Affective Processing in Tinnitus Patients Assessed by Functional Magnetic Resonance Imaging
Received: 27-Mar-2012 / Accepted Date: 23-May-2012 / Published Date: 28-May-2012 DOI: 10.4172/2161-119X.S3-003
Abstract
Background: Brain imaging studies suggested that the functional connectivity of various limbic, prefrontal, and temporal brain structures may play an important role in tinnitus.
Methods: We evaluated in affective processing of tinnitus patients by functional Magnetic Resonance Imaging (fMRI). Patients with tinnitus and healthy controls underwent fMRI (1.5 T scanner) during 4 blocks of auditory stimuli of different emotional quality: 1) unpleasant beep tones, 2) pleasant sounds of chimes, 3) neutral words, 4) words with affective valence, alternating with off-periods.
Results: The comparison of activation patterns (Statistical Parametric Mapping SPM 99) revealed significant differences in the limbic system, in prefrontal regions, temporal association cortices and striatal regions independent of affective relevance of stimuli.
Conclusion: Our results underline a differing affective perception of acoustic stimuli in tinnitus patients.
Abbreviations
BA: Brodmann Area; BDI: Beck’s Depression Inventory; fMRI: functional Magnetic Resonance Imaging; SPM: Statistical Parametric Mapping; TQ: Tinnitus Questionnaire
Introduction
Subjective tinnitus is a hyperactive hearing disorder commonly defined as a phantom sound perceived solely by the affected person without an external acoustic stimulus. The pathogenesis of subjective tinnitus is poorly understood; certain forms of subjective tinnitus are described and believed to involve both peripheral and central auditory pathways [1-5]. Neuronal plasticity was recently recognized as accountable for the restructuring of tonotopic maps in primary and secondary auditory regions [6-8]. Occasionally, tinnitus can cause a considerable amount of distress [1,4]. It has been suggested that subjects with tinnitus have elevated neuronal response to sound in the inferior colliculi [9]. Functional connectivity of various limbic, prefrontal, and temporal brain structures was proposed as a basis for tinnitus-related negative non-auditory symptoms such as stress, insomnia, anxiety, tension and depression (tinnitus-related distress) [3,4,10]. Moreover, functional connectivity between the auditory and somatosensory, attention, cognitive and emotional neural networks were suggested to be relevant for the pathogenesis of tinnitus [2,4,11,12]. The data has demonstrated the existence of a tinnitus network with long-range cortical connections extending beyond the central auditory system and including cingulate cortex, the insula, parahippocampal area, precuneus and amygdala. Such expansion of tinnitus network could be crucial for the continuous perception of tinnitus tone and distressrelated processes.
Criticism of earlier studies has been predominantly based on the audiometric differences between the groups. Tinnitus patients were often hearing impaired whereas the control subjects were not. In spite of this criticism, the concept of a positive correlation between changes in perception or reporting of tinnitus volume and the brain activity in the auditory cortex remains dominant [13]. Corroborating this concept, EEG studies performed in tinnitus patients have demonstrated alterations in the dorsal anterior cingulated cortex overlapping with the emotional component of pain matrix and distress-related areas [4].
Here, using functional Magnetic Resonance Imaging (fMRI), we examined cerebral processes devoted to perception and emotional processing (including distress symptoms) in tinnitus patients and in control subjects. Previous imaging studies [2,10,13-15] have demonstrated functional deficits in primary and secondary auditory cortex, dorsolateral prefrontal cortex and limbic system, occurring in tinnitus patients. Based on these findings, we hypothesized that the perception of tinnitus and different grades of distress could involve functional linkage of the brain areas mentioned above and may correlate with the complexity and the affective burden of the auditory stimuli.
Materials and Methods
Subjects
Twenty-three subjects (10 tinnitus patients and 13 healthy control subjects) participated in this study. The tinnitus group consisted of 5 men and 5 women with a mean age of 44 years; the control group included 5 men and 8 women, mean age 33 years (Tables 1 and 2). Tinnitus patients were examined by Otorhinolaryngologists. To be included in the study, the patients had to have normal hearing (maximal mean hearing loss of 20 dB over 0.5 to 6 kHz).
Tinnitus-related distress was assessed by a standard German Tinnitus Questionnaire (TQ) [16], an adaptation of Hallam’s TQ [17]. According to Hiller et al. [18], tinnitus is considered to be ‘compensated’ at a TQ level of ≤46 (no secondary symptoms) and ‘decompensated’ at a TQ level of >46 (permanent annoyance and psychological strain; accompanied by complaints like depression, anxiety, impaired sleep and concentration). Both groups were matched for depressive symptoms using the Beck’s Depression Inventory (BDI) [19]. None of the subjects with tinnitus or control group had a history of neurological or psychiatric diseases. Immediately after the scanning, the subjects estimated the affective value of the acoustic stimuli by paper-pencil rating on a 10 cm visual analogue scale with the pools: 0 cm = negative, 5 cm = neutral, 10 cm = positive.
Stimuli
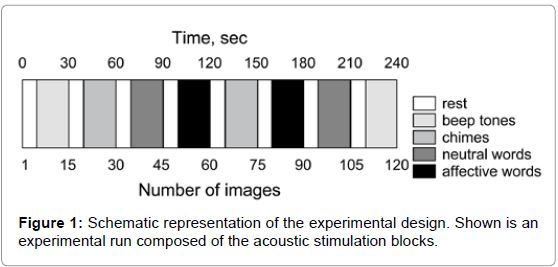
In order to visualize brain regions associated with acoustic, emotional and complex cognitive processing with emotional impact, subjects were asked to listen to blocks of 4 different acoustic stimuli. One block was designed of one of four different acoustic stimuli with different emotional impact: (A) acoustic stimulation with unpleasant beep tones (2 kHz), (B) pleasant sounds of chimes, (C) hearing of neutral words and (D) hearing of affectively-important words. The emotional word impact has been evaluated in a pilot experiment with 20 healthy volunteers to save the affective difference of stimulus material. The acoustic stimuli were presented via headphones that passively protected the subjects from a noise generated by scanner. Environmental noise was used as baseline condition (rest).
Task
Subjects were scanned during 2 experimental runs. All 4 acoustic stimuli were included twice in each run in a pseudo-randomized order (Figure 1), resulting in 4 repetitions of each stimulus during the whole experiment (total duration about 35 min, one block consists of only one type of stimulus, TR of 4 s). After 10 s of baseline (= 5 volumes), the acoustic stimuli were presented for 20 s each and were always separated by 10 s baseline phases (Figure 1). Twenty volumes were acquired during each acoustic condition (block) leading to a total of 80 volumes in addition to 40 baseline volumes. Two hundred and twenty volumes per run were acquired. The subjects were asked to silently listen to the stimuli with their eyes open.
It is known that MRI scanner noise can affect non-auditory brain areas; however, these effects were equalized since all subjects were exposed to the same experimental conditions. The time of testing was identical for all subjects.
MRI-Method
Magnetic resonance images were collected using 1.5 T whole body scanner (Siemens Magnetom Vision, Erlangen, Germany) with a standard head coil. A vacuum pad was used to minimize head movements. First, a T1-weighted localizer scan was recorded. Next, T2-weighted oblique scans were obtained (TR/TE 4500/128 ms, field of view 230 mm), primarily to aid Talairach transformation for data analysis. For the functional scans, an echo-planar sequence (TR/TE 4000/66 msec; flip angle 90 degrees; field of view 230 mm; matrix 128 x 128; slice thickness 6 mm, interslice gap 0.6 mm; in-plane resolution 1.8 x 1.8 mm) was used. Sixteen slices per volume adjusted at a transverseto- coronal angle of approximately 20° covering the whole brain with the exception of the most superior frontal and superior parietal lobe, inferior temporal pole, and cerebellum (most superior z about 60 and most inferior z about -25 according to Talairach and Tournoux [20] were obtained. Structural 3D data sets were acquired using a T1- weighted sagittal sequence with isotropic voxels (TR/TE 11.4/4.4 ms; flip angle 15 degrees; number of slices 160, matrix 256 x 256, field of view 256 mm, voxel size 1 mm3).
MRI-Analysis
The fMRI scans were analyzed using Statistical Parametric Mapping (SPM) 99 [21]. In order to remove effects of head movements, subsequent scans of each subject were first realigned using a ‘least squares’ approach with reference to the first scan. Motion artifact corrections were based on 3 parameters: translation x, translation y, and rotation α. In the next step, all images were transformed into a standard space [20] using a 2D affine transformation and then smoothed with a 5 mm FWHM (Full Width at Half Maximum) isotropic kernel to match lattice assumptions made by the SPM program. Images were re-sliced to 2 x 2 x 2 mm within stereotactic space and a high-pass frequency filter (128 s) was applied to remove low-frequency drifts.
In order to cope with the considerable inter-individual variance that usually arises from cognitive tasks, the random effects toolkit of the SPM 99 program package was used to perform the statistical data analysis. First, integrated pooled images of each experimental condition (A-D) were derived for each subject by computing the weighted mean of smoothed images from the separate experimental blocks in conjunction with an intra-subject global normalization. Second, the integrated images were entered into a multi-subject study (factor experimental condition, within subject analysis) and into a multisession study (factor experimental group: tinnitus patients vs. controls, between-subjects analysis). Resulting SPM scores (value of variance analyses of the statistical parametric mapping) were thresholded at p = 0.05 for height (u) and p = 0.05 for the spatial extent (k). Single subject studies were performed to derive patterns of individual activation for each experimental condition. Obtained data were used to compute regressions (Spearman’s correlation with highest statistical significance as regions of interest) between brain activities and the tinnitus or affective impairment assessed by TQ and BDI.
The study was approved by a local Ethics Committee and adhered to the Declaration of Helsinki regarding the use of human subjects.
Results
The scores of the individual subscales of TQ (indicating tinnitusrelated distress) are presented for individual patients in Table 1 and summarized in Table 2. Eight patients suffered from compensated tinnitus (TQ ≤46) and 2 patients from decompensated tinnitus (TQ ≥ 47).
None of the control subjects had relevant depression symptoms, as per standardized clinical test. Two tinnitus patients reached a threshold relevant for depressive disease, prior to our investigations. There were significant differences in depression between tinnitus patients and control subjects (BDI), but the absolute depression value in the tinnitus group was lower than the threshold level indicating clinically relevant depression (Table 3). The affective evaluation of stimuli was different for chimes.
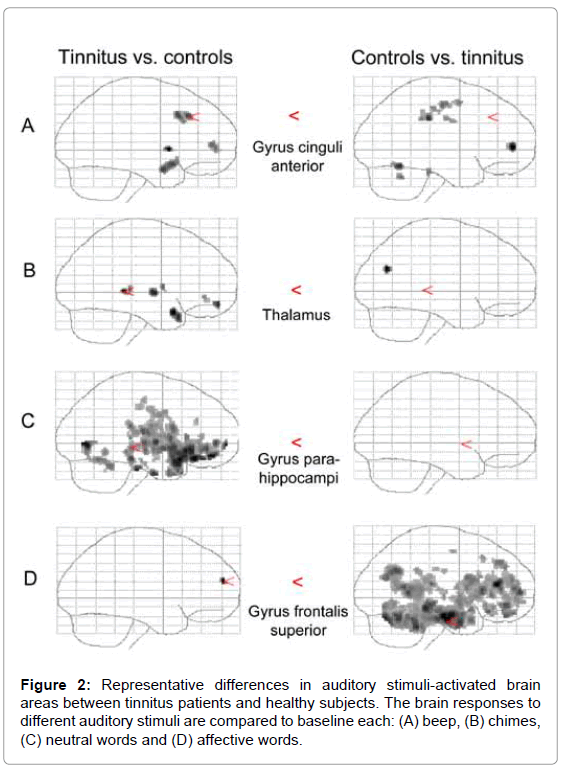
The fMRI data analysis revealed different activation patterns between tinnitus patients and control subjects. Principal spatial distribution of voxel clusters showing significant SPM Z-scores is illustrated in Figure 2. The x-, y-, and z-coordinates of local voxel maxima with reference to the standardized space of Talairach and Tournoux [20] are summarized in Table 4. Z-scores are reported that reach significance on an uncorrected voxel level. According to the paradigm, the following contrasts are reported only for the betweengroup analysis: beep tones vs. baseline (scanner-noise), chimes vs. baseline, neutral words vs. baseline and affective words vs. baseline.
Beep tones caused significant activation differences of nucleus caudatus, anterior part of gyrus cingulum and prefrontal regions (Brodmann areas 9 and 10), when comparing tinnitus group with the controls. The comparison of controls with tinnitus patients indicated activation differences of thalamus and posterior part of gyrus cingulum, in right prefrontal and frontal areas (Brodmann areas 6 and 10), and in somatosensory area (Brodmann area 2).
In the tinnitus group, the individual patterns of brain activation were correlated to the results of the TQ (tinnitus-evoked distress) and the depression score. Significant correlation coefficients were revealed between depression scores and activations of caudatus, thalamus, superior temporal gyrus (Brodmann area 28) and inferior frontal gyrus (Brodmann area 47) (r = 0.79; p < 0.001 for caudatus). Significant correlation was found between the depression score and significant deactivation in middle frontal gyrus (Brodmann area 10) (r = 0.85; p < 0.001).
| age | localization of tinnitus | gender | tinnitus distress | emotional distress | cognitive distress | intrusiveness | hearing problems | sleeping problems | somatic complaints | depression score | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 23 | Bilateral | m | 18 | 6 | 5 | 4 | 0 | 2 | 1 | 10 | |
| 53 | Right | f | 31 | 8 | 4 | 8 | 3 | 2 | 0 | 15 | |
| 34 | Right | f | 10 | 4 | 3 | 1 | 0 | 0 | 2 | 2 | |
| 41 | Bilateral | f | 25 | 9 | 3 | 10 | 4 | 3 | 2 | 50 | |
| 58 | Left | f | 55 | 15 | 12 | 12 | 6 | 5 | 5 | 32 | |
| 42 | Bilateral | m | 50 | 13 | 7 | 14 | 8 | 6 | 2 | 55 | |
| 36 | Bilateral | m | 23 | 5 | 4 | 9 | 3 | 2 | 0 | 18 | |
| 50 | Bilateral | m | 24 | 6 | 7 | 7 | 3 | 4 | 1 | 27 | |
| 64 | Bilateral | m | 30 | 7 | 5 | 9 | 5 | 2 | 3 | 8 | |
| 42 | Bilateral | f | 33 | 5 | 2 | 10 | 7 | 5 | 4 | 20 | |
Table 1: Individual patient’s characteristics and tinnitus impairment scores.
| Scale | Mean ± SD | Range |
|---|---|---|
| Tinnitus questionnaire | 29.9 ± 13.7 | 10.0 – 55.0 |
| Emotional distress | 7.8 ± 3.6 | 4.0 – 15.0 |
| Cognitive distress | 5.2 ± 2.9 | 2.0 – 12.0 |
| Intrusiveness | 8.4 ± 3.7 | 1.0 – 14.0 |
| Auditory perceptual difficulties | 3.0 ± 2.7 | 0 – 8.0 |
| Sleep disturbances | 3.1 ± 1.9 | 0 – 6.0 |
| Somatic complaints | 2.0 ± 1.6 | 0 – 5.0 |
| Beck’s depression inventory | 23.7 ± 17.6 | 2.0 – 55.0 |
| SD: standard deviation | ||
Table 2: Tinnitus impairment and depressive symptoms – mean scores for the tinnitus group. Given are the scores of the tinnitus questionnaire and its subscales and of the Beck’s depression inventory.
| Parameter | Controls | Patients | |
|---|---|---|---|
| Gender (female/male) | 8/5 | 5/5 | |
| Depression score | 7 | 24* | |
| Stimulus rating (VAS, range 0 neutral – 10 highly affective): | |||
| Beep tones | 1.6 | 2.0 | |
| Chimes | 8.8 | 7.1* | |
| Neutral words | 5.1 | 5.2 | |
| Affective words | 8.0 | 6.6 | |
*p < 0.05 vs. controls.
Table 3: Depression score and stimulus rating of healthy controls and tinnitus patients.
Chimes induced significantly different activation pattern between tinnitus patients and control subjects in following brain structure regions: (a) caudatus, thalamus and insula, (b) limbic structures: gyrus cinguli anterior, (c) prefrontal areas left (gyrus frontalis medius, Brodmann area 11) and (d) superior temporal areas (Brodmann area 38). In the between-group comparison of controls vs. tinnitus, significant activation differences were found in the left precuneus and the Brodmann area 39 (gyrus temporalis medius).
For the tinnitus group, the regressions of subjective impairment and fMRI activation pattern were significant in thalamus, superior temporal gyrus (Brodmann area 22), medial frontal gyrus (Brodmann area 10) and Gyrus precentralis. The correlation coefficient between depression score and activation in thalamus was r = 0.67 (p < 0.001). Significant correlations with Tinnitus-Distress-Syndromes were found inferior parietal (Brodmann area 40). Significant correlation scores for depression and significant deactivation were found in the anterior cingulum.
Neutral words induced relative over-activation in the tinnitus group in left frontal regions, in medial and inferior temporal regions (GTM, GTI), in the thalamus, gyrus lingualis, limbic regions: gyrus parahippocampi, gyrus cingulus and cerebellum in comparing both experimental groups. No significant effect was found for the betweengroup analysis controls vs. tinnitus patients. In the tinnitus group, significant regression scores between fMRI activation pattern and scores in depression inventory resulted in the lingual and fusiform gyrus, in the superior and medial temporal gyrus (Brodmann area 22), in the inferior frontal gyrus and the parahippocampal gyrus and the cerebellum. The correlation coefficient of depression score and activation in the lingual gyrus was r = 0.677 (p < 0.001).
Affective words induced significantly different activation only in the left prefrontal brain in comparing tinnitus group vs. controls. Controls had significant changes in activation of the left inferior frontal gyrus (Broca’s area, Brodmann area 46), prefrontal (Brodmann area 10) and temporal regions (gyrus temporalis medius), of the limbic cortex: parahippocampal gyrus, cingulate gyrus, in the precuneus, substantia nigra and pons, as compared to the tinnitus patients. Significant correlation was found only with significant deactivation. Depression scores (BDI) correlated with deactivation in caudate, putamen, cingulum, precuneus, middle and superior frontal gyrus (Brodmann area 9 and 10) and the insula. For example, the correlation coefficient between BDI scores and deactivation in caudate was 0.692 (p < 0.001). Tinnitus-related distress (TQ) correlated with deactivation in the anterior cingulum, Brodmann areas 4 and 40.
Discussion
The results of our study are partially in line with several other imaging studies that likewise found abnormal activation in the auditory cortex and the limbic system of tinnitus sufferers or healthy controls exposed to aversive noise [4,5,15,22-24]. Taken together, these studies suggest that the auditory cortex and limbic areas are always involved in the sounds processing by tinnitus patients, also the frontal and parietal cortex. The temporal regions and parietal association cortices are mainly involved in perceptual issues (loudness etc.) and in secondary processing concerning the character of the sound whereas the frontal regions are involved in with motivational attention of tinnitus (i.e., the tinnitus becoming a signal of high importance, so that it draws executive top-down attention) [25]. Prefrontal areas are seen as a “candidate for the integration of sensory and emotional aspects of tinnitus” [26]. Structures of the limbic system are involved in emotional aspects of tinnitus and in conjunction with frontal brain regions may be responsible for emotional distress and negative feedback loops seen in tinnitus [4,12,15]. It remains to be determined to what extend the tinnitus-related brain network is a cause or consequence of the problem. Bulk of published literature suggests that tinnitus-related sound perception and distress correlate with two separate mechanisms in the brain [4]. Our data suggest possible integration of several mechanisms into one network for acoustic processing and development of tinnitusrelated distress.
The major methodical limitation of our paper is small sample size. Unfortunately, extensive costs of fMRI reduced the possibility of extending the number of patients. Another drawback of our work is a lack of balance between the non-verbal auditory stimuli (negative and positive) and the verbal stimuli (emotional and neutral). These stimuli were chosen based on set-ups existing at that time and, unfortunately, cannot be redone.
In our study, we have not found differences between tinnitus patients and healthy subjects in the activation of primary and secondary acoustic cortices (Brodmann areas 41 and 42). This could be due to the large variability of AC morphology. However, we found the differences in the acoustic association areas and areas included in (episodic) memory processing (temporo-parietal association-cortex: angular gyrus, precuneus or posterior part of cingular gyrus; fronto-lateral association-cortex; basal ganglia and cerebellum). Other studies suggested that the amount of distress in tinnitus patients is related to the activation of amygdala-ACC-insula-parahippocampal area [4]. This distress network is also seen to be involved in affective aspects of pain [24].
| Activation in the tinnitus patients compared to controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Stimulus | Lobe | Structure | Side | BA | Z-score | x | y | z | P |
| A | Caudatus | 2.46 | -6 | 8 | 0 | 0.007 | |||
| limbic | G. cinguli anterior | L | 32 | 2.18 | -2 | 26 | 38 | 0.015 | |
| prefrontal | G. frontalis inferior | L | 9 | 2.19 | -44 | 2 | -22 | 0.014 | |
| prefrontal | G. frontalis medius | L | 9 | 2.14 | -48 | 18 | 38 | 0.016 | |
| prefrontal | G. frontalis superior | R | 10 | 1.97 | 30 | 52 | 2 | 0.024 | |
| B | Caudatus | L | 2.81 | 0 | 2 | 6 | 0.003 | ||
| sublobar | Thalamus | L | 2.84 | -2 | -30 | 4 | 0.002 | ||
| sublobar | Insula | L | 13 | 3.20 | -44 | -6 | 0 | 0.001 | |
| limbic | G. cinguli anterior | 32 | 2.65 | 0 | 42 | -6 | 0.004 | ||
| frontal | G. frontalis medius | R | 11 | 3.10 | 2 | 54 | -14 | 0.001 | |
| temporal | G. temporalis superior | R | 38 | 3.39 | 32 | 12 | -22 | 0 | |
| C | frontal | G. frontalis inferior | L | 47 | 3.45 | -38 | 30 | -18 | 0 |
| frontal | G. frontalis medius | L | 11 | 2.47 | -8 | 42 | -8 | 0.007 | |
| limbic | G. parahippocampi | L | 35 | 3.19 | -22 | -24 | -30 | 0.001 | |
| limbic | G. cinguli posterior | 31 | 1.98 | 14 | -24 | 44 | 0.024 | ||
| G. lingualis | R | 18 | 3.91 | 12 | -74 | 0 | 0 | ||
| sublobar | Thalamus | 1.97 | -14 | -18 | -2 | 0.024 | |||
| Cerebellum | 2.14 | -6 | -46 | -34 | 0.016 | ||||
| D | prefrontal | G. frontalis superior | L | 10 | 2.01 | -16 | 56 | 20 | 0.022 |
| Activation in controls compared to tinnitus patients | |||||||||
| Stimulus | Lobe | Structure | Side | BA | Z-score | x | y | z | P |
| A | sublobar | Thalamus | 2.18 | 36 | -53 | -12 | 0.003 | ||
| limbic | G. cinguli posterior | L/R | 31 | 1.84 | -14 | -30 | 36 | 0.001 | |
| prefrontal | G. frontalis inferior | R | 10 | 2.38 | 40 | 50 | 4 | 0.009 | |
| frontal | G. precentralis/frontalis* | R | 6 | 1.92 | -28 | -12 | 52 | 0.027 | |
| G. postcentralis | L | 2 | 2.20 | -40 | -30 | 36 | 0.014 | ||
| B | parietal | Precuneus | L | 7 | 2.90 | -22 | -70 | 26 | 0.002 |
| temporal | G. temporalis medius | L | 39 | 2.72 | -36 | -68 | 26 | 0.003 | |
| D | frontal | G. frontalis inferior | L | 46 | 3.83 | -46 | 36 | 16 | 0 |
| prefrontal | G. frontalis superior | L | 10 | 3.07 | -26 | 58 | 6 | 0.001 | |
| limbic | G. cinguli posterior | 23 | 3.17 | -8 | -58 | -24 | 0.001 | ||
| limbic | G. parahippocampi | L | 35 | 3.80 | -22 | -12 | -26 | 0 | |
| temporal | G. temporalis medius | L | 20 | 2.79 | -44 | -20 | -16 | 0.003 | |
| parietal | Precuneus | 7 | 3.00 | -6 | -60 | 32 | 0.001 | ||
| midbrain | Substantia nigra | 2.89 | -12 | -26 | -8 | 0.002 | |||
| Brainstem | Pons | 3.21 | -4 | -40 | -32 | 0.001 | |||
| A: beep; B: chimes; C: neutral words; D: affective words; G.: gyrus; L: left; R: right; BA: Brodman area; *gyrus frontalis medialis | |||||||||
Table 4: Significant signal differences in the between-group contrasts. Given are the responses to different auditory stimuli. vs. baseline.
In the controls and in the tinnitus group, we found activation of left parahippocampal gyrus during listening to the affective words and the neutral words. The parahippocampal gyrus is one of the areas preferentially active during encoding (relative to retrieval [27]), distinguishing unpleasant from neutral or pleasant emotion [28] and seems to be coupled with a noise-like character of the acoustic stimuli. Possibly in association with the tinnitus tone, tinnitus patients failed to distinguish between pleasant and unpleasant (affective and neutral).
The higher involvement of striatum (caudate, putamen, gyrus frontalis medius, gyrus frontalis inferior) seen in our study in tinnitus patients corroborates the hypothesis of Jastreboff, who suggested involvement of prefrontal cortex in the auditory attention directed towards tinnitus and in the emotional emphasizing of tinnitus [26].
Each of the striatal nuclei serves as an input point for signal circuits that originate in the cerebral cortex, pass through other basal ganglia and then return to the cerebral cortex via the thalamus. Previously performed fMRI studies confirm the involvement of (left) parietal and frontal sites in alerting (monitoring and regulation performance and arousal levels, especially left after a warning cue und usual right hemisphere activation as phasic and tonic influences) [29].
Furthermore, our data demonstrated stronger involvement of limbic structures of tinnitus patients in response to any auditory stimuli, as compared to the control subjects. Acoustic stimulation with tinnitus-like aversive beep tones had equally high emotional impact in both groups. However, the sound of pleasant chimes has activated the limbic, prefrontal and association areas significantly stronger in the tinnitus group than in healthy subjects. Also neutral words have significantly activated whole emotion-processing brain network in the tinnitus but not in the control group. This over activation seen in tinnitus patients could be associated with their general negative distress [30]. Interestingly, we have demonstrated activation of affectiveprocessing network of limbic, prefrontal and association-cortices in the control group only in case of the emotionally important words, as compared to the tinnitus group. Therefore, it is tempting to speculate that the prominent involvement of an emotional and attentional distress network could contribute to the development of negative mental symptoms associated with tinnitus, such as stress, insomnia, anxiety, tension and depression. Furthermore, these networks may also have an intensifying effect on tinnitus or could reinforce negative feedback-loops.
Regression analyses of our sample have suggested a latent (subclinical) depression or higher vulnerability towards emotional distress a cause for the tinnitus impairment.
Conclusions
Overall, the deficits in primary and secondary auditory cortex are one possible origin for the perception of auditory signals without internal or external source of sound [6,24,31]. Our present results have demonstrated altered cerebral response patterns in acoustic association cortices, striatum and the limbic system in response to different auditory stimuli in patients with tinnitus, as compared to the healthy subjects. In the tinnitus group, the resulting brain activity has not strictly corresponded to the affective value of stimuli, as compared to the control group; suggesting impairment in affective discrimination. Higher activation status found in limbic structures in response to acoustic stimuli suggests modified emotional processing in tinnitus patients, possibly contributing to the tinnitus-distress. This attentional and emotional state is tightly connected with tinnitus perception (unpleasantness of tinnitus tone) and related to tinnitus distress.
References
- Baguley DM, Axon P, Winter IM, Moffat DA (2002) The effect of vestibular nerve section upon tinnitus. Clin Otolaryngol Allied Sci 27: 219-226.
- Cacace AT (2003) Expanding the biological basis of tinnitus: crossmodal origins and the role of neuroplasticity. Hear Res 175: 112-132.
- Georgiewa P, Klapp BF, Fischer F, Reisshauer A, Juckel G, et al. (2006) An integrative model of developing tinnitus based on recent neurobiological findings. Med Hypotheses 66: 592-600.
- Vanneste S, Plazier M, der Loo E, de Heyning PV, Congedo M, et al. (2010) The neural correlates of tinnitus-related distress. Neuroimage 52: 470-480.
- Smits M, Kovacs S, de Ridder D, Peeters RR, van Hecke P, et al. (2007) Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology 49: 669-679.
- Muhlnickel W, Elbert T, Taub E, Flor H (1998) Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci U S A 95: 10340-10343.
- Møller AR (2007) The role of neural plasticity in tinnitus. Prog Brain Res 166: 37-45.
- Melcher JR, Levine RA, Bergevin C, Norris B (2009) The auditory midbrain of people with tinnitus: abnormal sound-evoked activity revisited. Hear Res 257: 63-74.
- Lockwood AH, Salvi RJ, Coad ML, Towsley ML, Wack DS, et al. (1998) The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology 50: 114-120.
- Levine RA (1999) Somatic (craniocervical) tinnitus and the dorsal cochlear nucleus hypothesis. Am J Otolaryngol 20: 351-362.
- Schlee W, Weisz N, Bertrand O, Hartmann T, Elbert T (2008) Using auditory steady state responses to outline the functional connectivity in the tinnitus brain. PLoS One 3: e3720.
- Melcher JR, Sigalovsky IS, Guinan JJ Jr, Levine RA (2000) Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol 83: 1058-1072.
- Arnold W, Bartenstein P, Oestreicher E, Romer W, Schwaiger M (1996) Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: a PET study with [18F]deoxyglucose. ORL J Otorhinolaryngol Relat Spec 58: 195-199.
- Mirz F, Gjedde A, Sødkilde-Jrgensen H, Pedersen CB (2000) Functional brain imaging of tinnitus-like perception induced by aversive auditory stimuli. Neuroreport 11: 633-637.
- Goebel G, Hiller W (1994) [The tinnitus questionnaire. A standard instrument for grading the degree of tinnitus. Results of a multicenter study with the tinnitus questionnaire]. HNO 42: 166-172.
- Hallam RS, Jakes SC, Hinchcliffe R (1988) Cognitive variables in tinnitus annoyance. Br J Clin Psychol 27: 213-222.
- Hiller W, Goebel G, Rief W (1994) Reliability of self-rated tinnitus distress and association with psychological symptom patterns. Br J Clin Psychol 33: 231-239.
- Beck AT (1995) Beck-Depressions-Inventar (BDI). Dt. Bearbeitung von M Hautzinger, M Bailer, H Worall, F Keller. Göttingen: Hogrefe.
- Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system - an approach to cerebral imaging. Stuttgart: Thieme.
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, et al. (1995) Spatial registration and normalization of images. Hum Brain Mapp 3: 165-189.
- Andersson G, Lyttkens L, Hirvela C, Furmark T, Tillfors M, et al. (2000) Regional cerebral blood flow during tinnitus: a PET case study with lidocaine and auditory stimulation. Acta Otolaryngol 120: 967-972.
- Mirz F, Gjedde A, Ishizu K, Pedersen CB (2000) Cortical networks subserving the perception of tinnitus--a PET study. Acta Otolaryngol Suppl 543: 241-243.
- Weisz N, Moratti S, Meinzer M, Dohrmann K, Elbert T (2005) Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Med 2: e153.
- Jastreboff PJ (1990) Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res 8: 221-254.
- Maguire EA, Frith CD, Morris RG (1999) The functional neuroanatomy of comprehension and memory: the importance of prior knowledge. Brain 122: 1839-1850.
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, et al. (1997) Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia 35: 1437-1444.
- Raz A, Buhle J (2006) Typologies of attentional networks. Nat Rev Neurosci 7: 367-379.
- Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, et al. (2002) Neural and behavioral substrates of mood and mood regulation. Biol Psychiatry 52: 478-502.
- Flor H, Hoffmann D, Struve M, Diesch E (2004) Auditory discrimination training for the treatment of tinnitus. Appl Psychophysiol Biofeedback 29: 113-120.
Citation: Georgiewa P, Bohner G, Rothemund Y, Klingebiel R, Olze H, et al. (2012) Affective Processing in Tinnitus Patients Assessed by Functional Magnetic Resonance Imaging. Otolaryngology S3:003. DOI: 10.4172/2161-119X.S3-003
Copyright: © 2012 Georgiewa P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15250
- [From(publication date): 1-2012 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 10448
- PDF downloads: 4802