Special Issue Article Open Access
Effects of Biostimulation and Bioaugmentation on The Degradation of Pyrene in Soil
| Ghaly AE*, Yusran A and Dave D | |
| Department of Process Engineering and Applied Science, Dalhousie University Halifax, Nova Scotia, Canada | |
| Corresponding Author : | Abdel Ghaly Professor Department of Process Engineering and Applied Science Dalhousie University Halifax Nova Scotia, Canada Tel: (902) 494-6014 E-mail: abdel.ghaly@dal.ca |
| Received: March 10, 2013; Accepted: June 26, 2013; Published: June 28, 2013 | |
| Citation: Ghaly AE, Yusran A, Dave D (2013) Effects of Biostimulation and Bioaugmentation on The Degradation of Pyrene in Soil. J Bioremed Biodeg S7:005. doi:10.4172/2155-6199.S7-005 | |
| Copyright: © 2013 Ghaly AE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Petroleum based products have been widely used as a source of energy for centuries. One of the main petroleum hydrocarbons of concern is Polycyclic Aromatic Hydrocarbon (PAHs) which include pyrene. Pyrene is a four ring PAHs commonly found in soils contaminated with petroleum based products. The effectiveness of the addition of nutrient (biostimulation) and Mycobacterium sp. (bioaugmentation) on biodegradation of pyrene in soil was evaluated. The addition of nutrients (biosimulation) and microorganisms (bioaugmentation) increased the number of viable cells over the control during the bioremediation process. The cell number increased by 40, 70, 59 and 132 fold for the control, biosimulation, bioaugmentation and combined bioaugmentation, respectively. A lag period of 0.5 d and a specific growth rate of 0.896 d-1 were observed with the combined biosimulation-bioaumentation treatment. The temperature results showed that the temperature of the combined biostimulation-bioaugmentation reached 41°C after two days of treatment while the maximum temperature of the control, biostimulation and bioaugmentation were within the range of 28-32°C. The moisture content decreased for all treatments reaching 45-57% but remained within the optimum range of 40-60% for bioremediation process. This was due to the fact that the moisture lost in the exhaust gas was not compensated by the water produced as a by-product of the organic matter degradation. The level of pyrene degradation was indicated by the decline in O2 concentration and the increase in CO2 concentration in the exhaust gas. The control, biostimulation and bioaugmentation treatments showed similar patterns of decreasing O2 and increasing CO2. However, the combined biostimulation-bioaugmentation treatment recorded a declining trend in O2 concentration and increasing trend in CO2 concentration in the exhaust gas at the beginning of experiment (first 7 days) followed by increasing trend in O2 concentration and decreasing trend in CO2concentration in the next 8 days of the experiment. The highest pyrene reduction in percentage (84.29%) was obtained through the combined bioaugmentation-biostimulation process followed by bioaugmentation (57.86%), biostimulation (50%) and control (37%) processes. Different pyrene degradation rates were observed during the various phases of microbial growth (lag, exponential and stationary) of combined bioaugmentation-biostimulation treatment.
| Keywords |
| Pyrene; Bioremediation; Bioaumentation; Biostimulation; Combined bioaugmentation-biostimulation; Nitrogen; Phosphorus |
| Introduction |
| The use of petroleum based products is very widespread in our modern society. Automobiles are powered by gasoline, homes are heated with oil and industrial and manufacturing activities derive energy from various hydrocarbon fuels. The transport of petroleum products by sea, rail, tanker trucks and pipelines is common and unavoidable. Releases of petroleum hydrocarbons to the environment occur during their extraction, processing, transportation, storage and use which often result in a serious and widespread contamination of the environment (soil, water and air) threatening human health and the economy [1-3]. |
| Polycyclic Aromatic Hydrocarbons (PAHs) are one group of the petroleum hydrocarbons that have been classified as hazardous organic chemicals [4]. PAHs are found in high concentrations on many abandoned industrial sites, particularly those associated with the petroleum production and wood preserving industries. They have also been introduced into the environment from leaking underground storage facilities, accidental spills and industrial processing [5-7] and as a result of pyrolysis or incomplete combustion of organic materials [8-11]. PAHs concentrations in soil samples from industrial countries, particularly in urban areas, have been found to increase over the last 100 years as a result of burning of fossil fuels [12,13]. |
| These compounds are of grave environmental and health concerns because of their toxic, carcinogenic and mutagenic properties [5,14,15]. The US Environmental Protection Agency (EPA) listed 16 PAHs as priority pollutants, 6 of them are known to be carcinogenic and the rest are either mutagenic or toxic [15,16]. Accumulation of PAHs in soil is of great concern because of their effect on soil microorganisms and the potential health risk from consuming the contaminated plants [17-19]. There is no specific amount or level of exposure from contaminated soil with PAHs that can be used as limits regarding health risk because the identification of most hazardous PAHs and the way they affect health is still uncertain compared to the recorded health risk from exposure to PAHs pollution in air [19]. Workers exposed to PAHs are known to be prone to scrotal cancer [20] and high level of PAHs exposure can also lead to lung cancer, prostate cancer and kidney cancer [20-22]. As the rapidity and range of environmental and health problems are increased, so does the need to remove PAHs from the environment. However, the current physico-chemical and biological treatment methods may not be effective in removing the contaminants to the safe level and may results in toxic by-products [23,24]. |
| Bioremediation is defined as a process in which microorganisms are used to degrade or transform contaminants to less toxic or nontoxic forms [3,25]. Microorganisms metabolize hazard wastes and toxic materials to environmentally friendly end products (usually carbon dioxide, water and biomass).The bioremediation technique has advantages over thermal and physical-chemical techniques in terms of being more environmentally friendly, cost effective and requires minimal site disruption and less energy [5]. Microorganisms require appropriate environmental conditions to successfully reduce, eliminate or transform contaminants presents in different media [24]. Although comprehensive information on the bacterial, fungal and algae metabolism of low molecular weight PAHs are presented in a number of reviews [26-28], the degradation of high molecular weight PAHs is less studied and poorly understood. |
| There was a believe that PAHs with four or more aromatic rings are considered resistant to microbial degradation and the low solubility or bioavailability of high molecular weight PAHs may cause a possible limitation in the degradation rate by dissolution and desorption [29,30]. So far, very few pure cultures (Rhodococcus sp. and Mycobacterium sp.) were found to utilize pyrene as sole carbon source [26,31,32]. However, Mycobacterium species such as Mycobacterium Vanbaalenii PYR-1 and Mycobacterium flavescens were able to degrade pyrene only when inorganic nutrients were supplemented in the medium [26,28,33-35]. Fungi are also known to be able to degrade pyrene and its derivative, benzo (a) pyrene [36]. |
| Kim et al. [34] reported that the degradation of pyrene is influences by environmental factors such as oxygen concentration, water solubility and pH value. Inorganic content in soil such as nitrogen and phosphorus can affect the rate of bioremediation activities as these nutrients are needed by microorganisms for cellular metabolism and growth [37]. Although the soil contains its own mineral nutrients, microbial metabolisms will deplete the source rapidly due to high concentration of organic carbon. Therefore, addition of mineral nutrients to the soil is usually carried out for an in situ bioremediation of site [37]. The optimum ratio of carbon: nitrogen: phosphorus (C:N:P) in the soil bioremediation activities is 100:10:1 [5,38-41]. |
| The aim of this study was to evaluate the effects of biosimulation and bioaugmentation on the degradation of pyrene in contaminated soil and associate microbial population. |
| Experimental apparatus |
| Figure 1 shows the bioremediation system used in the study. It included a bioreactor, mixing unit, an aeration unit, temperate measurement unit, data-logger and computer. |
| The bioreactor was constructed of 6.4 mm thick stainless steel. The sides of the bioreactor measured 340 mm×280 mm with a radius of 150 mm at the lower end. The top of the bioreactor (340 mm×800 mm) was held in place by 4 hinges placed on one side which allowed for the closing and opening of the top. Four locking clamps were provided on the other side to ensure easy and adequate locking of the top cover when the bioreactor was in operation. The top cover and end walls of the bioreactor were insulated with 25.4 mm thick styrofoam. The sides of the bioreactor were insulated by means of a 10 mm thick air space between double walls and a 25.4 mm thick layer of styrofoam placed on the outer wall of the reactor. A rubber gasket lining was used to prevent air leakage from the bioreactor during operation. |
| Inside the reactor, a 6.4 mm diameter solid stainless steel shaft was mounted on two bearings. There were 5 stainless steel collars on the shaft in which five bolts (101.6 mm long and 6.4 mm in diameter each) were mounted. The shaft was rotated (0-250 RPM) by a ¾ HP variable speed electric motor (Model No. 2Z846, Dayton, Ohio, USA). A speed controller (Model No. 60648, Dayton, Ohio, USA) controlled the speed of the motor. |
| There were three holes at the bottom of the reactor which were connected to a manifold by 6.4 mm diameter tygon tubing and used for aeration. An air flow meter (FM082-03G, Cole Parmer, Montreal, Quebec, Canada) was used to measure the airflow rate. The top cover had three 60 mm holes which were used as sampling ports. They were covered during operation with rubber stopper. |
| Temperature measurements were taken using ten thermocouples. The thermocouples T1-T3 were used to measure the temperature of the center of the contaminated soil. The thermocouples T4-T6 were located on the front wall of the reactor while thermocouples T7-T9 were located on the back wall of the reactor. The thermocouple T10 was used to measure the ambient air temperature. All thermocouples were linked to a datalogger (Model No. 525, Syscon International, South Bend, Indiana, USA) which was connected to a microcomputer. |
| Experimental Materials |
| Soil collection and preparation |
| The soil was obtained from a commercial farm in Truro, Nova Scotia, 100 km from Halifax. The top vegetation/trash cover of the soil was scraped away. The top 50 cm of the soil was removed with a shovel. The soil was placed in a heavy duty polyethylene bags. Each bag was filled with about 50 kg of soil and sealed. The bags were transported from the collection site to the Waste Management Laboratory in the Department of Process Engineering and Applied Science, Dalhousie University, Halifax, Nova Scotia. The soil was washed 3 times with water to minimize the level of nutrients in the soil. The soil nitrogen and phosphorus contents were measured. The soil characteristics are presented in Table 1. According to Wilson and Jones [5], the optimum moisture content of soil for PAH degradation is between 30 and 70%. In this experiment the moisture content was adjusted at 60% of the dry weight of soil. |
| Pyrene collection and preparation |
| The chemical formulae and structure of pyrene are shown in Table 2. Pyrene was bought from SIGMA (Oakville, Ontario, Canada) in a powder form in a 100 mg container. The direction of preparing the liquid form given by the supplier was followed. According to Tsai et al. [42], diesel contains approximately 7% pyrene. Diesel contamination level of 10g diesel/kg soil is commonly encountered in hydrocarbon contaminated soils [43,44]. Pyrene was, therefore, added to the soil at a rate of 700 mg of pyrene per kg of oil. The required amount of pyrene was estimated to be 29.4g (37.3mL). |
| Nutrients |
| The nutrients added to the soil included carbon, nitrogen, phosphorus and micronutrients. Used cooking oil was used to provide bio-available carbon as recommended by Alkoaik and Ghaly [45] and Ghaly et al. [3]. It was obtained from local fast food restaurant from Halifax, Nova Scotia. Urea [CO(NH2)2] was added as nitrogen source to adjust the C:N ratio to 30:1. The choice of urea was based on the work by Ghaly and Pyke [46], Alkoaik and Ghaly [45] and Ghaly et al. [3], which indicated that it is an effective source of nitrogen in initiating and maintaining intense respiratory activity. It was purchased from Halifax Seed Company in Halifax Nova Scotia. Na2H2PO4 was used as a source of phosphorus to adjust the N:P ratio to 4:1. The choice of Na2H2PO4 was based on the work of Leys et al. [47]. The micronutrients added to the soil were K, Na, Cl, Mg, S, Ca and Fe. The composition of the supplemental nutrient solution is shown in Table 3. This was based on the work by Liebeg and Curtright [48]. The chemical reagents used to prepare the nutrient solutions were obtained from Fisher scientific (Ottawa, Ontario, Canada). |
| Bulking material |
| Wood shavings were used as the bulking agent for this process. The choice of wood shavings was based on the work of Ghaly et al. [44]. They were collected from a wood lumber mill in Sackville, Nova Scotia in small polyethylene bags (~5 kg). |
| Experimental Procedure |
| Inoculum preparation |
| Mycobacterium vanbaalenii was used in this experiment. It is a non-native microorganism and has the ability to grow on pyrene and different other types of PAH as a sole source of carbon. Mycobacterium sp. was brought from the American Type Culture Collection (Manassas, Virginia, USA). For the isolation and cultivation of the Mycobacterium species, the Lowenstein-Jensen medium was used which was also brought from the American Type Culture Collection. Lowenstein-Jensen agar medium was used for slants and Petri dishes and Lowenstein-Jensen broth was used as liquid medium. The pH was adjusted to 7.0. The agar medium was prepared by mixing the Lowenstein-Jensen medium with 15 g of agar and 1L of water and was autoclaved (Sterilmatic Autoclave, Market Forge Industries Inc., Everett, Massachusetts, USA) at 125°C for 20 minutes. After the agar was cooled to about 70°C, approximately 25 mL and 20 mL of the medium were poured onto each Petri dish and slant tube, respectively. The Petri dish and slants were cooled to solidify. The broth medium was autoclaved (Sterilmatic Autoclave, Market Forge Industries Inc., Everett, Massachusetts, USA) at 125°C for 20 min and cooled to room temperature before use. |
| The steps for preparing the Mycobacterium sp. spore suspension are shown in Figure 2. The freeze dried culture was hydrated in Lowenstein-Jensen broth and then plated on Lowenstein-Jensen agar. Colonies started forming on the agar after 48 hours. Spore suspension of Mycobacterium sp. was prepared by cutting 1 cm2 Petri dish culture into 25 mL sterile Lowenstein-Jensen broth medium. In order to activate the microbial culture, the inoculated broth was incubated at 50°C in a furnace (Isotemp® oven, Model 106G, Fisher Scientific, Hampton, New Hampshire, USA) for 72 hours. Then, colony forming units (CFU) were determined using the plate counts method. An amount of 5 mL media containing the CFU of 1.0×106/mL was transferred into Fernbach flasks containing 250 mL of liquid cultural media and agitated on rotary shaker (Series G-25 Incubator Shaker, New Brunswick Company, New Jersey, USA) at 120 rpm and 50°C for 72 hours. The final cultures were used as inoculum in the experiment in the amount of 10% (by weight) of composting materials. |
| Compost mixture preparation |
| The characteristics of materials used in making the compost mixture are shown in Table 4. The volume of the bioreactors is approximately 133 L. The optimum loading for this particular bioreactor is about 75% of the total volume (100L). The ratio of soil to bulking agent reported in the literature varied from 1:3 [49] to 1:1 [45]. Therefore, a soil: bulking agent of 1:2 (by volume) was chosen. 30 L of soil and 60 L of wood shavings were mixed together. A pure culture of mycobacterium species was used to augment the native soil microbes for the degradation of pyrene and was only added (10% v/v) to the compost mixtures of the bioaugmentation and combined bioaugmentation-biostimulation treatments. The micronutrients (1L of supplemental nutrients solution) were added to the compostmixtures of the biostimulation and combined bioaugmentation-biostimulation treatments. The required amount of cooking oil was added to the compost mixtures. Alkoaik and Ghaly [45] and Ghaly et al. [3] recommended the addition of 36 mL of cooking oil per kg (dry weight) of the compost mixture. The required cooking oil was estimated to be 1.6 L. Pyrene was added to the mixture at a rate of 700 mg/kg soil. The required amount of 29.4g (37.3mL) of pyrene was added to the mixture. The moisture content of the mixture was brought to 60% by adding water to the compostmixture and the C:N:P ratio was adjusted to 120:4:1 by the addition of urea and Na2H2PO4. The C:N ratio of 30:1 was recommended by Ghaly et al. [3] and the N:P ratio of 4:1 was within the range of 1.5:1-10:1 recommended by several researches [5,38,39,50]. The quantity of nitrogen, phosphorous and water required were calculated as follows: |
| Nitrogen required = (total carbon / desired C:N) - (total available nitrogen) (1) |
| Phosphorus required = (total nitrogen/ desired N:P) - (total available phosphorus) (2) |
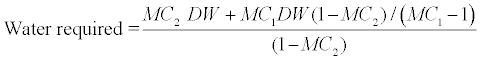 (3) (3) |
| Where: |
| MC1 is the initial moisture content (%) |
| MC2 is the final moisture content (%) |
| DW is the dry weight (kg) |
| The experiment protocol |
| Four experiments were carried out: control, biostimulation, bioaugmentation and combined biostimulation -bioaugmentation. The reactor was filled with the compost mixture of a given treatment. The airflow and mixing speed were adjusted to 2.5 L/min (1.5 v/v/h) and 25 rpm, respectively. The reactor temperatures were recorded on an hourly basis for the duration of the experiment. Gas samples were taken on a daily basis from the inlet air and exhaust gas streams using syringes and evacuated vials. These samples were stored at room temperature until the time of analysis. Samples were also taken on a daily basis from the compost mixture for moisture content, pyrene and microbial analyses. The samples were collected by scooping approximately 50 mL of materials into glass bottles. The bottles were capped and refrigerated until needed for analysis. Each experiment was run for 2 weeks. After the experiment was terminated the reactor was cleaned and prepared for the next run. |
| Sampling and analysis |
| The microbial growth and total phosphorus were carried out according to the procedures described by Page et al. and Black et al. [51,52], respectively. The total kjeldahl nitrogen and ammonia nitrogen were determined using a kjeltech auto analyzer (Tecator Model 1030, FOSS Teacator AB, Höganäs, Sweden). The proximate analysis, ultimate analysis, moisture content and pyrene analysis were carried out as follows. |
| Proximate analysis |
| The proximate analysis was performed to determine the weight fractions of volatiles, ash and fixed carbon in the soil, wood shavings and oil samples. The ASTM Standard Method for Proximate Analysis of Coal and Coke (D-3172-73 through D-3174-82 and D-3175-82) was used (ASTM, 2007a). The values of volatiles and ash were determined on dry matter basis. The fixed carbon was then obtained by subtracting from 100 the sum of volatile matter and ash contents. |
| Ultimate analysis |
| The ultimate analysis was performed to determine the elemental composition of the soil, wood shavings and oil samples. The weight fractions of carbon, hydrogen, nitrogen, sulfur, chlorine and ash were determined and the weight fraction of oxygen in the samples was calculated by the difference. Carbon, hydrogen and nitrogen weight fractions were determined using a Perkin-Elmer LECO CHN Elemental Analyzer (Model No. 240, International Equipment Company, Needham Heights, Massachusetts). Sulfur was determined using the LECO induction furnace (LECO S-Analyzer, International Equipment Company, Needham Heights, Massachusetts). Chlorine was determined by following the Mercuric Nitrate Method given in Standard Methods for the Examination of Water and Wastewater (AOAC, 2005). The ASTM Standard Test Method for Ash in Wood (D-1102-84) was followed to determine the ash percentage in the peat sample (ASTM, 2007b). Because of the lack of a simple, direct method for determining oxygen in biomass fuels, it is usually estimated by subtracting the sum of carbon, hydrogen, nitrogen, sulfur, chlorine and ash from 100. |
| Moisture content |
| Moisture content tests were performed on the samples by the oven drying method, according to the ASTM D 3173-73 procedure (ASTM, 2007c). Samples weighing approximately 4 - 5 g each were placed in large aluminum dishes. The dishes containing the samples were placed in an air forced drying oven (Isotemp Oven Model No. 655F, Fisher Scientific, Toronto, Ontario) at 105°C for at least 24 hours. The moisture content of the samples was then calculated on wet basis as follows: |
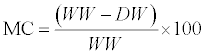 (4) (4) |
| Where: |
| MC is the moisture content on wet basis (%) |
| WW is the wet weight of the sample (kg) |
| DW is the dry weight of the sample (kg) |
| The moisture losses were monitored in two parts: (a) the portion collected by the desiccant and (b) the portion collected in the water trap. The moisture absorbed by the desiccant was calculated by subtracting the mass of the desiccant before starting the experiment from its mass following the experimental run. The water collected in the water trap was measured and added to the amount absorbed. The total accounted for total moisture losses. |
| Pyrene |
| The pyrene contents in the exhaust gas and the compostmixture were determined using a gas chromatograph (Model No. 5890-SII, Hewlett Packard, Atlanta, Georgia). The gas chromatograph was calibrated by injecting 1.0 μL of the hexane extracted mixture onto a 25 m×0.2 mm, 0.33 μm film thickness, 5% diphenyl siloxane megabore capillary column. A split ratio of 5:1 was employed using the split mode of the injection port. The injection port was set at 180°C and the flame ionization detector was set at 250°C. The oven containing the column was first maintained at 40°C for 4 minutes and then increased at a rate of 10°C/min until a final temperature of 350°C was reached. This final temperature was held for 5 minutes. The carrier gas, helium, was held at a flow rate of 1.2 mL/min. In order to perform this analysis on the soil samples, hexane extraction had to be performed on the mixture samples first. |
| Results and Discussion |
| Microbial growth |
| The microbial growth is shown in Figure 3. The addition of nutrient (biostimulation) or microorganisms (bioaugmentation) increased the number of viable cells over that of the control during the 15 day treatment. The cell content increased from the initial value of 2.5×106 cells/g mixture to 102.2×106cells/g mixture (41 fold) and 175.4×106 cells/g mixture (70 fold) for the control and biosimulation treatments, respectively. However, the addition of nutrients and/or microbes significantly increased the number of viable cells from the initial cell content of 3.8×106 cells/g mixture to 225.1×106 and 503.2×106 for the bioaugmentation and combined biosimulation-bioaugmentation treatments, respectively. |
| The specific growth rate and lag period were determined accordingly to the procedure described by Ghaly et al. [53] as shown in Figure 4. The lag period and specific growth rate (μ) were 0.5 d and 0.896 d-1, respectively. Fellie et al. [54] studied the biodegradation of benzene, toluene, ethylbenzene and xylene isomers (BTEX) by a heavy metaladapted environmental bacterial consortium and reported significant decrease in the specific growth rate (μ) when the centration of each individual BTEX was increased from 10 mg/L to 500 mg/L. They observed the highest specific growth rate of 0.14 h-1 and the shortest lag period of 8 h while degrading benzene with 10 mg/L concentration. Rhee et al. [55] studied the degradation of pyridine (3 mM) in a batch reactor and reported a lag period of 13 h and a specific growth rate of 0.08 h-1 using denitrifying bacteria isolated from industrial wastewater. Obayori et al. [56] studied the degradation of engine oil by Pseudomonas sp. Strain LP1 and reported a lag period of 5.71 d and a specific growth rate of 0.122 h-1. |
| Chaineau et al. [50] studied the effects of nutrient concentration on biodegradation of crude oil and associate soil microbial population and found that the addition of nutrients sharply increased the number of viable microorganisms during the first 30 days of the 180 day study. Liebeg and Curtright [48] studied the effects of macro and micro nutrients addition on the degradation of PAHs in soil and observed significant increases in the microbial activities of the degrading microbial population as measured by the rate of O2 consumption. Cerniglia [27] reported that the PAH degrading bacteria decreased to an insufficient level when inorganic nutrient concentration in soil was low as the slower-growing pollutant degrading bacteria was outcompeted by other soil microorganisms. However, other researchers [47,57,58] found no effect of nutrient addition on the degradation of PAH’s in creosote contaminated sites and concluded that the microbial population was capable of metabolizing PAH’s under low nutrients. |
| Temperature |
| Temperature records obtained from the nine thermocouple indicated that the bioreactor had a homogenous temperature distribution. The variations in the temperature among the various locations in the bioreactor were very small (coefficient of variation was in the range of 2.1-4.8%). The average temperatures for each treatment were plotted versus time as shown in Figure 5. The results showed a lag period of about 24 h for all treatments during which the microbial population acclimatized to the new media. The lag period was followed by a period of a rapid temperature increase during which the temperature of the mixture rose from the initial temperature of 21°C to the maximum temperatures of 28°C, 30°C, 32°C and 41°C for the control, biostimulation, bioaugmentation and combined bioaugmentation-biostimulation treatments, respectively. The steady state condition was reached after 4h for the control and bioaugmentation treatments and after 2h for the biostimulation and combined bioaugmentation-biostimulation treatments. |
| The rise in the temperature demonstrated the conversion of the complex organic carbon (mineralization) into CO2 and H2O and release of energy. A part of this energy is needed for synthesis of structural cell parts and substances within the cells (such as enzyme, proteins, cell wall etc.) as well as for cell growth and multiplications whereas the rest is converted into heat giving a rise to the reactor temperature. The utilization of organic carbon for energy and cell growth can be described as follows. |
| Cell respiration |
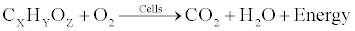 (5) (5) |
| Cell growth |
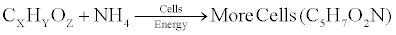 (6) (6) |
| Cai et al. [59] reported a steady state temperature around 55°C in a composting process for removal of PAHs. Ghaly et al. [3] reported steady state temperatures of 32°C for intermittent mixing and 40°C for continuous mixing during bioremediation of soil contaminated with toluene. Alkoaik and Ghaly [45] reported a gradual temperature increase up to 55°C during the first 52 days of bioremediation of soil contaminated with used motor oil which was then followed by a decline. Ghaly and Pyke [46] reported a gradual increase in temperature up to 47°C in the first 6 days during the bioremediation of peat contaminated with oil which was followed by faster decline in the temperature. Jørgensen et al. [49] reported that the thermophilic temperature is delayed to the 20th-25th day for soil contaminated with petroleum hydrocarbon. In this study, the combined bioaugmentationbiostimualtion treatment reached the thermophilic temperature on 2nd day. The temperatures of other treatments remainedin the mesophilic range. Ghaly et al. [3] reported a temperature in the thermophillic range after two days while bioremediating soil contaminated with toluene. |
| Moisture content |
| Moisture is usually lost through the exhaust gas during bioremediation [3]. The initial moisture content of the mixture was 60% which declined to 57, 52, 50 and 45% for the control, biostimulation, bioaugmentation and combined bioaugmentation-biostimulation treatment, respectively (Figure 6). Alkoaik and Ghaly [45] reported a decline in the moisture content from the initial value of 60% to 35% while treating soil contaminated with motor oil. Rojas-Avelizapa et al. [60] reported a reduction in moisture content from the initial value of 60% to 30-36% during the treatment of petroleum hydrocarbons by biopiling. In this study, the final moisture content remained with the optimum range of 40-60% for bioremediation [3,35]. This was due to compensating of water lost in the exhaust with the water produced as a by-product of the organic matter degradation process [45]. |
| The amount of moisture lost from the bioreactor over the 15 day treatment period was 176.97, 416.66, 500.00 and 681.99 g/kg of initial mixture for the control, biostimulation, bioaugmentation and combined bioaugmentation-biostimulation treatments, respectively. Beck-Friis et al. [61] reported moisture losses due respiration of 220 g water/kg of initial dry weight of compost material. Ghaly et al. [3] reported moisture losses of 257-636 g water/kg soil-wood shaving mixture during bioremediation of toluene depending on the levels of aeration and mixing. |
| Exhaust gas |
| The initial composition of the inlet air (N2, O2, Ar and CO2) was determined at the start of the experiments whereas the concentrations of O2 and CO2 in the exhaust air were monitored on a daily basis. The inlet air had 20.94% O2, 78.10% N2, 0.93% Ar and 0.025% CO2. The changes in O2 and CO2 concentrations in the exhaust gas are shown in Figure 7. The oxygen concentration in the exhaust gas declined with time from the initial value of 20.94% reaching values of 11.1, 7.2, and 9.1 % for the control, biostimulation and bioaugmentation treatment, respectively. The CO2 in the exhaust gas increased from the initial value of 0.025% reaching values of 3.7, 4.0 and 4.0% for the control, biostimulation and bioaugmentation treatment, respectively. However, for the combined bioaugmentation-biostimulation treatment, the O2 declined from the initial value of 20.94% to 5.0% on days 6-8 and then increased to 10.2% by the end of experiment where as the CO2 increased from the initial value of 0.025% to 5.0% by the 8th day and then declined to 3.0% by the end of the experiment. Ghaly et al. [3] reported similar changes in the O2 and CO2 concentration of the exhaust gas from a bioremediation treatment of soil contaminated with toluene. They also reported peaks of CH4 and C2H6 during the process. Breedveld and Sparrevik [58] reported significant increases in CO2 with the addition of nutrients (N, P and K) during bioremediation of PAHs. They reported CO2 production rate of 65 mg/kg/d for a soil treated with nutrient compared to 25 mg/kg/d for the untreated soil. Cajthaml et al. [62] reported fast increases in CO2 production and O2 consumption during the first 6 days of 42 days of composting soils contaminated with PAHs. Saner et al. [63] reported that increasing the aeration rate and mixing increased the CO2 concentration in the exhaust gas during the bioremediation of soil contaminated with diesel oil. Ghaly et al. [3] reported higher O2 consumption and CO2 production with continuous mixing compared to intermittent mixing during bioremediation of soil contaminated with toluene. |
| Pyrene |
| The first observation was made on day 6. Upon opening the bioreactor, a warm moist air with slightly oily smell odour was detected. The volume of mixture was reduced due to the loss of moisture and break down of wood savings. The second visual observation was made at the end of the experiment (day 15). The material was reduced to denser clay like material with brownish colour. |
| The changes in pyrene concentration in the soil-wood saving mixture are shown in Figure 8. The pyrene concentration decreased with time from the initial concentration of 700 mg/kg mixture reaching 500, 350, 295 and 110 mg/kg mixture which resulted in a removal efficiency of 35.71, 50.00, 57.86 and 84.29% for the control, biostimulation, bioaugmentation and combined bioaugmentation and biosimulation treatments, respectively. |
| Chaineau et al. [50] studied the effect of nutrient concentration on the biodegradation of crude oil and associated microbial population in soil and reported a maximum degradation of 62% in 90 days with the addition of nutrient compared to 47% degradation with natural attenuation. Leys et al. [47] studied the effect of C:N:P ratio on the degradation of PAH’s using sphingomonas in soil and concluded that the PAHs degradation rate was not affected by excess N or P nor when N and P were 10 times lower indicating that the microorganisms were capable of metabolizing PAHs under low nutrient condition. Liebeg and Curtright [48] investigated the effect of macro and micro nutrients on PAHs degradation in soil and found the O2 consumption rate and PAHs degradation rate at low levels of macronutrients (N, P and K) and high levels of micronutrients (K2, Cl, Mg, Na, Ca and Fe) were need with phosphorus on the dominant macronutrient. Breedveld and Sparrevik [58] studied the effect of nutrients addition as biodegradation rate of PAHs in various soil strata at a creosote contaminated site and found the N:P ration to have a significant impact on the degradation rates of various PAHs. Other researchers [50,64-66] reported that different nutrients levels are needed for the optimum degradation of aliphatic and aromatic hydrocarbons and recommended a N:P ration is in the range of 2.4:1-10:1. The N:P ratio of 4:1 used in this study is with the reported range. Carmichael et al. [57] studied the effects of inorganic and organic supplements on the microbial degradation of phenanthrene and pyrene in soils and found that the supplements increased the microbial population of the heterotrophic microorganisms and PAHs mineralization. They noticed that the PAHs degradation community at different sites to be unique in their response to materials added in attempt to stimulate PAHs degradation. Simarro et al. [67] studied the effect of nutrients on degradation of PAHs and found that the addition of glucose to PAHs as bioavailable carbon source increased the number of microbes and enhanced the degradation rate. They suggested a C:N:P ration of 100:21:16 and NaNO3 as a nitrogen source. |
| In this study, the reduction in pyrene was biological in nature and volatilization did not contribute to the removal of pyrene from the soil-wood shaving mixture as the exhaust gas samples did not show any trace of pyrene. The biodegradation of organic substances such as pyrene can be described using a first order model [44,68,69]. |
| Ct=C0e-kt (7) |
| Where, |
| Ct = Concentration of the pyrene at the time t (mg/kg) |
| C0 = Initial concentration of pyrene (mg/kg) |
| k = Rate constant (h-1) |
| t = Time (h) |
| The change in pyrene for the combined bioaugmentationbiostimulation treatment seems to fit the first order model. A plot of ln (Ct/C0) versus time yields a straight line with a slope equal to k as shown in Figure 9. The results showed that a degradation constant during the initial start up period (days0-2) was 0.051d-1 which increased during the exponential microbial growth phase (days2-8) to 0.162d-1 and then declined during the stationary phase (days 8-15) to 0.1 d-1. Ghaly et al. [3] bioaugmented soil-wood mixture contaminated with a toluene and reported a degradation rate of 0.24 h-1 and 0.17 h-1 for intermitted mixing and continuous mixing, respectively. Ghaly et al. [44] reported a degradation rate constant in the range of 0.010-0. 026 h-1 (depending on the initial concentration) while composting tomato plant residues contaminated with pirimipuos-methyl. Ghaly and Dave [70] reported different degradation rates for the lag period (0.0025 h-1) and exponential growth period (0.071 h-1) while degrading the fungicide captan (144 mg/L) contaminated wastewater under batch mode of operation for 5 days. Pimda and Bunnag [71] studied the biodegradation of 10% used motor oil using single and mixed cultures of Nostoc hatei and Synechocystis aquatilis and reported the highest degradation rate constant of 0.0667 day-1 at the end of 14 day by using single culture N. hatei. |
| Adesodun and Mbagwu [72] used bacteria isolated from oilpolluted sites to degrade the waste-lubricating petroleum oil with varied concentrations in soil amended with animal droppings (cow dung, poultry manure and pig wastes) and reported the highest degradation rate constant of 0.3834 d-1 while treating 0.5% spent oil contaminated soil amended with poultry manure. |
| Conclusion |
| The addition of nutrients (biosimulation) and microorganisms (bioaugmentation) increased the number of viable cells over the control during the bioremediation process. The cell number increased by 40, 70, 59 and 132 fold for the control, biosimulation, bioaugmentation and combined bioaugmentation, respectively. A lag period of 0.5 d and a specific growth rate of 0.896 d-1 were observed with the combined biostimulation-bioaugmentation treatment. The temperature of the combined biostimulation-bioaugmentation reached 41°C after two days of treatment while the maximum temperature of the control, biostimulation and bioaugmentation were within the range of 28-32°C. The moisture content decreased for all treatments reaching 45-57% but remained within the optimum range of 40-60% for bioremediation process. The level of pyrene degradation was indicated by the decline in O2 concentration and the increase in CO2 concentration in the exhaust gas. The control, biostimulation and bioaugmentation treatments showed similar patterns of decreasing O2 and increasing patterns of CO2. However, the combined biostimulation-bioaugmentation treatment recorded a declining trend in O2 concentration and increasing trend in CO2 concentration in the exhaust gas at the beginning of experiment (first 7 days) followed by increasing trend in O2 concentration and decreasing trend in CO2 concentration in the next 8 days of the experiment. The highest pyrene reduction in percentage (84.29%) was obtained through the combined bioaugmentation-biostimulation process followed by bioaugmentation (57.86%), biostimulation (50%) and control (37%) processes. Different pyrene degradation rates were observed during the various phases of microbial growth (lag, exponential and stationary) of the combined bioaugmentation-biostimulation treatment. |
| Acknowledgement |
| This research was supported by the National Science and Engineering Research Council (NSERC) of Canada. |
References
|
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 18123
- [From(publication date):
specialissue-2013 - Dec 19, 2025] - Breakdown by view type
- HTML page views : 13217
- PDF downloads : 4906
