Adenocarcinoma NOS of The Maxillary Sinus: Clinical and Histopathological Features with Therapeutic Considerations
Received: 06-Jun-2011 / Accepted Date: 03-Jul-2011 / Published Date: 23-Jul-2011 DOI: 10.4172/2161-119X.1000103
Abstract
Malignant tumours of the nasal cavities and paranasal sinuses are uncommon. They constitute less than one per cent of all tumours and less than three per cent of head and neck tumours. Carcinoma of the maxillary sinus is the most common of the sinonasal malignancies. In this anatomical site a case of adenocarcinoma, not otherwise specified, was documented, mainly from a histological perspective and discussed considering all types of differential diagnoses.
Keywords: Adenocarcinoma NOS, Immunohistochemistry, Differential diagnosis.
246925Adenocarcinoma not otherwise specified (NOS), is a malignant epithelial salivary gland tumor with glandular or ductal adenocarcinomatous differentiation but without other specific histologic features, allowing for a more definitive classification and that characterize the other defined types of salivary carcinoma. The modifying term “not otherwise specified” should be included because most other epithelial salivary gland malignancies are also adenocarcinomas [1]. Clinically, it may be considered one of the more common malignant salivary gland neoplasm and third most common behind mucoepidermoid carcinoma and acinic cell adenocarcinoma. More common in women than in man it occurs over a wide age range, but is most frequently seen in the fifth-eight decades of life. It may occur in both major and minor salivary glands. Among minor salivary gland sites most commonly occurs in intraoral sites, particularly the palate [1,2]. On the other hand adenocarcinoma can be considered a glandular malignancy of the sinonasal tract, excluding defined types of salivary gland carcinoma (also adenocarcinoma NOS). Two main categories of adenocarcinoma are recognized: intestinal-type, and nonintestinal- type adenocarcinoma, which can be further divided into low-grade and high-grade subtypes [3,4]. Overall, adenocarcinomas and salivary-type carcinomas (also adenocarcinoma NOS) comprise 10-20% of all sinonasal primary malignant tumours. In this case report we describe a case of adenocarcinoma, NOS, occurring in the maxillary sinus of a 65 aged female patient.
Case Report
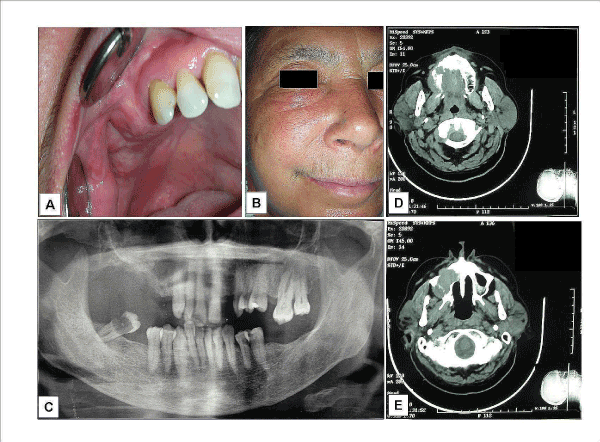
A 65-year-old Italian woman was referred to the “Clinica Odontoiatrica” of the Second University of Naples, Napoli (Italy) complaining of swelling in the right upper edentulous molar area and fornix. A recent study have identified the role of work exposure to organic dusts in patients with malignant paranasal sinus tumors [5]. In our case, anamnestic exposure to wood dust was not documented, so that we have excluded an occupational disease. At the clinical examination (Figure 1A) the swelling appeared as a soft elastic mass, about 30 mm long and 15 mm large, covered by a normal and not necrotic nor sore mucous epithelium; it was not compressible and painful when palpated. The teeth mesially to the lesion did not show mobility, with roots well-preserved and not notched. Palpation of the neck region did not show regional lymph node in the level I-VI and not distant metastases were found. She had no nasal congestion but felt a tension in the sub-ocular area (Figure 1B). For these reasons, she consulted our hospital and we advised her to perform all the radiological exams before treating her surgically. A panorex (Figure 1B) showed radiopacity in the right maxillary sinus, and a slight resorption of the maxillary tuber in its caudal portion. A postero-anterior radiographic view of the skull showed radiopacity corresponding to the maxillary sinus and maxillary process, with the absence of a clear tuberosity line. Axial computed tomography images reported a large radiopaque neoformation involving the right sinus and the alveolar process of the right maxilla and involving the entire right palatal process. (Figure 1D,E)
Figure 1: Adenocarcinoma not otherwise specified (NOS): clinical (A-B) and radiographical (C-E) appearance. A) At the clinical examination the swelling appeared as a soft elastic mass, covered by a normal appearing. B). Patient showed cheek swelling that was extended to the sub-orbital area. C) The panorex showed radiopacity in the right maxillary sinus, and a slight resorption of the maxillary tuber in its caudal portion. D-E) Axial computed tomography images reported a large radiopaque neoformation involving the alveolar process (D) and the right sinus eroding its postero-lateral and anterior wall (E) and involving the entire right palatal process.
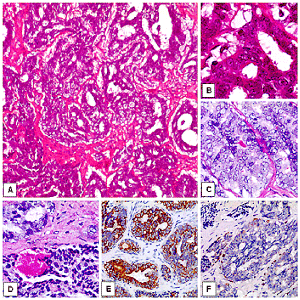
Figure 2: Adenocarcinoma not otherwise specified (NOS): morphological (A-D) and immunohistochemical findings (E-F). A) The evidence of glandular and ductal features oriented for an epithelial origin of the neoplasia. The tumor was histologically graded as moderated differentiated adenocarcinoma (H&E, original magnification x4). B) A detail of A, with glandular representative histological findings and absence of a myoepithelial layer (H&E, original magnification x40). C-D) Focally, PAS positive material was observed within the neoplastic gland (C, H&E, original magnification x40; D, H&E, original magnification x20). E) The mass stained positive for cytocheratins AE1/AE3 (LSAB-HRP, nuclear counterstaining with haematoxylin, original magnification x10). F) The case has shown focal staining for p63 protein (LSAB-HRP, nuclear counterstaining with haematoxylin, original magnification x10).
It was previously (six month before) treated by conservative enucleation and bone courettage in other local hospital after incorrect diagnosis of a benign tumor. Original diagnosis was performed in a non-specialized hospital and the lesion was erroneously considered benign although location and bone destruction. After local recurrence, lesion was treated with right emimaxillectomy. The maxillectomy defect has created a significant rehabilitative issue, as it has created patient speech, deglutition, cosmetic and orbital problems. The main aims of the consequently surgical reconstruction can be here synthetized: 1. consistently obtain a healing wound, 2. restore palatal competence and function, 3. support the orbit or fill the orbital cavity in exenteration 4. obliterate the maxillectomy defect, and 5. re-store facial contour. Our patient underwent successful obturator prosthetic rehabilitation with fair to good speech, deglutition, and cosmetic results.
Patient was lost at follow-up. In a recent work of Huber GF, adenocarcinoma of the paranasal sinuses was found to have almost 80% 5-year survival if adequately treated surgically and with adjuvant intensity-modulated radiotherapy [6]. In our case, unfortunately, we have not notice about her outcome and her subsequently type of clinical management, apart the post-surgical reconstruction.
The histopathological diagnosis of malignant neoplasia was consecutively made at the Section of Anatomic Pathology of the University of Foggia - Foggia. Microscopic evaluation was performed by two pathologists, determining the histotype, the degree of differentiation according to WHO grading system, and establishing tumor extent according to the TNM system.
Gross findings
On gross examination this tumor recurrence was a poorly demarcated tan-white mass measuring 3 cm in diameter. Cut section was grey white in colour.
Microscopic features
On microscopic examination the neoplasm displayed histologic heterogeneity with presence of infiltrative cells showing evidence of glandular and ductal structures with an absence of squamous differentiation or differentiation indicative of other tumor type, Tumor cells were arranged in solid sheets, cords, nests, glands, cystic spaces and microglandular cribriform pattern diffusely infiltrating the fibrous stroma. Focal areas of necrosis were also seen. PAS staining clearly delineated the mucin secreting cells. The tumor was histologically graded as moderated differentiated and examined by a panel of immune-phenotyping antibodies.
Immunohistochemical findings
Immunohistochemistry was performed on the sections mounted on poly-L-lysine-coated glass slides, by standard LSAB-HRP technique, using a complete panel of specific monoclonal antibodies against: TTF-1 (mouse monoclonal - CELL MARQUE – dilution at 1:100 30 min with EDTA), p63 (mouse monoclonal - BIOCARE – prediluted, with EDTA), EgR (rabbit monoclonal - DIAGNOSTIC BIOSYSTEMS - dilution at 1:100 with EDTA), PgR (mouse monoclonal - NEOMARKERS - dilution at 1:180 overnight, with EDTA), EMA (mouse monoclonal - BIOGENEX – dilution at 1:70), CEA (rabbit monoclonal - DAKO – dilution at 1:300 with EDTA), GCDFP15 (mouse monoclonal - NEOMARKERS – dilution at 1:50 with EDTA), S-100 (mouse monoclonal - BIOGENEX - dilution at 1:200), CK AE1/ AE3 (mouse monoclonal - NEOMARKERS – dilution at 1: 150 with EDTA), CK7 (mouse monoclonal - ZYMED - dilution at 1:80 with EDTA), CK20 (mouse monoclonal - BIOCARE - dilution at 1:50 with EDTA) and alfa-actin (mouse monoclonal - CELL MARQUE – dilution at 1:200). We have chosen CKAE1/AE3, CK7, EMA and CEA to confirm epithelial differentiation; S100, Actin and p63 were helpful to identify the presence or the absence of a myoepithelial component. In order to exclude a metastatic disease were used TTF1 for eventually paranasal sinus localization of a lung cancer; EgR, PgR, and GCDFP15 to exclude a mammary origin, CK20 to consider the possibility of a metastatic colic cancer.
In our case, the mass stained positive for cytocheratins AE1/AE3, cytokeratin 7, S100 and, focally, for p63. Negative results were observed for TTF-1, EgR, PgR, EMA, CEA, GCDFP15, Actin and cytokeratin 20.The mass stained positive for cytocheratins AE1/AE3, cytokeratin 7, S100 and, focally, for p63. Negative results were observed for TTF-1, EgR, PgR, EMA, CEA, GCDFP15, Actin and cytokeratin 20. According to these results we have confirmed the epithelial origin of the neoplasia, excluding the possibility of a sebaceous carcinoma, a myoepithelial tumor or a lung or breast metastasis.
Discussion
Malignant tumours of sinonasal tract are uncommon. They constitute less than 1% of all malignancies in the body and about 3% of head and neck cancers. The incidence is approximately 1 in 100,000 people per year [7]. Generally, the incidence in males is twice that of females. These tumors are most frequently found during the fifth to the seventh decades. Because a majority of them present at advanced stages, it is difficult to determine the primary site of the tumor. When these tumors do occur in the sinonasal tract, the most common site is the maxillary antrum; other sites, in order of decreasing incidence, include the nasal cavity, nasopharynx, and ethmoid sinuses. This pattern most likely reflects the relative distribution of minor salivary glands in this area of the upper aerodigestive tract.
Adenocarcinomas make up 4% to 8% of all sinonasal cancer [8]. Sinonasal tract adenomatous tumors have been presumed to arise from submucosal mucoserous glands or represent intraosseous extension of minor salivary gland tumours of the sinus mucosa [8,9]. The antrum, ethmoid and nasal cavity are the most common locations for adenocarcinoma NOS. Acherson and Macbeth reported a significantly high incidence of adenocarcinoma of the paranasal sinus in High Wycombe, England, among furniture workers [10]. These tumors usually present with nasal obstruction and facial pain. They are sizable partially capsulated tumors involving the adjacent bone, with a clear locally infiltrative growth pattern and with multiple necrotic areas. Many histological features have been described: low grade adenocarcinomas with mucin secreting ductular, tubulocystic and papillary structures; intermediate adenocarcinomas with prevalent trabecular pattern and high grade adenocarcinomas with marked pleomorphism, cellular atypia and high mitotic rate. Enteric-type adenocarcinomas of the sinus may also resemble moderately differentiated colon adenocarcinoma [11]. The location as well as the extent of the mucosal lesion within the maxillary sinus has prognostic significance. Historically (1933) Ohngren’s oblique line, connecting the medial canthus of the eye to the angle of the mandible, is used to divide the maxillary sinus into an anteroinferior portion (infrastructure), which is associated with a good prognosis, and a superoposterior portion (suprastructure), which has a poor prognosis. The poorer outcome associated with superoposterior cancers reflects early access of these tumors to critical structures, including the eye, cribriform plate, sphenoid sinuses, nasopharynx, skull base, pterygoids, and infratemporal fossa [12]. The currently used American Joint Committee TMN classification is based upon Ohngren’s original description. It emphasizes the size, as well as the extension of tumors. This uniform staging system is not perfect, but allows various different centers to report their experiences in a manner that lends itself to objective comparison and interpretation [12].
Prior to a major exploration or resection, a tissue diagnosis must be made. This can be achieved by an intranasal biopsy using endoscopic sinus surgery techniques or by transoral or transcutansous procedures such as Caldwell-Luc or external ethmoidectomy [13].
Adenocarcinoma NOS is characterized by a considerable variability in cytology and architectural structures, an infiltrative growth into parenchyma or surrounding tissues and lack of features that characterize other types of adenocarcinomas. Moreover, the cytologic variability is useful for grading these tumours. We have to remember that in low-grade tumours often the bland nuclear morphology could erroneously suggests benignity. Our case was previously treated by a conservative enucleation, that has not been radical. The wrong diagnosis of benignity could be due to the superficially observation of minimal variability of cell nuclear size, shape, and rare mitoses.
Because this tumour does not have pathognomonic histopathologic features, the differential diagnosis is the rule. While immunohistochemical studies may be useful in this evaluation, it should be remembered that adenocarcinoma NOS has non-specific immunoreactivity.
We excluded the possibility of an intestinal type of adenocarcinoma, not only morphologically, but also because the mass did not stain for CK20. When performing a histological diagnosis of an adenomatous lesion of the sinonasal tract, acinic cell carcinoma could be considered in differential diagnosis, although larger nuclei and abundant cytoplasm are usually distinguishing histological features of adenocarcinoma – NOS [14,15]. Also the negativity for CEA excluded the diagnosis of acinic cell carcinoma. Pleomorphic adenoma, basal cell monomorphic adenoma and polymorphous low grade adenocarcinoma are extremely rare in the sinonasal tract to offer a common diagnostic difficulty. Moreover, the presence of highly atypical cells contrasted with a diagnosis of a polymorphous low grade adenocarcinoma. Diagnosis of mucoepidermoid carcinoma, acantolytic squamous cell carcinoma, basal cell adenocarcinoma and clear cell carcinoma was ruled out respectively for the complete lacking of epidermoid and basaloid aspects and for the absence of clear neoplastic cells. Before making a definitive diagnosis of adenocarcinoma, recommended histochemical stains for intracellular mucin were performed to ruled out a squamous cancer.
The focal positivity obtained for only few myoepithelial markers did not convince us for a malignant myoepithelial neoplasia. Patient did not have anamnestic history of a previous pleomorphous adenoma in the same anatomical site, so that we exclude also the possibility of a carcinoma ex pleomorphous adenoma.
Morphologically we also considered to exclude the diagnosis of an adenoid cystic carcinoma, with tubular and cribriform architectural pattern. Our case was lacking of cylindromatous microcystic spaces, filled with hyaline or basophilic material.
Generally, a specific tumor should not be forced into one of the recognized salivary type neoplasm diagnostic categories. It is often reasonable to withhold a salivary gland type diagnosis for most high grade, poorly differentiated adenocarcinomas of the sinonasal tract.
The possibility of a metastasis from distant region should always be considered. Carcinomas from the kidney, prostate, lung, breast, gastrointestinal tract, uterus, testis, thyroid, adrenal gland, pancreas, cutaneous sebaceous carcinoma and melanoma have all been reported to rarely metastasize to the nasal cavity and paranasal sinuses [16-18].
Positron emission tomography, also called PET imaging or a PET scan, is a type of nuclear medicine imaging. Nowadays, PET and PET/CT scans are performed to detect a cancer (particularly unknown primitive cancer), determine whether a cancer has spread in the body,assess the effectiveness of a treatment plan, such as cancer therapy, determine if a cancer has returned, relapsed or metastasized after treatment. The information provided by nuclear medicine examinations is unique and often unattainable using other imaging procedures. For many diseases, nuclear medicine scans yield the most useful information needed to make a diagnosis or to determine appropriate treatment, if any. Nuclear medicine is less expensive and may yield more precise information than exploratory surgery. By identifying changes in the body at the cellular level, PET imaging may detect the early onset of disease before it is evident on other imaging tests such as CT or MRI. Anyway, in our case patient did not undergo PET. Only by axial computed tomography a large radiopaque neoformation involving the right sinus, the alveolar process of the right maxilla and the entire right palatal process was reported, without images of other body lesions. This fact helped us to consider sinus mass as a primitive tumor. Generally, when a differential diagnosis between a primary tumor of the maxillary sinus and a metastasis has to be carried out, detailed immunohistochemical analysis should be taken into account and performed.
References
- Beale FA, Garrett PG (1983) Cancer of the paranasal sinuses with particular reference to maxillary sinus cancer. J Otolaryngol 12: 377-382.
- Bridger GP, Mendelsohn MS, Baldwin M, Smee R(1991) Paranasal sinus cancer. Aust N Z J Surg 61: 290-294.
- Barnes L WHO histological classification of tumours of the nasal cavity and paranasal sinuses. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, eds. World Health Organization Classification of Tumours: Pathology and Genetics of Head and Neck Tumours. Lyon, France: IARC Press; 2005.
- Patel S, Shah JP (2009) Part II: Head and neck sites. In: Edge SB, Byrd DR, Carducci MA, Compton CA, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer.
- Cantu G, Solero CL, Mariani L, Lo Vullo S, Riccio S, et al. (2011) Intestinal type adenocarcinoma of the ethmoid sinus in wood and leather workers: A retrospective study of 153 cases. Head Neck 33: 535-42.
- Huber GF, Gengler C, Walter C, Roth T, Huber A, et al. (2011) Adenocarcinoma of the nasal cavity and paranasal sinuses: single-institution review of diagnosis, histology, and outcome. J Otolaryngol Head Neck Surg 40: 34-9.
- Adams G (1986) Malignant tumors of the paranasal sinuses and nasal cavity. In: McGuarrie DG editor Head and Neck Cancer Chicago: Year Book Medical Publishers 311-334.
- Gamoh S, Kakimoto N, Tamaki J, Ozono K, Chang Chien CC, et al. (2004) Adenocarcinoma in the maxillary sinus: a case report Oral Radiol 20: 72-75.
- Chow JM, Leonett JP, Mafee MF (1993) Epithelial tumors of the paranasal sinuses and nasal cavity. Radiol Clin North Am 31: 61-73.
- Acheson ED, Cowdell RH, Hadfield E, Macbeth RG (1968) Nasal Cancer in Woodworkers in the Furniture Industry. Br Med J 2: 587-596.
- Barnes,Leon MD (1986) Intestinal-Type Adenocarcinoma of the Nasal Cavity and Paranasal Sinuses. The American Journal of Surgical Pathology 10: 151- 226.
- Har-El A, Hadar T, Krespi YP, Abraham A, Sidi J (1988) An analysis of staging systems for carcinoma of the maxillary sinus. Ear Nose Throat J 67: 511-520.
- Harrison DF (1971) The management of malignant tumors of the nasal sinuses. Otolaryngol Clin North Am 4: 159-177.
- Ihrler S, Blasenbreu-Vogt S, Sendelhofert A, Lang S, Zietz C, et al. (2002) Differential diagnosis of salivary acinic cell carcinoma and adenocarcinoma (NOS):A comparison of (immuno-) Histochemical Markers. Pathol Res Pract 198: 777-83.
- Mardi K, Singh S (2002) Primary mucoepidermoid carcinoma of maxillary sinus- a rare case report. The Internet Journal of Otorhinolaryngology 10: 1.
- Cama E, Agostino S, Ricci R, Scarano E (2002) A Rare Case of Metastases to the Maxillary Sinus from Sigmoid Colon Adenocarcinoma ORL 64: 364-367.
- Kent SE, Majumdar B (1985) Metastatic tumours in the maxillary sinus A report of two cases and a review of the literature. The Journal of Laryngology & Otology 99: 459-462.
- Prescher A, Brors D (2001) Metastases to the paranasal sinuses: case report and review of the literature. Laryngorhinootologie 80: 583-94.
Citation: Santoro A, Laino L, Contaldo M, Pannone G, Guida A, et al. (2011) Adenocarcinoma NOS of the Maxillary Sinus: Clinical and Histopathological Features with Therapeutic Considerations. Otolaryngol 1:103. DOI: 10.4172/2161-119X.1000103
Copyright: © 2011 Santoro A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 28415
- [From(publication date): 8-2011 - Nov 22, 2025]
- Breakdown by view type
- HTML page views: 23123
- PDF downloads: 5292