conditions at 37°C and 5 % CO2 showed that the CaP coating remained attached to the PCU substrate throughout the period of immersion (0–14 days). Moreover, there was evidence of further deposition of calcium and phosphorus from the immersion medium onto the disc surface. It is hypothesised that the monetite (DCPA) initially present in the CaP coating dissolved within the first hour of immersion in the immersion medium, due to its instability at pH above 7.0 [34]. This increased the pH of the immersion solution, and may have initiated the deposition of calcium and phosphorus (as CaP) on the disc surface. Further, the CaP coating may have provided nucleation sites for the re-precipitation of CaP, which caused a measured drop in both the calcium and phosphorus concentrations and the pH of the immersion medium. Whether the further deposition of CaP on the disc surface provides an increase in the osteoconductivity or bioactivity of the disc needs to be investigated.
Acknowledgements
This work was generously supported by the Engineering and Physical Sciences Research Council (EPSRC) and Ranier Technology Ltd., Cambridge, U.K. The authors are also grateful to Ranier Technology for providing poly carbonate urethane substrate material.
References
- Lacefield WR (1998) Current status of ceramic coatings for dental implants. Implant Dent 7: 315-322.
- Hench LL (1991) Bioceramics: From Concept to Clinic. J Am Ceram Soc 74: 1487-1510.
- Lo WJ, Grant DM (1999) Hydroxyapatite thin films deposited onto uncoated and (Ti, Al, V) N-coated Ti alloys. J Biomed Mater Res Part A 46: 408-417.
- Sepulveda P, Jones JR, Hench LL (2002) In vitro dissolution of melt-derived 45S5 and sol-gel derived 58S bioactive glasses. J Biomed Mater Res Part A 61: 301-311.
- Mavropoulos E, Rossi AM, da Rocha NCC, Soares GA, Moreira JC, et al. (2003) Dissolution of calcium-deficient hydroxyapatite synthesized at different conditions. Mater Characterization 50: 203-207.
- Wang J, Layrolle P, Stigter M, De Groot K (2004) Biomimetic and electrolytic calcium phosphate coatings on titanium alloy: physicochemical characteristics and cell attachment. Biomater 25: 583-592.
- Boyd AR, Meenan BJ, Leyland NS (2006) Surface characterisation of the evolving nature of radio frequency (RF) magnetron sputter deposited calcium phosphate thin films after exposure to physiological solution. Surf & Coat Technol 200: 6002-6013.
- Lelah MD, Cooper SL (1986) Polyurethanes in Medicine. CRC Press.
- Gogolewski S (1991) In vitro and in vivo molecular stability of medical polyurethanes: A review. Trends in Polym Science, 1: 47-61.
- Zhao Q, Topham N, Anderson JM, Hiltner A, Lodoen G, et al. (1991) Foreignbody giant cells and polyurethane biostability: in vivo correlation of cell adhesion and surface cracking. J Biomed Mater Res 25: 177-183.
- Stokes K, McVenes R, Anderson JM (1995) Polyurethane elastomer biostability. J Biomater Applications 9: 321-354.
- Bergeron M, Lévesque S, Guidoin R (2001) Biomedical Applications of Polyurethanes. In Vermette P, Griesser HJ, Laroche G, and Guidoin R, eds., Biomedical Applications of Polyurethanes pp. 220-251 Eurekah.com.
- Pinchuk L (1994) A review of the biostability and carcinogenicity of polyurethanes in medicine and the new generation of 'biostable' polyurethanes. J Biomater Sci Polym Ed 6: 225-267.
- Christenson EM, Dadsetan M, Wiggins M, Anderson JM, et al. (2004) Poly(carbonate urethane) and poly(ether urethane) biodegradation: In vivo studies. J Biomed Mater Res 69: 407-416.
- Wiggins MJ, MacEwan M, Anderson JM, Hiltner A (2004) Effect of soft segment chemistry on polyurethane biostability during In vitro fatigue loading. J Biomed Mater Res. Part A 68: 668-683.
- Szycher M, McArthur WA (1985) Surface fissuring of polyurethanes following in vivo exposure. In Fraker AC and Griffin CD, eds., Corrosion and Degradation of Implant Mater: Second Symposium pp. 308-320. ASTM International, American Society for Testing and Materials.
- Szycher M (1988) Biostability of polyurethane elastomers: a critical review. J Biomater Applications 3: 297-402.
- Tang YW, Labow RS, Santerre JP (2001) Enzyme-induced biodegradation of polycarbonate polyurethanes: Dependence on hard-segment concentration. J Biomed Mater Res Part A 56: 516-528.
- Tanzi MC, Mantovani D, Petrini P, Guidoin R, Laroche G (1997) Chemical stability of polyether urethanes versus polycarbonate urethanes. J Biomed Mater Res Part A 36: 550-559.
- Hsu SH, Kao YC, Lin ZC (2004) Enhanced Biocompatibility in Biostable Poly(carbonate)urethane. Macromol Biosci 4: 464-470.
- Miyazaki T, Ohtsuki C, Akioka Y, Tanihara M, Nakao J, et al. (2003) Apatite deposition on polyamide films containing carboxyl group in a biomimetic solution. J Mater Sci: Mater in Med 14: 569-574.
- Kawai T, Ohtsuki C, Kamitakahara M, Hosoya K, Tanihara M, et al. (2007) In vitro apatite formation on polyamide containing carboxyl groups modified with silanol groups. J Mater Sci Mater in Med 18: 1037-1042.
- Eceiza A, Martin MD, De La Caba K, Kortaberria G, Gabilondo N, et al. (2008) Thermoplastic polyurethane elastomers based on polycarbonate diols with different soft segment molecular weight and chemical structure: Mechanical and thermal properties. Polym Eng & Sci 48: 297-306.
- Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T (1990) Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J Biomed Mater Res 24: 721-734.
- Socrates G (1994) Infrared Characteristic Group Frequencies. John Wiley & Sons, second ed.
- Feddes B, González P, Serra J, Pou J, Chiussi S, et al. (2009) Characterization of Thin Calcium Phosphate Coating. In León B and Jansen J, eds., Thin Calcium Phosphate Coatings for Medical Implants pp. 25-66. Springer.
- Liu Y, Hunziker E (2009) Biomimetic Coatings and Their Biological Functionalization. In León B and Jansen J, eds., Thin Calcium Phosphate Coatings for Medical Implants, pp. 301-314. Springer.
- Kokubo T, Takadama (2006) H. How useful is SBF in predicting in vivo bone bioactivity? Biomater 27: 2907-2915.
- Arifuzzaman SM, Rohani S (2004) Experimental study of brushite precipitation. J Crystal Growth 267: 624-634.
- Asaoka N (1996) Calcium Phosphate Coatings for Biomed Application. Master's thesis, Interdisciplinary Research Centre in Biomed Mater, UK.
- Prado da Silva MH, Lima JHC, Soares GA, Elias CN, De Andrade MC, et al. (2001) Transformation of monetite to hydroxyapatite in bioactive coatings on titanium. Surf & Coat Technol 137: 270-276.
- Barrere F, Van Blitterswijk C, De Groot K, Layrolle P (2002) Influence of ionic strength and carbonate on the Ca-P coating formation from SBF×5 solution. Biomater 23: 1921-1930.
- Müller L, Müller FA (2006) Preparation of SBF with different content and its influence on the composition of biomimetic apatites. Acta Biomaterialia 2: 181-189.
- Elliott JC (1994) Structure and Chemistry of the Apatites and Other Calcium Orthophosphates. Elsevier.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 16414
- [From(publication date):
October-2011 - Sep 22, 2024] - Breakdown by view type
- HTML page views : 11909
- PDF downloads : 4505