Biochemical Composition during the Embryonic Development and Freshly Hatched Zoea of Macrobrachium idae (Heller, 1862)
Received: 14-Oct-2013 / Accepted Date: 04-Nov-2013 / Published Date: 09-Nov-2013 DOI: 10.4172/2157-7617.1000171
Abstract
In the present study an attempt has been made to know the biochemical composition of freshly hatched zoea of Macrobrachium idea. The content of protein in egg was gradually decreased from egg stage-I (63.98%) to egg stage-IV (51.85%), carbohydrate increased from egg stage-I (3.06%) to freshly hatched 1st zoea (4.06%), lipid values showed a decreasing trend from egg stage-I (14.97%) to freshly hatched 1st zoea (4.86%) and moisture increased from egg stage-I (61.68%) to freshly hatched 1st zoea stage (78.80%). The fatty acid composition of egg stage and zoea was in the range of 32.56-41.60 % for saturated, 26.09-36.22 % for monounsaturated and 9.07-11.92 % for polyunsaturated respectively and they were high in egg stages - I, III and IV. The unsaturated fatty acid contents were higher than the saturated fatty acid content. The amino acids showed increasing trends from egg stage-I to IV. Among the amino acids leucine, lysine, valine, methionine and arginine increased gradually as development progressed in egg stages and isoleucine, phenylalanine, histidine, tryptophan and threonine had a change of high-low-high.
Keywords: Palaemonid prawn; Macrobrachium idea; Biochemical; Zoea; Fatty acid
5604Introduction
Crab products are significantly important sources of nutrition in the human diet. Decapods crustaceans and other marine benthic invertebrates with complex life cycles develop through a planktonic larval and a benthic juvenile–adult phase. The larvae show dramatic growth and morphogenetic changes, which are affected by environmental conditions. Changes in the biochemical composition of decapod crustacean larvae were studied in several species [1-3]. They typically show a high percentage of protein (>30%), followed by lipids (<20%), chitin (<15%) and free carbohydrates (<5%). The variation is mainly depends on the environmental factors such as temperature [4,5], food availability [6-10] and salinity [3,11]. The Palaemonid females extrude their eggs and carry them under the abdomen until hatching and the eggs are rich in yolk substances that are used as embryonic development progresses. The utilization of yolk substances closely correlates with the embryonic development. Protein is one of the main components of yolk, plays an important role in both morphogenesis and energy supply in embryos [12,13]. During the embryonic development stages, lipid is not only a kind of energy source, but also components of biological membranes and pigments of compound eyes. It is, therefore, important to understand the metabolic changes occurring in larvae as they progress through developmental stages. There is little information available on the biochemical composition during embryonic development and freshly hatched zoea of palaemonid prawn in general and no information about Macrobrachium idea in particular. So the present study was designed to know the biochemical changes in the embryonic and freshly hatched first zoea stages of edible prawn, Macrobrachium idea.
Materials and Methods
The animals were collected from Ponnanthittu (Lat.11°28’41’N; Long. 79°45’30’E) waters which is located 2 km south to Parangipettai and connected with Vellar estuary. The sizes of the animals collected were ranged from 35 to 108mm in length. Totally 240 specimens were collected and transported to the laboratory in live condition. After reaching the laboratory they were washed carefully with distilled water to remove dust and algal particles and ice killed.
The freshly collected berried females were segregated into four arbitrary stages as described by [14-17]. The color and diameter of the eggs were noted before segregation and small clumps of eggs was snipped from random locations in each clutch using sharp scissors. All the developing embryos were examined with a MEIJI binocular dissecting microscope (100x) to ensure that only viable embryos were sampled and the colour of the embryo were also observed. The diameter of the eggs was measured using a micrometer mounted in the ocular of a dissecting microscope. Eggs were classified into 4 stages based on the following characteristic features (Table 1).
| Egg stages | Colour | Shape | Diameter |
|---|---|---|---|
| Egg stage-I | Opaque, greenish | Round or oval | 0.45mm |
| Egg stage – II | Translucent, light green | Oval with a narrow peri-vitelline space at one end and small transparent plate (the blastoderm) were easily distinguished | 0.57mm |
| Egg stage-III | Translucent, brownish-yellow | The embryo further developed with anterior transparent plate and differentiated into cephalic lobe | 0.58mm |
| Egg stage – IV | Transparent, dull whitish in colour | Developed with conspicuous black eye spots at the anterior and rudiments of cephalothoracic appendages were developed, the posterior region was coiled | 0.65mm |
Table 1: Characteristic features of different egg stages.
The eggs were blot dried with filter paper and transferred to the previously weighed dry paper. The dry weight of the eggs was determined after drying them in a hot-air oven at 60ºC for 24 hours. Then the samples were stored in desiccators with calcium chloride until further analysis.
Analysis of proximate composition
The protein, carbohydrate and lipid contents were estimated by adopting the standard methods of [18-20] respectively.
Moisture: The body tissue as a whole was dried in an electric oven at 60°C until a constant weight was reached and then it was weighed in an electronic balance to the nearest 0.1 mg. The percentage of dry matter in the body was calculated. The differences in weight between wet and dried tissues represented the weight of water in the body tissue, which was expressed as percentage.
Estimation of fatty acids
The fatty and methyl esters of the sample was injected into the gas chromatography (GC-6890) capillary column coated with 5% phenyl silicon at a temperature from 170º C to 300º C for 23.33 minutes. Flame ionization detector was used for analysis. Based on the retention time, the different fatty acids in the samples were identified. Triplicate was maintained for each experiment.
Estimation of amino acids
The amino acids were determined by HPLC analyzer (Lachromehitachi). Five microliter of amino acids standards mixture sample were injected into the column (DENALIC18 5MICROMM 4.6mm x 150mm). The flow rate was about 1 ml per minute, ambient temperature of 23ºC was maintained and sample was detected at 254 nm by following the method of [21].
Results
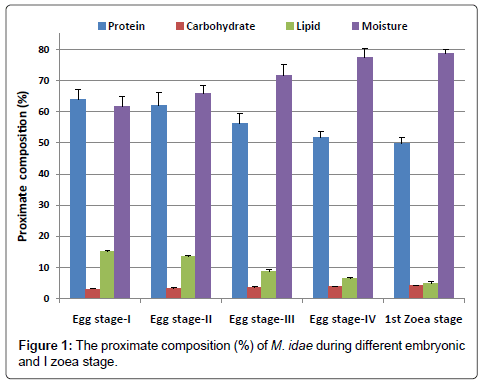
The proximate composition of four egg stages and 1st zoeal stage of Macrobrachium idea are presented in Figure 1.
Protein
The protein content of egg was gradually decreased from egg stage-1 (63.98%) to egg stage-IV (51.85%) and the protein content in Ist zoeal stage was found to be 49.69%.
Carbohydrate
The carbohydrate content was increased from egg stage-I (3.06%) to freshly hatched Ist zoea (4.06%).
Lipid
Lipid values showed a decreasing trend from egg stage-I (14.97%) to freshly hatched Ist zoea (4.86%).
Moisture
The water content uniformly increased from egg stage I (61.68%) to freshly hatched Ist zoea stage (78.80%).
Fatty acids
The percentage composition of fatty acids in each egg stage and I zoea are presented in Table 2. The fatty acid composition of egg stage and zoea was in the range of 32.56-41.60% for saturated, 26.09-36.22% for monounsaturated and 9.07-11.92% for polyunsaturated respectively and they were high in egg stages - I, III and IV. The unsaturated fatty acid contents were higher than the saturated fatty acid content. A total of nine saturated, nine monounsaturated and five polyunsaturated fatty acids were indentified. Among saturated fatty acids, palmitic acid (C16:0) was maximum in all egg stages and in Ist zoea. It was maximum in egg stage I than other stages. The palmitic acid was followed by stearic acid (C18:0) which is high in egg stage- III and myristic acid (C14:0) in egg stage- I followed by C17:0, C15:0 and C12:0 respectively in egg stages-I and III. The total saturated fatty acids decreased from egg stage I to 1st zoea.
| Fatty acids (%) | Egg stage-I | Egg stage-II | Egg stage-III | Egg stage-IV | 1st zoea stage |
|---|---|---|---|---|---|
| C12:0 | 1.31 ± 0.19 | 1.17 ± 0.08 | 0.45 ± 0.03 | 0.60 ± 0.07 | 0.87 ± 0.03 |
| C13:0 | 0.51 ± 0.05 | 0.24 ± 0.03 | 0.16 ± 0.02 | 0.17 ± 0.04 | 0.24 ± 0.01 |
| C14:0 | 3.88 ± 0.12 | 3.78 ± 0.13 | 3.25 ± 0.07 | 3.42 ± 0.34 | 3.11 ± 0.12 |
| C15:0 | 1.08 ± 0.2 | 1.20 ± 0.06 | 1.36 ± 0.07 | 0.94 ± 0.04 | 0.89 ± 0.03 |
| C16:0 | 25.48 ± 0.52 | 24.14 ± 0.35 | 23.86 ± 0.31 | 23.60 ± 0.34 | 20.91 ± 0.41 |
| C17:0 | 1.98 ± 0.08 | 1.82 ± 0.06 | 1.85 ± 0.19 | 1.38 ± 0.08 | 1.05 ± 0.04 |
| C18:0 | 6.58 ± 0.18 | 5.47 ± 0.13 | 7.66 ± 0.18 | 4.79 ± 0.24 | 4.81 ± 0.16 |
| C19:0 | 0.49 ± 0.06 | 0.29 ± 0.04 | 0.24 ± 0.03 | 0.27 ± 0.03 | 0.19 ± 0.07 |
| C20:0 | 0.26 ± 0.05 | 0.37 ± 0.04 | 0.41 ± 0.06 | 0.50 ± 0.02 | 0.46 ± 0.02 |
| Σ SFA | 41.60a | 38.51c | 39.26b | 35.70d | 32.56e |
| C14:1n5c | 0.16 ± 0.03 | 0.17 ± 0.09 | 0.13 ± 0.02 | 0.17 ± 0.04 | 0.13 ± 0.02 |
| C16:1n5c | 0.62 ± 0.04 | 0.55 ± 0.04 | 0.49 ± 0.03 | 0.27 ± 0.04 | 0.55 ± 0.03 |
| C16:1n9c | 0.41 ± 0.02 | 0.25 ± 0.02 | 0.33 ± 0.02 | 0.42 ± 0.01 | 1.19 ± 0.10 |
| C17:1n8c | 1.05 ± 0.04 | 1.22 ± 0.07 | 1.42 ± 0.05 | 0.98 ± 0.07 | 1.44 ± 0.07 |
| C17:1n6c | 0.29 ± 0.01 | 0.23 ± 0.04 | 0.25 ± 0.04 | 0.19 ± 0.05 | 0.21 ± 0.04 |
| C18:1n9c | 23.96 ± 1.23 | 19.84 ± 0.73 | 24.94 ± 0.77 | 22.30 ± 0.39 | 18.77 ± 0.41 |
| C18:1n7c | 6.22 ± 0.75 | 5.62 ± 0.59 | 8.06 ± 0.24 | 3.54 ± 0.31 | 3.25 ± 0.22 |
| C18:1n5c | 0.45 ± 0.06 | 0.28 ± 0.06 | 0.25 ± 0.09 | 0.32 ± 0.08 | 0.35 ± 0.06 |
| C19:1n9c | 0.25 ± 0.06 | 0.20 ± 0.03 | 0.35 ± 0.04 | 0.25 ± 0.07 | 0.18 ± 0.05 |
| Σ MUFA | 33.44b | 28.38c | 36.22a | 28.44c | 26.09d |
| C18:2n6c | 6.45 ± 0.49 | 5.46 ± 0.32 | 5.73 ± 0.31 | 6.99 ± 0.20 | 5.57 ± 0.73 |
| C18:3n6c | 0.67 ± 0.05 | 0.51 ± 0.03 | 0.50 ± 0.03 | 0.71 ± 0.07 | 0.46 ± 0.08 |
| C20:2n6c | 0.70 ± 0.08 | 0.77 ± 0.04 | 0.92 ± 0.07 | 1.12 ± 0.11 | 0.93 ± 0.04 |
| C20:3n6c | 1.55 ± 0.06 | 1.61 ± 0.02 | 1.79 ± 0.06 | 1.17 ± 0.06 | 0.60 ± 0.05 |
| C20:4n6c | 0.84 ± 0.03 | 0.73 ± 0.05 | 1.75 ± 0.05 | 1.91 ± 0.04 | 2.04 ± 0.08 |
| Σ PUFA | 10.22b | 9.07c | 10.7b | 11.92a | 9.60c |
| Unidentified | 14.74 | 24.02 | 13.81 | 23.93 | 31.74 |
| PUFA/SFA | 0.24 | 0.23 | 0.27 | 0.33 | 0.29 |
Table 2: Fatty acid composition (%) in egg stages and 1st zoeal stage of M. idae.
Among the monounsaturated fatty acids, C18:1n9c (Oleic) was maximum and it was followed by C18:1n7c in all the stages. These two fatty acids were recorded high in the egg stage-III. The other monosaturated fatty acids recorded were in megre in their percentage composition. The total amount of monounsaturated fatty acids were gradually decreased from egg stage I to 1st zoea and the decrement was statistically significant. The total amount of polyunsaturated fatty acids was irregular in eggs as well as zoeal stage. Among 5 polyunsaturated fatty acids, C18:2n6c was found to be maximum followed by C20:3n6c and C20:4n6c. The polyunsaturated fatty acids were showed significant variation between egg stage and zoea. The maximum PUFA/SFA ratio found in the III egg stage (0.33) and minimum in egg stage II (0.23).
Amino acids
The total essential amino acids were showed increasing trend from the egg stages I to IV. However they were little bit low in Ist zoea stage when compared to egg stage IV. Totally 10 essential amino acids were observed. Among them leucine, lysine, valine methionine and arginine was increased gradually as development progressed in egg stages. However isoleucine, phenylalanine, histidine, tryptophan and threonine had a change of ‘high-low-high’. As in essential amino acids, non essential amino acids were also followed almost similar trend. Among total 9 amino acids, 5 amino acids (glutamine, asparagine, glycine, cystine and alanine) were gradually increased from egg stage- I to IV. But other non essential amino acids (serine, tyrosine, proline and taurine) were irregular. The total aminoacid content was showed increasing trends from egg stage- I to IV (Table 3).
| Amino acids | Egg stage-I | Egg stage-II | Egg stage-III | Egg stage-IV | 1st zoea stage |
|---|---|---|---|---|---|
| Leucine | 3.43 ± 0.31 | 3.37 ± 0.23 | 3.56 ± 0.39 | 4.17 ± 0.13 | 3.84 ± 0.09 |
| Lysine | 3.34 ± 0.32 | 3.35 ± 0.17 | 3.50 ± 0.27 | 3.65 ± 0.13 | 3.73 ± 0.05 |
| Valine | 2.78 ± 0.11 | 2.83 ± 0.06 | 3.26 ± 0.08 | 3.27 ± 0.18 | 3.25 ± 0.17 |
| Isoleucine | 2.41 ± 0.12 | 2.39 ± 0.23 | 2.54 ± 0.07 | 2.31 ± 0.20 | 2.19 ± 0.03 |
| Phenylalanine | 2.19 ± 0.10 | 2.11 ± 0.11 | 2.17 ± 0.04 | 2.18 ± 0.07 | 2.20 ± 0.05 |
| Threonine | 1.84 ± 0.08 | 2.10 ± 0.06 | 2.16 ± 0.10 | 2.15 ± 0.07 | 2.14 ± 0.15 |
| Methionine | 1.10 ± 0.04 | 1.24 ± 0.06 | 1.29 ± 0.06 | 1.47 ± 0.08 | 1.48 ± 0.07 |
| Arginine | 2.91 ± 0.09 | 3.15 ± 0.06 | 3.21 ± 0.04 | 3.33 ± 0.10 | 3.40 ± 0.07 |
| Histidine | 1.48 ± 0.04 | 1.47 ± 0.08 | 1.45 ± 0.03 | 1.54 ± 0.06 | 1.40 ± 0.06 |
| Tryptophan | 1.81 ± 0.06 | 1.90 ± 0.06 | 1.61 ± 0.06 | 1.25 ± 0.04 | 1.20 ± 0.07 |
| Total EAA | 23.33 | 23.94 | 24.77 | 25.34 | 24.87 |
| Gultamine | 5.13 ± 0.13 | 5.20 ± 0.04 | 5.49 ± 0.12 | 6.08 ± 0.05 | 5.77 ± 0.07 |
| Asparagine | 3.34 ± 0.05 | 3.49 ± 0.05 | 3.64 ± 0.17 | 4.10 ± 0.10 | 3.83 ± 0.04 |
| Glycine | 2.07 ± 0.04 | 2.16 ± 0.07 | 2.23 ± 0.10 | 2.49 ± 0.02 | 2.53 ± 0.07 |
| Alanine | 2.11 ± 0.06 | 2.17 ± 0.06 | 2.24 ± 0.09 | 2.76 ± 0.04 | 2.52 ± 0.11 |
| Serine | 2.10 ± 0.04 | 2.02 ± 0.03 | 2.20 ± 0.04 | 2.24 ± 0.06 | 2.05 ± 0.07 |
| Cystine | 1.68 ± 0.09 | 2.05 ± 0.04 | 2.30 ± 0.07 | 2.68 ± 0.07 | 2.39 ± 0.05 |
| Tyrosine | 1.75 ± 0.05 | 2.06 ± 0.06 | 1.86 ± 0.07 | 2.13 ± 0.07 | 2.20 ± 0.12 |
| Proline | 1.73 ± 0.04 | 1.77 ± 0.07 | 1.89 ± 0.09 | 1.86 ± 0.04 | 1.79 ± 0.05 |
| Taurine | 1.82 ± 0.04 | 1.76 ± 0.04 | 1.38 ± 0.06 | 1.38 ± 0.06 | 1.19 ± 0.08 |
| Total NEAA | 21.74 | 22.71 | 23.25 | 25.75 | 24.32 |
| Total Amino acids (EAA+NEAA) | 44.07d | 43.65e | 48.02c | 50.09a | 49.19b |
Table 3: Essential and non essential amino acids (%, dry weight) in M. idae during different embryonic development stages and Ist zoeal stage.
Discussion
In the present study the protein content in egg stage I was maximum (63.98%) due to the deposit of yolk. But the protein levels started decreasing when the development progresed. As the embryo developing, the yolk deposited is utilised for the development. This may be the reason for the low value of protein seen in egg stage IV. According to [22] the protein values decreased gradually as developing stages proceeds in P. pelagicus and P. sanguinolentus. Similar observation was made by [23] in Emerita holthuisi and [24] in Thalamita crenata. Needham [25] stated that the terrestrial animal utilised fat as the source of energy for their development whereas in aquatic forms, protein was the source of energy. The protein content of the yolk is important for the tissue differentiation and organization particularly for the cuticle layers, muscle, the digestive and nervous system [26]. Barnes and Pandian [27,28] reported that the protein in developing eggs is progressively depleted and they also suggested the possible utilization of protein during embryogenesis to meet the metabolic demand.
The present study reveals that carbohydrate value showed an increasing trend from egg stages I to IV. The carbohydrate played major part for the development of earlier stages in M. rosenbergii [29]. Sumitra [30] also reported gradual increasing trend in M. idella of Cochin backwaters. But in P.pelagicus and P. sanguinolentus [22] and T. crenata [24] the carbohydrate value showed gradual decrease when development proceeded. [31,32] stated that the carbohydrate showed an increasing trend from egg stage-I to IV in M. idella idella as in the present study.
Lipid plays a central role in the embryonic metabolism as they represent the most important energy source and form atleast 60% of the total energy expenditure of the developing crustacean embryo [33]. Large decapod eggs have a relatively high fat percentage and low density when compared to small eggs [34]. Lipid value obtained in the present study varied between 14.97% (stage-I) to 6.36% (stage-IV) which is comparatively higher than that of M. idella idella available from Cochin backwaters [30] and Vellar estuary [31]. Ammar [32] reported more or less similar values in M. idella idella. However, lipid values shown gradual increase with development in P.pelagicus and P.sanguinolentus [22]. Needham [25] classified the crustacean eggs as cleidoic and non – cleidoic types of eggs. The cleidoic eggs are not dependent on the environment for water and salt (ash); oxidation of protein is suppressed to considerable extend and fat oxidation is greatly enhanced, serving as main source for the embryonic metabolism. A similar pattern has been reported for Callinectes sapidus [35] and Xantho bidentatus [26]. The crab Caridina nilotica and prawn M. malcolmsonii derived more energy from fat [36,37].
Developing eggs of crustaceans absorb water [38,39]. In M. rosenbergii the egg volume is increased slowly from fertilized egg stage to zoea stage indicated that there were prominent correlation between egg volume and water content [29].The absorption of water increases the internal osmotic pressure resulting in osmotic hatching of egg and accumulation of water decreases the specific gravity of developing eggs and finally the free floating planktonic larvae released. The increase in water content of the eggs is due to the absorption of water through the egg membrane of the retention of metabolic water since water is the ‘byproduct’ of respiration. The oxidation of 100 gm of lipid releases 107.7 gm of water, the same amount of protein produces 111.3 gm of water and carbohydrate oxidation liberates 55.5 gm of water [40]. The weight of eggs during zoea stage increased since fertilized stage, mainly because of the increased water content. Water provided a liquid environment for embryo and the higher water pressure during zoea stage might be cause of the embryo breaking through the egg membrane in preparation for hatching [29].
In the present study the lipid acted as the most important energy source in the embryonic metabolism of M. idae. The total lipid content of egg stage-I (14.97 ± 0.18%) expected for formation of the organs, lipid was mainly used as a source of energy. Rapid decrease of total lipids in egg stages-III and IV was closely associated with the formation and development of many organ anlages. Brain anlage and heart anlage came into being. Compound eyes turned larger, hemolymph started to flow and heart beats at about 200 times per minutes in zoea stage [41,42]. More energy was needed and some fatty acids turned into component of organs in these two stages. In zoea stage, lipids in yolk were used up. The zoea stage of M. rosenbergii lasted about 90 h, and more energy was needed. So some fatty acids were probably used in the synthesis of organs during these stages [29]. The consumption pattern of different fatty acids in eggs of M. rosenbergii during the embryonic development did not differ markedly from that of other crustaceans [43-45]. The most important fatty acids were C16:0, C18:0, C16:1, C18:1n-9 and C18:2n-6 in eggs of M. rosenbergii. In the present study most important fatty acids components in M. idae were C16:0, C18:0, C18:1n-9 as in M. rosenbergii. The first zoea stage of M. idae is nonfeeding stage, the remaining nutriment favours their independence of external energy resources when external feeding begins and would increase the changes for the first successful moult. Yao [29] reported that in M. rosenbergii the remaining yolks in the eggs are utilized by the first larval stage as the first larva is non-feeding stage.
It has been revealed that the amino acids [46], monosaccharides [47] and nucleosides [48] could be transported into the embryos of the marine invertebrates. Rosa [45] assumed that embryos of N. norvegicus could absorb some compounds. In the present study, the content of essential amino acids increased in eggs, may be the result of organic compounds could be transported into the embryos of M. idae. Even though the amino acids (EAA) and Total Amino Acids (TAA) contributed to the completion of the embryonic development of M .idae the ratio of EAA and TAA content remained unchanged during different embryonic development stages. But when compared first zoea stage to the egg stages the EAA and TAA were less than egg stages. It may be because of the first zoea stage does not feed externally and utilizes the yolk present inside the zoea stage. And the zoea doesn’t have the capacity of absolving organic compounds from the external water source as in the egg stages. The unchanged EAA and TAA were also determined in the eggs of M. rosenbergii [29].
The increase in TAA is indicative of the fact that protein in yolk act as the main structural substance during embryonic development of M. idae. Increases in TAA were also reported in the eggs of N. norvegicus [45] and in M. rosenbergii [29]. Quantitively the most important amino acids were leucine, lysine, valine, arginine and glutamic acid during the embryonic development of M. idae. Regarding the function of single amino acid, leucine is a ketone-producing amino acid. It could be transformed into acetyl-CoA and acetyl-acetic acid, which are important intermediates in carbohydrate and lipid metabolism [49]. Arginine was proven to be crucial in energy metabolism by maintaining glycolysis under hypoxic conditions [50]. Valine is a carbohydrateproducing amino acid and may be associated with carbohydrate metabolism through citric acid cycle. Content of glutamic acid was high during embryonic development, which may have resulted from nitrogen metabolism in the eggs of M. idae. Glutamic acid is turned into glutamine, which is deaminated to produce NH3 [49]. NH3 can be excreted along with Cl-. An increase in the content of NH4Cl after the blastula stage also suggests that NH4+ and Cl- are being excreted together. Tyrosine can be used to synthesize melanin [49], which plays a central role in the accumulation of compound eye pigments. High content of these amino acids is closely correlated with their important role in the embryo.
References
- Anger K, Harms J (1990) Elemental (CHN) and proximate biochemical composition of decapod crustacean larvae. Comp Biochem Physiol 97: 69-80.
- Anger K (1991) Developmental changes in the bioenergetics of decapod larvae. Mem Queensl Mus 31: 289-308.
- Anger K (1998) Patterns of growth and chemical composition in decapod crustacean larvae. Invertebr Reprod Dev 33: 159-176.
- Dawirs R, Dietrich A (1986) Temperature and laboratory feeding rates in Carcinus maenas L. (Decapoda, Portunidae) larvae reared in the laboratory. Mar Biol 93: 133-147.
- Anger K (1987) Energetics of spider crab Hyas araneus megalopa in relation to temperature and the moult cycle. Mar Ecol Prog Ser 36: 115-122.
- Anger K, Dawirs R (1982) Elemental composition (CHN) and energy in growing and starving larvae of Hyas araneus (Decapoda, Majidae). Fish Bull 80: 419-433.
- Rhyne AL, Lin J (2004) Effects of different diets on larval development in a peppermint shrimp (Lysmata sp.). Aquac Res 35: 1179-1185.
- Dawirs RR (1987) Influence of limited starvation periods on growth and elemental composition (C, H, N) of Carcinus maenas (Decapoda: Portunidae) larvae reared in the laboratory. Mar Biol 93: 543-549.
- Harms J, Anger K, Klaus S, Seeger B (1991) Nutritional effects on ingestion rate, digestive enzyme activity growth and biochemical composition of Hyas araneus (Decapoda Majidae) larvae. J Exp Mar Biol Ecol 145: 233-265.
- Harms J, Meyer-Harms B, Dawirs R, Anger K (1994) Growth and physiology of Carcinus maenas (Decapoda, Portunidae) larvae in the field and in laboratory experiments. Mar Ecol Prog Ser108: 107-118.
- Pfaff K (1997) Einflu ß der Salinitat auf den Stoffbestand der Larvenstadien einer marinen Dekapodenart. MSc thesis, University of Darmstadt, Darmstadt, Germany.
- Holland DL (1978) Lipid reserves and energy metabolism in the larvae of benthic marine invertebrates. Alins DC, Sargent JR (edn.). Biochem Biophy Perspec Mar Biol 85-123.
- Luo W, Zhou ZL, Zhao YL (2004) Analysis on the contents of protein and amino acids in Cherax quqdricarinatus during different embryonic development stages. J Eas Chi Norm Univ 1: 88-92.
- Rodriguez A (1977) Contribution al conocimiento de la biologiay pesca dellangostino Penaeus kerathurus. Forskal 1775. del Golfo de Cadiz (region Sudatlantica Espanola). Investigacion Pesq 41: 603-635.
- Rodriguez A (1985) Biologia del langostino Penaeus kerathurus. Forskal., 1775. del Golfo de Cadiz. I Reproduction. Investigacion pesq 49: 581-595.
- Ajith Kumar M (1990) Studies on the proximate composition of the prawn Macrobrachium idella (Hilgendorf). M. Phil Thesis, Annamalai University, India.
- Dinakaran GK (2010) Mating behaviour, embryonic development, biochemical composition and mass seed production of edible prawn Macrobrachium idella idella (Hilgendorf, 1898). Ph.D Thesis, Annamalai University, India.
- Raymont JEG, Austin J, Linford E (1964) Biochemical studies on Marine zooplankton .I. The biochemical composition of Neomysis integer. J Cons Perm Explor Mar 28: 354-363.
- Dubois M, Giles KA, Hamilton JK, Rebors PA, Smith F (1956) Calorimetric method for determination of sugar and related substances. Analyt Chem 28: 350-356.
- Folch J, Lees M, Sloane-Stanley GH (1956) A Simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497-509.
- Baker DH, Han Y (1994) Ideal amino acid profile for broiler chicks during the first three weeks post hatching. Poultry Sci 73: 1441-1447.
- Radhakrishnan N (1979) Studies on portunid crabs of Porto Novo. (Crustacea: Decapoda: Brachyura ). Ph. D. thesis, Annamalai university, India.
- Sumitra Vijayaraghavan, Wafar MVM, Joseph P Royan (1975) Changes in the biochemical composition and energy utilization in developmental stages of mole crab Emerita holthuisi Sankolli. Mahasagar 18: 165-170.
- Krishnan T (1988) Yolk utilization, larval development and effects of salinity and starvation on the edible estuarine crab, Thalamita crenata (Latreille) reared in the laboratory. Ph.D. Thesis, Annamalai University, India.
- Needham J (1950) Biochemistry and morphogenesis. Cambridge Univ. press, Cambridge, UK.
- Babu DE (1987) Observations on the embryonic development and energy source in the crab Xantho bidentatus. Mar Biol 95: 123-127.
- Barnes H (1965) Studies in the biochemistry of Cirripede eggs. I changes in the general biochemical composition during development of Balanus balanoides and B. balanus. J Mar Biol Ass UK 45: 321-339.
- Pandian TJ (1972) Egg incubation and yolk utilization in the isopod Ligia oceanica. Proc Natn Inst Sci Indian 38: 430-441.
- Yao J, Zhao Y, Wang Q, Zhong Z, Hu X, et al. (2006) Biochemical compositions and digestive enzyme activities during the embryonic development of prawn, Macrobrachium rosenbergii. Elsevier. Aquacult 253: 573-582.
- Sumitra V, Easterson DCV (1974) Biochemical changes and energy utilization in developing stages of the estuarine prawn Macrobrachium idella (Hilgendorf). J Mar Biol Ass India 16: 275-279.
- Glencross BD, Smith DM, Thomas MR, Williams KC (2002) Optimising the essential fatty acids in the diet for weight gain of the prawn Penaeus monodon. Aquacult 204: 85- 99.
- Ammar D, Muller YMR, Nazair EM (2001) Biologia Reproductive De Macrobrachium Olfersii (Wiegman) (Crustacea, Decapoda, Palamonidae) Coletados Na Ilha De Santa Catarina, Brasil. Revta Bras Zool 18: 529-537.
- Wehrtmann LS, Graeve M (1998) Lipid composition and utilization in developing eggs of two tropical marine caridean shrimps (Deccapoda: Caridea: Alpheida: Palaemonidae). Comp Biochem Physiol 121: 457-463.
- Salmon M, Kettler MK (1987) The importance of behavioural and biochemical differences between fiddler crab taxa, with special reference to Uca rapax (Smith) and U. virens (Salmon and Atsaides). Contri Mar Sci 30: 63-76.
- Amsler MO, George RY (1984) Seasonal variation in the biochemical composition of the embryos of Callinectes sapidus Rathbun. J Crust Biol 4:546-553.
- Ponnuchamy R, Ayyappan S, Ravichandran R, Katare S (1979) Yolk and copper utilization during embryogenesis of the fresh water prawn Cardina nilobica. ProcIndian Acad Sci 88: 353-362.
- Mathavan S, Murugadoss S, Marian MP (1986) Ontogenetic changes in the composition and energy budget of Macrobrachium malcolmsonii. The first Asian Fisheries Fourm. Asian Fisher Soc Manil Phillipin 647-650.
- Pandian TJ (1970) Ecophysiological studies on the developing eggs and embryos of European lobster Homarus gammarus. Mar Biol 5: 154-167.
- Herringt PJ (1974) Size, density and lipid content of some decapod eggs. Deep-SeaRes 21: 91-94.
- Baldwin E (1964) An introduction to comparative embryology. W.B. saunders co., Philadelphia, USA.
- Zhao YL, Wang Q, Du N SH (1998) Embryonic development of the giant fresh water prawn, macrobrachium rosenbergii (Crustacea;Decapoda): I Morphogenesis of external structures of embryo (Chinese). Acta Zool Sinica 44: 249-256.
- Petersen S, Anger K (1997) Chemical and Physiological changes during embryonic development of the spider crab, Hyas araneus L. (Decapoda: Majidae). Comp Biochem Physiol B 117: 299-306.
- Morais S, Narciso L, Calado R, Nunes ML, Rosa R (2002) Lipid dynamics during the embryonic development of Plesionika martia martia (Decapoda; Pandalidae), Palaemon serratus and Palaemon elegans (Deccapoda; Palaemonidae): relation to metabolic consumbtion. Mar Ecol Prog Ser 242: 195-204.
- Li H, Zhao YL, Wang Q (2003) Variations in biochemical composition during embryonic development of Macrobrachium nipponense (Chinese). J Fisher China 27: 545- 549.
- Rosa RS, Morais, Cslado R (2003) Biochemical changes during the embryonic development of Norway lobster, Nephrops norvegicus. Aqua 221: 507-522.
- Litaay M, De Silva SS, Gunasekeran RM (2001) Changes in amino acid profiles during embryonic development of the blacklip abalone Haliotis rubru. Aquacult Living Resour 14: 335-342.
- Monroy A, Tollis H (1961) Uptake of radioactive glucose and amino acids and their utilization for incorporation into proteins during maturation and fertilization of the eggs of Asterias forbesiii and Spsiula solidissima. Biol Bull 147: 456-466.
- Schneider EG, Whitten DJ (1987) Uptake and metabolism of nucleosides by embryos of the sea urchin Strongylocentrotus purpuratus. Exp Cell Res 168: 1-14.
- Shen T, Wang JY (1990) Biochemistry. [M]. Higher Education Publisher. pp 67-86.
- Gade G, Grieshaber MK (1986) Pyruvate reductase catalyze the formation of lactate and opines in anaerobic invertebrates. Comp Biochem Physiol 83: 255-272.
Citation: Soundarapandian P, Sudhakar S, Varadharajan D, Dinakaran GK (2013) Biochemical Composition during the Embryonic Development and Freshly Hatched Zoea of Macrobrachium idea (Heller, 1862). J Earth Sci Clim Change 5: 171. DOI: 10.4172/2157-7617.1000171
Copyright: ©2013 Soundarapandian P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16375
- [From(publication date): 1-2014 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 11396
- PDF downloads: 4979