Research Article Open Access
Augmenting Composting Microbial Community with Thermophilic Cellulolytic Organisms for Enhanced Degradation of Phenolic Compoundsin Creosote Treated Wood Waste
| Abdel E Ghaly*, Deepika Dave and Bopeng Zhang | |
| Department of Process Engineering and Applied Science, Dalhousie University, Halifax, Nova Scotia, Canada | |
| Corresponding Author : | Abdel E Ghaly Professor Department of Process Engineering and Applied Science Dalhousie University, Halifax, Nova Scotia, Canada Tel: (902)494-6014 Email: abdel.ghaly@dal.ca |
| Received January 23, 2012; Accepted March 05, 2012; Published March 07, 2012 | |
| Citation: Ghaly AE, Dave D, Zhang B(2012) Augmenting Composting Microbial Community with Thermophilic Cellulolytic Organisms for Enhanced Degradation of Phenolic Compounds in Creosote Treated Wood Waste. J Bioremed Biodegrad 3:139. doi:10.4172/2155-6199.1000139 | |
| Copyright: © 2012 Ghaly AE, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Creosote is widely used as a wood preservative in railway sleepers, utility poles, bridges, building foundations, fences, stakes for vegetables and fruits, garden furniture and outdoor recreational facilities. Contamination of soil and water and threat to human and animals health are the major constraints to disposal of creosote-treated wood waste. Composting provides a treatment option for creosote-treated wood waste and production of a value- added product. The aim of this study was to test the effectiveness of inoculating the composting process with three thermophilic-cellulolytic microorganisms ( T. curvata , T. aurantiacus and T. fusca ) in degrading phenols in creosote treated wood waste. Used cooking oil was added into the composting system as a bio-available carbon source. The temperature, pH, moisture content, solids, total carbon, nitrogen, phenols, cellulose and lignin were monitored. The temperature profiles showed that the thermophilic phase (>45 ?C) was achieved and successfully maintained due to the addition of used cooking oil. The moisture content decreased because the water produced by microbial respiration did not compensate for the water vapour lost with the exhaust gases. The breakdown of organic nitrogen to ammonium caused an initial increase in the pH which was then decreased due to the formation of organic acids from the decomposition of fats and the loss of ammonia with the exhaust gases. The inoculated experiments achieved higher reductions in volatile solids, total carbon, TKN, phenols, cellulose and lignin compared to the control. Different degradation rates were observed in the psychrophilic, mesophilic and thermophilic stages of composting. The product from the inoculated experiment had improved stability and phytotoxicity compared to that of the control (uninocualted). The inoculation of thermophilic-cellulolytic microorganisms ( T. curvata , T. aurantiacus and T. fusca ) accelerated the composting process and resulted in higher degradation of phenolic compounds, lignocellulose and lignin.
| Keywords |
| Wood waste; Phenolic compounds; Cellulose; Lignin; Composting; Degradation; Cellulolytic; Mesophilic; Thermophilic; Stability; Phytotoxicity |
| Introduction |
| Creosote is a strong fungicide and genotoxicant (at a concentration of 1-2%) and is used to expand the service life of wood. Creosote is widely used as a wood preservative and waterproofing agent for railway ties, cross ties, bridges, utility poles, building foundation and fences, stacks for agricultural production and furniture and outdoor recreational facilities [1,2]. |
| Creosote contains 75% of polycyclic aromatic hydrocarbons (PAHs), 2-17% phenolic compounds, and 10-18% heterocyclic organic compounds. Polycyclic aromatic hydrocarbons (PAHs) and phenols are responsible for the carcinogenic and genotoxic properties of creosote and severely effect living creatures including human. Direct or indirect human exposure to creosote treated wood may cause damage to kidney, liver, bladder, eyes and skin cancer [1]. Release of components such as PAHs and furans and the possible migration of phenolic compounds into soil and groundwater are major constraints in reuse and disposal of creosote-treated wood wastes. Proper treatment of creosote-treated wood waste is crucial. |
| Composting has been used for the degradation of environmental contaminants such as pesticides and petroleum compounds [3]. Composting could be used as an, environmentally friendly and effective process for degrading pollutants in creosote-treated wood waste that are not easily degraded in the environment with conventional disposal practices such as combustion and land filling. Also, wood substance can be converted into humus and plant nutrients during the volumedecreasing composting processes of mineralization and humification. |
| The final value-added product is free of contaminants and could be used as a soil amendment [4-6]. As a bioremediation technique, composting has several other advantages including: (a) elevated thermophilic temperature phase (which means a higher level of biological activity), (b) less management and maintenance costs and (c) large treatment capability. |
| However, typical municipal waste composting processes still take several months to several years to finish, during which time thermophilic phase (<45°C) may last for only a few hours or days [7,8]. A possible way to extend the thermophilic phase is to provide additional bioavailable carbon and augment the composting microbial population with thermophilic-cellulolytic microbes (Thermomonospora curvata, Thermoascus aurantiacus and Thermobifida fusca) that would help in the decomposition of wood component. T. aurantiacus is thermophilic ascomycetous, has an optimum growth temperature of 45°C and produces a pool of stable enzymes (cellulases, hemicellulases, amylases and xylanases) [9]. The cellulases secreted by T. aurantiacus have optimum temperature in the range of 65-75°C [10]. T. curvata is a filamentous actinomycete, can grow at temperature between 40°C and 60°C, produces single heat sensitive aleurospores on aerial hyphae and recognized as a major cellulose decomposer in municipal solid waste composting system and produces a pool of extracellular enzymes including endoglucanases, β-glucosidase, amylases, pectinases, and xylanases [11]. T. fusca is a filamentous thermophilic actinomycete, has the ability to degrade cellulose and hemicelluloses and produces extracellular cellulolytic enzymes including β-1,4-endoglucanases, β-1,4-exoglucanases, and processive endoglucanase. The optimum temperature for the extracellular lignocellulolytic enzyme produced by T. fusca is 50°C [12,13]. |
| The main objective of this study was to evaluate the ability of three thermophilic cellulolytic microbes (Thermoascus aurantiacus and Thermomonospora curvata, Thermobifida fusca) to degrade phenolic compounds and cellulose in creosote-treated wood waste, while at the same time produce a marketable compost/soil conditioner. The focus was on phenolic compounds because (a) extensive studies have been conducted on PAHs but few reports were found in the literature on phenolic compounds and (b) phenolic compounds are water soluble which makes them easy to be monitored. |
| Experimental Apparatus |
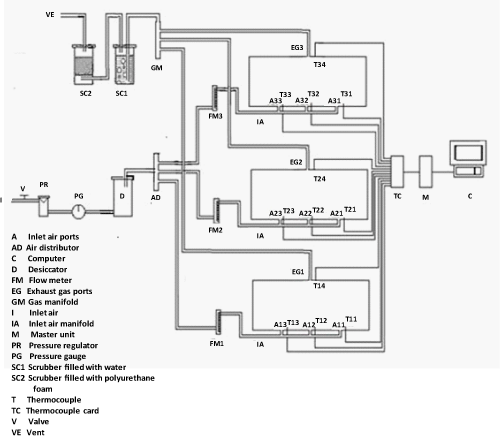
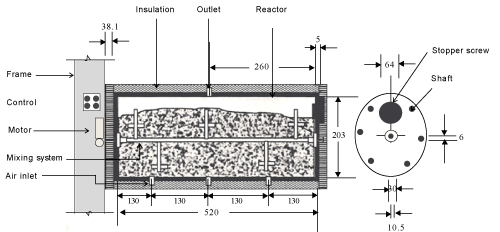
| The experiments were carried out in a specially designed multiple bioreactor composting system (Figure 1). Three bioreactors made of a polyvinyl chloride cylinder (PVC/711, IPS Corporation, Gardena, Canada) were horizontally fastened into a main frame. Each cylinder had an inside diameter of 203 mm, a length of 520 mm and a wall thickness of 5 mm which provided a space for 3.5 kg (wet-basis) of the compost mixture plus 25% of the volume as a head space (Figure 2). A fixed circular PVC plate of 203 mm diameter and 6 mm thickness was glued into the back end of the cylinder. A removable circular plexiglas plate of 203 mm diameter and 6 mm thickness was installed on the front end of the cylinder. A circular window of 64 mm diameter was left on the removable circular plate for sampling purposes. The window was closed with a rubber stopper (No.13) when it was not in use. The cylinders were insulated with a 38.1 mm thick fibreglass while the removable circular plates were insulated with a 38.1 mm thick Styrofoam layer. |
| Each bioreactor had three holes at the bottom which were used for supplying air to the bioreactor. A hole at the top was used for the exhaust gas. The pressure regulated air passed through a desiccator and then through a flow meter (No 32461-14, Cole-Parmer Instrument Company, Vernon Hills, Illinois, USA). The flow meter had a 10 cm scale and a range of 0.0566-0.566 m3/h. The exhaust gas passed through a scrubber that contained water to get rid of aerosol and water soluble organic compounds, then through another scrubber to eliminate possible airborne PAH compounds in the exhaust gas. |
| Inside each bioreactor, a removable 10.5-mm diameter solid stainless steel shaft was mounted on two bearings (Figure 2). There were 5 stainless steel collars on the shaft on each of which, a bolt of 69 mm length and 6 mm diameter was mounted. A thermally protected electric motor (Model No. 127P1486/B, D.C., Sigma Instruments Inc., Braintree, Massachusetts, USA) provided power to rotate the mixing shaft (at 6 rpm). |
| The data acquisition unit consisted of a master unit (Multiscan 1200, Omega, Stamford, Connecticut, USA), thermocouple/volt scanning card (MTC/24, Omega, Stamford, Connecticut, USA), Tempview software (Omega, Stamford, Connecticut, USA), temperature sensors (type T thermocouples, Cole Parmer, Chicago, Illinois, USA) and a personal computer. The thermocouples were inserted through specially constructed fitting. The thermocouples on the bottom of the bioreactors were located far enough from the inlet air holes (65 mm away) to minimize the influence of inlet air temperature. |
| Experimental Procedure |
| Collection and preparation of wood waste |
| C&D wood was collected from a C&D site in Yartmouth, Nova Scotia and screened to remove visible non-biodegradable materials. Then, it was sieved using USA Standard Testing Sieve with 12.5 mm opening (USA Standard Testing Sieve, ATM, Milwaukee, Wisconsin, USA). Fresh compost (Miller Compost Corporation, Dartmouth, Nova Scotia, Canada) was mixed with the wood waste at a ratio of 1:1 as a source of composting microbial culture and bioavailable nutrients. The moisture content was adjusted to 60% using distilled water. Urea [CO(NH2)2] was added as a nitrogen source. |
| Preparation of inoculums |
| The three microorganisms (table 1) were obtained from the American Type Culture Collection (Manassas, Virginia, USA) and used collectively in one of the experiments. Broth culture, slant solid media and Petri dish solid media were used to grow the three microorganisms. For T. aurantiacus, potato dextrose agar (PDA) medium was used for slants and Petri dishes, and potato dextrose broth was used as a liquid medium. For T. fusca, Trypticase-yeast extract-glucose (TYG) medium was used for slants and Petri dishes, and Trypticase-yeast extract-glucose (TYG) broth medium was used as a liquid medium. For T. curvata, Hichey-Tresner agar was used for slants and Petri dishes, and Hichey-Tresner broth medium was used as a liquid medium. All media were made using distilled water and the reagents listed in Table 2 (Sigma Aldrich, St. Louis, Missouri). |
| Suspensions of T. aurantiacus, T. fusca and T. curvata were prepared by cutting 1 cm2 Petri dish culture into 25 ml sterile potato dextrose, TYG and Hichey-Tresner liquid media, respectively. The inoculated media were incubated in an incubator (Isotemp® oven, Model 106G, Fisher Scientific, Hampton, New Hampshire) for 48 h at 48°C. Plate counts were then conducted to detect colony forming units (CFU). An amount of 5 ml of each medium (containing approximately CFU of 2.0 × 106/ml) were transferred into 6 Fernbach flasks containing 250 ml of designated liquid cultural medium and agitated on rotary shaker (Series G-25 Incubator Shaker, New Brunswick Company, New Jersey, USA) at 120 rpm and 45°C for 48 hours. The final cultures were collected from the Fernbach flasks, mixed together and used as an inoculum. |
| Experimental protocol |
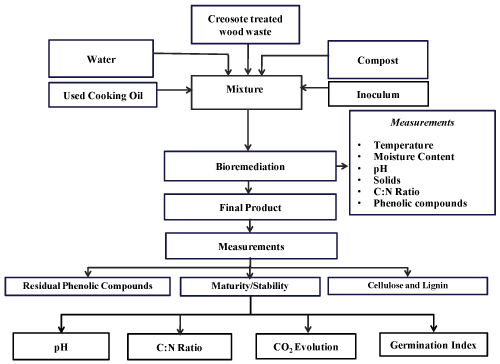
| A summary of the experimental procedure performed on creosote treated wood waste samples and the various parameters measured during the treatment process is presented in Figure 3. After adding the inoculum (100 mL/kg), used cooking oil (as a source of bioavailable carbon) and water (to adjust moisture content of 60%), approximately 3.5 kg of the final mixture were placed in each bioreactor. Two experiments were carried out: (a) a control (uninoculated) experiment and (b) an inoculated experiment (with three microorganisms added to the compost mixture). Three replicates were conducted for each experiment using the three bioreactors simultaneously. Each experiment lasted for 15 days. The pressure-regulated air was supplied continuously to the bottom of the bioreactor and the air flow rate was measured by a flow meter (No 32461-14, Cole-Parmer Instrument Company, Vernon Hills, Illinois, USA) and adjusted to 0.05 m3/h. The temperature was monitored throughout the process and the data was stored in a Microsoft Excel® file in the computer every 30 min. The temperature profile of each experiment was generated using the average temperature of 12 thermocouples in each of three bioreactors. An amount of 36 ml used cooking oil were added into each bioreactor every 12 h for the duration of the experiment as a source of bioavailable carbon as recommended by Ghaly et al. [14]. Samples were collected every 3 days and analyzed for pH, moisture content, total carbon, solids, total kjeldahl nitrogen, phenolic compounds, cellulose and lignin. The experiment was determined after 15 days and the quality, stability and maturity of the end product were evaluated by quantifying C:N ratio, pH, CO2 evolution, phytotxicity, degradation of cellulose, lignin and phenolic compounds. |
| Experimental Analysis |
| Moisture content and pH |
| The moisture content (MC) was measured following the ASTM (D4442-07) oven-drying method [15]. Slurry contained about 10 g of material and 50 ml distilled water was used to measure the pH value using a pH meter (Fisher Accumet®, Model 805 MP, Fisher Scientific, Montréal, Quebec, Canada). |
| Total carbon |
| Approximately 1.0 g of the material was used for total carbon analysis. Carbon dioxide was determined with a Leco carbon analyzer (Model 516-000, Leco Corporation, St. Joseph, Michigan, USA) along with an induction furnace (Leco HF2O Furnace, Leco Corporation, St. Joseph, Michigan, USA) at the Mineral Engineering Centre of Dalhousie University, Halifax, Nova Scotia. |
| Solids |
| The solids analyses were performed according to the procedures described in the USEPA Method 1684 [16]. Ash contents were determined by burning samples (approximately 1g) in muffle furnace (Isotemp® Muffle Furnace, Model 186A, Fisher Scientific, Montréal, Quebec, Canada) at a temperature of 550°C for 30 minutes. |
| Total kjeldahl nitrogen |
| The total kjeldahl nitrogen (TKN) was determined at Maxxam Analytical Testing Laboratory in Mississauga, Ontario, following the procedure of USEPA Method 351.2 [17]. |
| Phenolic compounds |
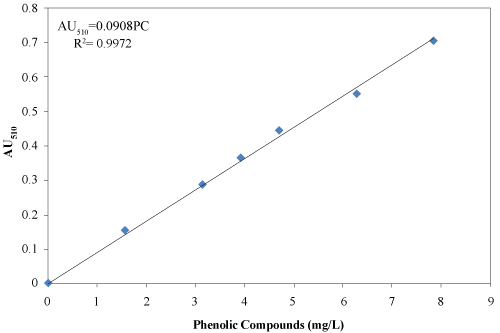
| Phenolic compounds were extracted from 3 g material with 50 ml deionized water and centrifuged for 20 min at 2400 rpm. The supernatant was vacuum filtered through a 0.4 μm polycarbonate filter paper (45 mm diameter polycarbonate filter paper, Fisher Scientific, Montreal, Quebec, Canada) as described by Chantigny et al. [18]. The supernatant was transferred into a flask and analyzed for the presence of phenolic compounds using the 4-aminoantipyrine colorimetric test following the ASTM procedure [19]. The absorbance was measured at 510 nm using spectrophotometer (Spectronic 601, Milton Roy, Ivy land, Pennsylvania, USA). A standard curve was developed as shown in Figure 4. |
| The germination index (GI) |
| The germination index (GI) was measured following the procedure described by Jiang et al. [20]. About 10 g of final compost sample were mixed with 100 mL distilled water. Ten cress seeds (Lepidium sativum L.) were placed on the filter paper (Whatman® 40, Whatman Inc., Clifton, New Jersey) in a sterilized Petri dish. Then, 5.0 ml of the extract was transferred into the filter paper. Three replicates were carried out for each sample. The Petri dishes were incubated at 25Ã?Â?C in the dark for 48 hours. The results were evaluated by counting the number of germinated seeds and measuring the length of roots. The germination index (GI) was determined as follows: |
 (1) (1) |
| CO2 evolution |
| CO2 evolution was determined as described by Benito et al. [21]. Approximately 25 g of the final compost were pre-incubated at room temperature for 3 days. The moisture content was adjusted to 60% and each sample was separately sealed in containers containing a beaker with 10 ml of 1 M NaOH solution. The samples were incubated at 25°C and the CO2 generated was determined by titrating NaOH solution with 1 M HCl solution every day for 5 consecutive days. The rate of CO2 evolution was calculated as mg C-CO2 per gram compost per day. |
| Cellulose and lignin contents |
| The cellulose and lignin contents were measured following the Standard Methods published by AOAC International [22] for acid detergent fiber (ADF) and acid detergent lignin (ADL). Cellulose was estimated as the difference between ADF and ADL. Lignin was estimated as the difference between ADL and ash content as described by Yu et al. [23]. |
| Results and Discussion |
| Temperature |
| The organic materials were broken down (to supply carbon and nitrogen and other nutrients for the synthesis of new microbial cells) and utilized by microorganisms for energy and cell growth as follows: |
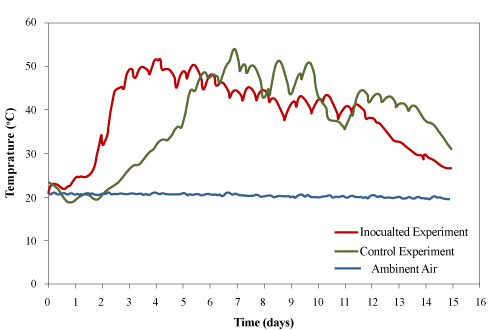
| After loading the materials into the bioreactors (at 23±1°C), the utilization of bio-available carbon (used cooking oil and other biodegradable organic matters in the wood waste) generated heat inside the bioreactors which increased the temperature. However, there was an initial lag time during which the microbial population adjusted to the new environmental conditions inside the reactor. The peak temperatures for control and the inoculated experiments were 51.7°C and 54.5°C, respectively. The temperature was maintained above 45°C for 99 and 108 h and above 40°C for 186 and 191 h for the control and inoculated experiments, respectively. The fluctuation in the temperature was caused by opening the sampling port every 12 hours for adding the used cooking oil (bio-available carbon). |
| Tang et al. [24] composted cattle manure with rice straw in a laboratory-scale composting system and observed a peak temperature of 65°C. Loser et al. [4] composted PAH-contaminated pine wood waste with liquid hog manure (as a nitrogen and mineral source) and reported a maximum temperature of 42°C. The higher temperature achieved in present study may due to the addition of bio-available carbon (used cooking oil) into the system and the use of better thermally insulated bioreactors. |
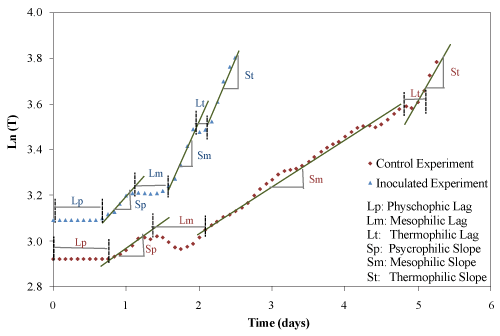
| The temperature profiles of both trials were similar to microbial growth profiles (Figure 5). There are three groups of microorganisms characterized by their optimum growth temperature: psychrophilic (-10°C to 20°C), mesophilic (15°C to 45°C) and thermophilic (35°C to 68°C). In this study, the lag and exponential phases were clearly identified for the physchrophilic, mesophilic and thermophilic phases. The duration of lag phases and the rates of temperature increase for the psychrophilic, mesophilic and thermophilic stages were determined graphically according to the procedure described by Ghaly et al. [25] as shown in Figure 6 and the results are presented in Table 3. The duration of psychrophilic lag phase was 17 and 16 h for the control and inoculated experiments, respectively. The duration of the mesophilic lag phase was 18 and 10 h and started after 30 and 27 h for the control and inoculated experiment, respectively. The duration of thermophilic lag phase was 8 and 6 h and started after 112 h and 46 h for the control and inoculated experiments, respectively. The rates of temperature increase for the psychrophilic, mesophilic and thermophilic stages of the inoculated equipment were much higher by (45%, 176% and 955%, respectively) than those of the control experiment. In the control experiment, the rates of temperature increase for the mesophilic and thermophilic were 25% and 95% higher than that of the psycrophilic stage. However, in the inoculated experiment, the rates of temperature increase for the mesophilic and thermophilic stages were 138% and 162% higher than that of the psycrophilic stage. |
| Moisture content |
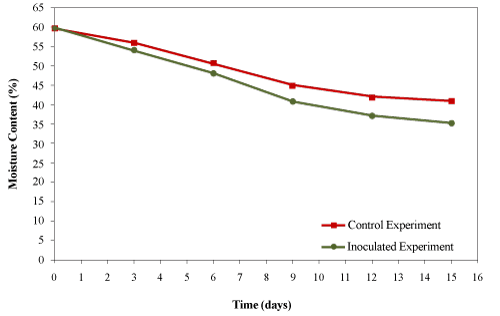
| Water is the media for nutrient transportation and metabolic reactions. The availability of nutrients and contaminants to microorganisms is affected by the water content in their microenvironment especially in the thin liquid layers on the surfaces of particles. The optimum moisture content (MC) is in the range of 50- 70%. If too much water fills the voids, the pore space that allows air diffusion would be limited [8]. In present study, the initial moisture content of the compost mixture was 59.69 ± 0.77% which was within the optimal range. However, the moisture content decreased significantly for both trials (Figure 7) during the 15 days of composting due to the water loss through vapour with the exhaust gases. |
| Guardia et al. [26] composted food waste with wood chips and observed a decrease in the moisture content of the mixture from 63.4% to 50.5% after 37 days of composting. Ghaly et al. [27] reported a reduction in moisture content from 60% to 43% after 15 days of composting creosote treated wood waste. Zhu [28] reported a decrease in the moisture content (from 70.39% to 35.87%) during composting of swine manure with corncob in a pilot-scale aerated static bin system after 60 days. Changa et al. [29] composted three types of manure (dairy manure amended with wheat straw, dairy manure amended with sawdust and swine manure amended with sawdust and ground wood pallets) in windrows for 120 days and reported that the moisture content of the dairy manure-straw compost decreased from 67% to 52% after 98 days, the moisture content of dairy manure- sawdust compost decreased from 65% to 56% after 112 days and the moisture content of the hog manure compost decreased from 65% to 48% after 84 days. The moisture contents for the control and inoculated experiments were 42.9% and 35.3%, respectively which was not within the optimal range but was still high enough for the composting process to processed. |
| pH |
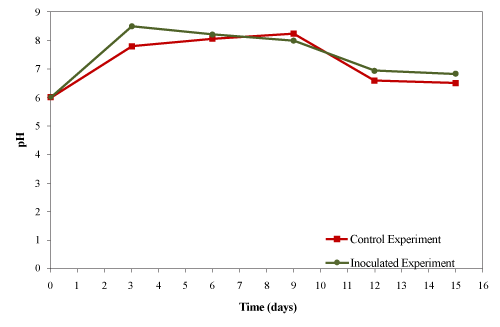
| The initial material was acidic (pH 6.0) which changed to basic (pH 8.3) for the control in the first week of composting and then decreased to a weak acidic (pH 6.6) by the end of experiment as shown in Figure 8. In the inoculated experiment, the pH increased to 8.2 on the 4th day and gradually decreased back to neutral (pH 7.2). |
| Ghaly et al. [27] reported that the initial pH of 6 increased to 8.5 on day 5 and then decreased to 5.5 after 15 days of composing creosote treated wood waste. Ramaswamy et al. [30] reported that the initial pH of 6.7 increased to 8.7 on the 12th day and then gradually declined to 8.1 after 38 days of composting pharmaceuticals (salinomycin-antibiotics) in poultry manure. They concluded that production of ammonia as a result of microbial activities increased the pH while the release an H+ ion due to the volatilization of ammonia nitrogen slightly decreased the pH in the latter part of the experiment. Tang et al. [24] while composting cattle manure with rice straw observed an increase in the pH from 8.0 to 9.2 in the first week which then decreased back to 8.6 after 21 days. In the present study, production of ammonia from the decomposition of organic nitrogen caused the initial pH increase while the decomposition of fats resulted in the formation of organic acids that caused the final drop in the pH. |
| Solids |
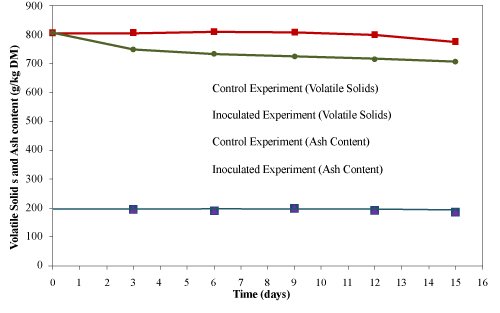
| The initial volatile solids and ash contents of the material were 805 ± 10 g/kg material and 195 ± 12 g/kg material (dry basis), respectively (Figure 9). The ash content remained unchanged till the end of the composting process, while the volatile solids content decreased to 775 ± 12 and 707 ± 12 g/kg material (dry basis) for the control and inoculated experiments, respectively. Microorganisms utilized volatile solids as a major energy source for their growth during the composting process that results into the reduction of volatile solids [31]. Ivanov et al. [32] reported 21% reduction in volatile solids after 10 days during the composting of sewage sludge and food waste. Garland et al. [33] reported 47% reduction in volatile solids after 21 d composting of inedible plant residues. Ghaly et al. [27] reported very small reduction in total solids (0.1-4.6%) while composting wood waste for 15 days. In the present study, the reduction in volatile solid were very small (3.7 and 12.2% for the control and the inoculated experiments, respectively) due to the continuous addition of used cooking oil into the system as bio-available carbon source. |
| Total carbon |
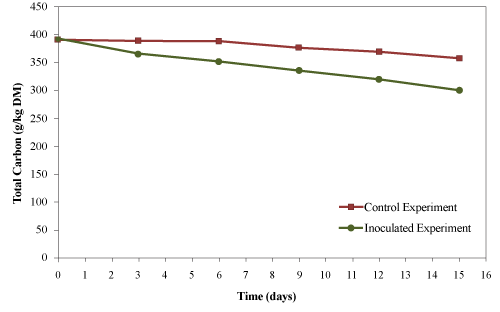
| The initial total carbon of the mixture was 392 g/kg material (dry basis). The final total carbon for the control and the inoculated experiments were 358 g/kg material (dry basis) and 302 g/kg material (dry basis), respectively (Figure 10). The total carbon reductions were 8.7 and 22.9% for the control and inoculated experiment, respectively. The reduction of total carbon was higher (2.6 folds) in inoculated experiment compared to control indicating a higher levels of decomposition in inoculated experiments. |
| Ravindran and Sekaran [34] reported a decrease in total carbon from 55.6% to 27.9% after 49 days of composting animal flesh generated from tannery industries. Zhu [28] reported a reduction in total carbon from 398.47 to 318.11 g/kg (20.16%) during composting of swine manure with for 60 days. Bautista et al. [35] reported a 67% reduction in the total carbon after composting a mixture of swine manure, sawdust and wood bark for 18 days. Changa et al. [29] reported reductions of 64%, 63% and 52% in the total carbon after 98 days of composting dairy manure amended with sawdust, dairy manure amended with wheat straw and swine manure amended with sawdust, respectively. Ghaly et al. [27] reported reductions in the total carbon in the range of 1.5- 8.7% after 15 days of composting creosote treated wood waste. The low reduction in the total carbon obtained in present study may be due to the continuous addition of used cooking oil as a carbon source for microorganisms, shorter composting time and lower biodegradability of the wood waste. |
| Total kjeldahl nitrogen |
| The initial TKN was 24.94 g/kg material (dry basis) which decreased to 12.5 and 2.9 g/kg material (dry basis) by the end of experiment (Figure 11), resulting in TKN reductions of 50.2% and 88.4% for the control and the inoculated experiments, respectively. The reductions in TKN (50.2-88.4%) were more rapid and much higher than the reductions in the total carbon (8.7-22.9%) due to the higher initial nitrogen content, immobilization of NH4 by microorganisms and the loss of NH3 with the exhaust gas. The higher temperature and/or longer thermophilic phase resulted in higher rate of organic nitrogen decomposition and increased nitrogen loss in the inoculated experiment. Several researchers [27,36-39] stated that when the initial C:N ratio is low (<20), the nitrogen is lost via NH3 volatilization and high temperature will accelerate the volatilization process. Ghaly et al. [27] composted creosote treated wood waste for 15 days and reported nitrogen losses of 31.9-50.2%. Tiquia et al. [36] composted manure with an initial C:N ratio in the range of 9:1-12:1 and reported nitrogen losses of 35-45% after 42 days. Tiquia and Tam [37] reported a nitrogen reduction of 59% while composting chicken litter with an initial C:N ratio of 14.5:1. Beck-Friis et al. [39] reported nitrogen reduction of 24-33% while composting household wastes with an initial C:N ratios of 21-23:1 under controlled conditions for 22-31 days. |
| C:N ratio |
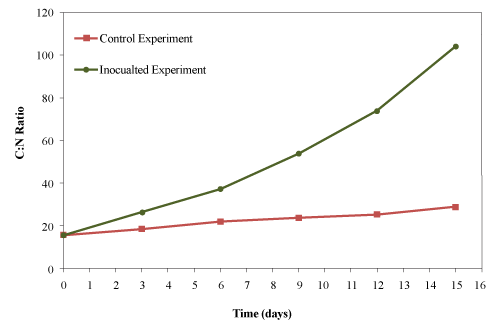
| The C:N ratio was calculated from total carbon and total kjeldahl nitrogen data. The initial C:N ratio of the mixture was 15.6:1 which increased to 28.6:1 and 104:1 for the control and the inoculated experiments, respectively (Figure 12). |
| Carbon and nitrogen are the main two building blocks of microorganisms. Carbon serves primarily as an energy source for the microorganisms and a small fraction of the carbon is incorporated into the microbial cells. Nitrogen is critical for microbial growth and it is a constituent of protein that forms over 50% of dry bacterial cell mass [8]. A balance between carbon and nitrogen amount (C:N ratio) is very essential factor in the composting process and their relative amount in compost is an indicative of compost quality. Microbial growth will slow down if the availability of nitrogen and results in longer decomposition time for the available carbon. On the other hand, a surplus of a nitrogen beyond the microbial necessities is often lost as NH3 with the exhaust gas [8]. C:N ratio at 25-30 is considered the optimum ratio for composting [28]. The C:N ratio decreases in a biological decomposition system if the organic carbon is oxidized to CO2 faster than the organic nitrogen is converted to ammonium and then oxidized to NO3 -. Nitrogen can remain relatively stable if the balance between mineralization of organic nitrogen to NH4 and the immobilization of NH4 to organic nitrogen (microbial growth) is maintained during the composting process [38]. |
| Lemus et al. [40] reported 35-40% reduction in the C:N ratio when composting yard trimmings and 60-64% when composting synthetic food waste. Li et al. [41] reported 50% reduction in C:N ratio after 12 days of composting of poultry litter, municipal solid waste and corn straw. Ghaly et al. [27] reported an increase in the C:N ratio (from 15.6:1 to 28.6:1) while composting creosote treated wood waste. In the present study, the C:N ratio increased by 1.8 fold for the control and 6.6 fold for the inoculated experiment due to continuous addition of used cooking oil and the rapid consumption of nitrogen by microorganisms. |
| Phenolic compounds |
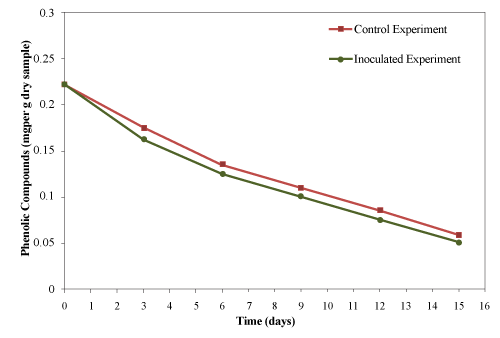
| The initial concentration of PC was 0.222 ± 0.010 mg /g material (dry basis) which decreased gradually reaching the final concentration of 0.058 and 0.051 mg/g material (dry basis) for control and inoculated experiment, respectively as shown in Figure 13. Approximately, 73.9% and 77% of the phenolic compounds were degraded from control and inoculated experiment, respectively in 15 days. Only 3.1% increase were achieved by the addition of thermophilic cellulytic organism. |
| McMahon et al. [42] reported 66% degradation of creosote components in the C&D wood waste after 10 days of composting. Galli et al. [43] reported 75% degradation of phenolic compounds in creosotetreated wood using white-rot fungus after 30 days. Atagana et al. [44] reported complete degradation of heavily creosote-contaminated soil during composting with cattle manure and vegetable waste after 19 months. In present study, the higher degradation of PC was due to the addition of bio-available carbon (used cooking oil) which helped to achieve a higher temperature. The degradation of organic substrate can be described with the following first order model [7]: |
| Ct = C0 (4) |
| Where: |
| Ct is the concentration of the organic substrate at time t (mg/kg) |
| C0 is the initial concentration of the organic substrate (mg/kg) |
| k is the rate constant |
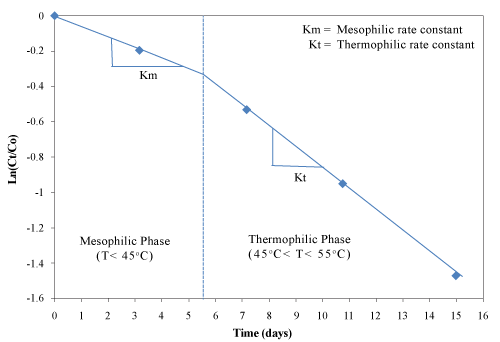
| The value Ln(Ct/C0) has a linear relationship with the time t within given temperature range. The linear relationship between Ln(Ct/C0) and time for phenolic compounds was determined graphically for the mesophilic and thermophilic temperature zones as shown in Figure 14. The rate constant (k) was determined from the slope of the lines (Table 4). The mesophilic rate constant was 0.0027 and 0.0026 h-1 for the control and the inoculated experimental trials, respectively. During the thermophilic phase, the rate constant of the inoculated experimental trial was higher (0.0051 h-1) compared with the control (0.0047 h-1). The rate constant of PC degradation was correlated well with reductions in volatile solids, total carbon and TKN. |
| Lignocellulose |
| The results of the degradation of cellulose and lignin are shown in Table 5. The initial content of cellulose was 24.8 ± 1.5% which decreased after 15 days to 19.2 and 16.8% resulting in reductions of 22.6 and 32.3% for the control and inoculated experiments, respectively. The initial content of lignin was 19.8 ± 0.9% which decreased after 15 days to 16.3 and 13.7% resulting in reductions of 17.7% and 30.8% for the control and the inoculated experiments, respectively. |
| Ghaly et al. [27] composted wood waste for 15 days and reported reductions in cellulose and lignin of 19.8% and 16.3%, respectively. Yu et al. [23] reported degradation of cellulose and lignin of 11% and 18% on day 15 and 30% and 25% on day 45, respectively. Rodriguez et al. [45] reported 20-27% and 3% mineralization of C14-labeled lignin from wheat straw and pine wood in 28 days of composting, respectively. Liers et al. [46] reported 10% degradation of lignin with inoculation of Paecilomyces inflatus strain BKT02 after 12 weeks of composting. The present study resulted in the degradation of 22.6 and 32.3% of cellulose and 17.7 and 30.8% (lignin in a shorter period of time) for the control and inoculated experiments, respectively. This may be due to the addition used cooking oil as a bioavailable carbon which increased the degradation rate of these compounds by the microorganisms. |
| Maturity and stability tests |
| CO2 evolution and germination index (GI) are widely accepted as standard parameters for assessing the maturity and stability of the compost. CO2 evolution can provide useful information on the metabolic activities in composting material while the decomposition of phytotoxic organic substances, which indicates the degree of maturity of compost, can be revealed by seed germination rate and the root length of the ensuing plants. Stable and mature compost can be identified with the lower CO2 evolution and higher GI values [47,48]. |
| The CO2 evolution rate and germination index result are shown in Table 6. The CO2 evolution rates from the final products were 3.18 and 2.62 mg CO2-C/g VS- day for the control and inoculated experiments, respectively. The initial GI values were 0% and 0% for the control and the inoculated experiments which improved significantly after 15 days reaching 20% and 75% for the control and inoculated experiments, respectively. |
| Michel et al. [49] reported that the rate of CO2 evolution was decreased from initial value of 3 to 1 mg CO2/g VS/h after 23 days of composting dairy manure with sawdust amended windrow. Wong et al. [3] reported that the CO2 evolution rate rapidly declined from 7.33 to 3.23 mg CO2/g VS/d and 5.51 to 3.38 mg CO2/g VS/d in 3 weeks and gradually declined to 2.43 mg CO2/g VS/d and 1.89 CO2/g VS/d after 7 weeks of in-vessel composting of high sludge (1 sludge: 1.5 horse stable straw bedding waste) and low sludge (1 sludge: 2.9 horse stable straw bedding waste), respectively. The final products for control and inoculated experiment had a threshold of under 8 mg CO2-C per g carbon per day and are considered stable according to Gomez and Lima [50]. Rekha et al. [51] reported 49% and 95% increase in GI values in sediment from upper and lower stratus after 14 weeks of composting of contaminated lake sediments. Wong et al. [3] reported gradual increase in GI from 22% to 84% and 10% to 72% as the compost became stabilized reaching 84 and 72% after 7 weeks of in-vessel composting with high sludge (1 sludge: 1.5 horse stable straw bedding waste) and low sludge (1 sludge: 2.9 horse stable straw bedding waste). In present study, 20% and 75% of GI values were obtained only a short period of time (15 days) for the control and inoculated experiment, respectively. The results indicated that the final product of inoculated experiment had more stability and maturity than the control experiment. |
| Conclusions |
| The lag phases for the psychrophilic, mesophilic and thermophilic stages were clearly identified in the temperature profiles of both trials. The thermophilic phase (>45°C) was successfully achieved in the control and the inoculated experiments due to the addition of used cooking oil as bioavailable carbon. The water produced by microbial respiration did not compensate for the water lost as vapour with the exhaust gases resulting in decreasing moisture content over time. The final moisture content of 43% for the control was within the optimum range for composting of 40-60% while that of the inoculated experiment was low (35.3%) and may have affected the degradation rate. The pH fluctuation during the composting was due to the breakdown of organic nitrogen to ammonium at the beginning of the process and the formation of organic acids from decomposition of fats and grease and the loss of ammonia with the exhaust gases in the latter stage. The inoculated experiments achieved higher reductions in volatile solids, total carbon, TKN, phenolic compounds, cellulose and lignin compared to the control. Different degradation rates were observed in the psychrophilic, mesophilic and thermophilic stages of composting. The product from the inoculated experiment had improved stability and phytotoxicity compared to that of the control (uninoculated). The inoculation of thermophilic-cellulolytic microorganisms (T. curvata, T. aurantiacus and T. fusca ) accelerated the composting process and as a result higher degradation levels of phenolic compounds lignocellulose and lignin were achieved. |
| Acknowledgements |
| This research was supported by the National Science and Engineering Research Council (NSERC) of Canada. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
| Table 4 | Table 5 | Table 6 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
 |
 |
 |
 |
 |
| Figure 6 | Figure 7 | Figure 8 | Figure 9 | Figure 10 |
 |
 |
 |
 |
| Figure 11 | Figure 12 | Figure 13 | Figure 14 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15119
- [From(publication date):
March-2012 - Dec 20, 2025] - Breakdown by view type
- HTML page views : 10348
- PDF downloads : 4771
