Research Article Open Access
Bioconcentration of Trace Metals in the Freshwater Snail Melanoides tuberculata (Mollusca: Thiaridae) from Alaro Stream Ecosystem of South West Nigeria
Emmanuel Teryila Tyokumbur* and Tonye Grace OkorieDepartment of Zoology, University of Ibadan, Ibadan, Nigeria
- *Corresponding Author:
- Emmanuel Teryila Tyokumbur
Department of Zoology
University of Ibadan, Nigeria
E-mail: e.tyokumbur@mail.ui.edu.ng
Received date: March 30, 2013; Accepted date: May 20, 2013; Published date: May 22, 2013
Citation: Tyokumbur ET, Okorie TG (2013) Bioconcentration of Trace Metals in the Freshwater Snail Melanoides tuberculata (Mollusca: Thiaridae) from Alaro Stream Ecosystem of South West Nigeria. J Ecosys Ecograph 3:124. doi:10.4172/2157-7625.1000124
Copyright: © 2013 Tyokumbur ET, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
The level and tissue bioconcentration of V, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Mo, Ag, Cd and Pb were determined in the viscera and shell of the freshwater snail, Melanoides tuberculata in the upstream and downstream of Alaro stream, South West Nigeria. Trace metal concentrations in snail and sediment samples collected from six stations were measured using an Inductively Coupled Plasma-Mass Spectrometry (ICP-MS).
The mean trace metal concentrations (ppm) of V, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Mo, Ag, Cd and Pb in the snail were estimated to be 2.10-3.26, 28.31-33.32, 1668.21-2398.43, 1.75-3.98, 6.18-8.12, 33.86-89.23, 12.10- 66.35, 0.78-0.96, 9.86-11.35, 1.44-1.73, 1.98-2.33, 2.88-3.22 and 10.12-14.12 respectively. The study shows that M. tuberculata is capable of bioconcentrating trace metals in magnified quantity than that found in the sediment. The tissue concentration pattern was correlated with the amount of trace metals in the sediments. Bioconcentration of the trace metals V, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Mo, Ag, Cd and Pb varied between the viscera and the shell with the viscera having the highest concentration of all the trace metals. Results of the study indicate that ingestion of sediments and algae may be the main uptake route of the trace metals in Melanoides tuberculata.
Keywords
Bioconcentration; Trace metals; Freshwater snail; Sediment; Alaro stream
Introduction
Trace metals in the aquatic environment can be traced to both natural and anthropogenic sources. Pollutants such as trace metals can be bioconcentrated by aquatic biota such as macrophytes, macroinvertebrates and fish [1]. Bioconcentration measurements refer to the monitoring of uptake and retention of pollutants like trace metals in the organs or tissues of organisms. Bioconcentration takes place if the rate of uptake of pollutants by organisms exceeds the rate of elimination or excretion [2,3]. When the chemicals bioconcentrated are toxic, bioconcentration becomes an environmental threat to the organism concerned. Hence toxicity occurs along the food chain when the contaminated species or substance is consumed or eaten by an organism on the higher trophic level [4].
Terrestrial and aquatic molluscs are widely used as bioindicators due to their seeming reflection of environmental trace metal contamination [5]. Trace metals that are either essential or non-essential have been known to be highly tissue concentrated by invertebrates especially mollusca species [6]. Mussels can accumulate Cd in their tissues in the magnitude of up to 100,000 times higher than that found in the water in which they live [7]. Bioconcentration of trace metals and other pollutants has been reported in snail species such as Helix aspersa, Leptoxis praerosa and Lymnaea stagnalis [8,9].
The aims and objectives of the study are: as a result of the harmful effects of trace metals on aquatic ecosystem, it is important to monitor the bioconcentration of metals in the aquatic ecosystem which will serve as an indication of the temporal and spatial extent of trace metal contamination and an assessment of the potential impact on ecosystem health; to assess sediment trace metal concentration in Alaro Stream; and to calculate the Bioconcentration Factor (BF) to ascertain whether it exceeds 1 for use of the freshwater snail for clean-up of trace metalcontaminated freshwater ecosystems
Materials and Methods
Study area: The study area is the Alaro Stream which forms part of the hydro-ecological system of the Oluyole Industrial Estate which receives effluents from diverse sources of trace metal pollution. Effluents from both natural and anthropogenic sources are discharged into it directly or indirectly through run-off, leaching or seepage especially during the rainy season or as windblown materials during the dry season. Oluyole industrial estate is located between latitude 7o 21’N -7o22’N and longitude 3o50’-3o52’E. Alaro stream flows into Oluyole in a west-south-east direction from its source at Agaloke near Apata in Ibadan. It joins river Ona at the south east end of a meat processing factory as its main tributary. The stream receives effluents from diverse industries.
In this study, the bioconcentration of trace metals in Melanoides tuberculata (Mollusca: Thiaridae) a freshwater snail was assessed in both upstream (2 control stations) and downstream of effluents discharge (4 stations) in the Alaro stream ecosystem of Southwest Nigeria. Six sampling stations were selected based on the presence of the sessile mollusc species (M. tuberculata). Two of the sampling stations serving as the control were located upstream of the industrial effluent discharge into the stream while four sampling stations were located downstream of the effluent outfall.
Sampling and processing of snails and sediments: Snail samples were collected at the sampling stations using a hand trowel and then placed in plastic containers filled with the stream water. A sediment sample was collected at the spot on the sampling station where the freshwater snail (M. tuberculata) was collected. Sampling was carried out on monthly basis for four months.
The freshwater snail M. tuberculata has a red-rimmed shell, with the characteristic red spots apparent. Specimen length is approximately 20 mm. It has an elongate, conical shell, which is usually light brown, marked with rust-colored spots. An operculum is present. The average shell length is about 20-27 mm or 30-36 mm, but exceptional specimens may be up to 80 mm long. The shells have 10-15 whorls. It is native to subtropical and tropical northern Africa and southern Asia. Nonindigenous distribution is encountered in many parts of Africa, Europe and United States of America [10,11].
The snail sample was dissected to remove the viscera and shell into separate aluminum foils. These were then dried in an oven at 105°C for 6 hours. After which, the samples were pulverized into powdery form and packaged into labeled plastic sachets. Each sample (0.5 g) was weighed out and digested using 2-ml trace metal grade HNO3 to boil down to a clear solution in Teflon tubes at 105°C for 1hour in a heat block after which 1-ml H2O2 was added. After simmering down, the samples were diluted to the 10 ml mark with MilliQ water. These were then transferred into deionized water-rinsed test tubes for the Inductively Coupled Plasma-Mass Spectrometer (ICP-MS) analyses. Agilent 7700 ICP-MS was used for the analyses of the samples because it combines a high-temperature ICP (Inductively Coupled Plasma) source with a mass spectrometer, which converts the atoms of the elements in the sample to ions that are separated according to their mass/charge ratios by a quadruple mass analyzer (MS). The Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) was preferred to other facilities because it is rapid, precise, accurate, and extremely sensitive multielement analytical technique for the determination of trace metals in solid sample materials.
Sediment samples were air-dried on white polythene sheets and grounded to fine powder in a mortar. The fine powder was sealed in labeled polythene sachet. 0.5 g of each soil sample was digested using 2ml technical grade HNO3 in beakers at 95°C for 1hour after which 2 ml H2O2 was added. After cooling, the samples were decanted and diluted with milliQ water to the 10 ml mark for ICP-MS analyses.
Quality assurance of results was determined with National Institute of Standards and Technology’s (NIST) Standard Reference Material (SRM) number 1577 bovine liver for reproducibility of the tissue samples (viscera and shell) of the freshwater snail (Melanoides tuberculata).Recoveries of quality determination ranged from 74.1% (Zn)-104.54% (Ag), which was credible to proceed with the analyses. NIST SRM number 2709 San Joaquin soil was used for quality assurance in the sediments and the recoveries were in the range of 52.21% (V) - 100.17% (Pb).
Results and Discussion
The results were analyzed using SPSS statistical software package and Excel. The relationship between the trace metal concentration in the tissues (viscera and shell) of freshwater snail (Melanoides tuberculata) and the sediments was determined using linear regression analysis samples while the differences between the various data sets was carried out using Analysis of Variance (ANOVA).
The Bioconcentration Factor (BF) describes the ratio of the trace metals in the body tissue of the freshwater snail Melanoides tuberculata in relation to the trace metals in the immediate environment, the sediments
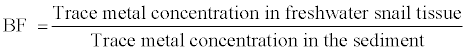
Since trace metals in freshwater snails are derived mainly from the sediments also known trace metal sink, only this point source impacting accumulating the effluent outfall was assessed, calculated and presented as part of Table 1 and 2.
| Trace metal | Month | Body tissue | Sediment | tissue/ sediment ratio (BF) |
|---|---|---|---|---|
| V | August | 2.14 ± 1.98 | 0.52 ± 0.25 | 4.11 |
| September | 2.10 ± 1.72 | 0.58 ± 0.23 | 3.62 | |
| October | 3.26 ± 2.87 | 0.68 ± 0.19 | 4.79 | |
| Mn | August | 40.14 ± 3.22 | 15.20 ± 2.32 | 2.64 |
| September | 28.31 ± 2.64 | 14.12 ± 0.54 | 2.00 | |
| October | 33.32 ± 4.82 | 14.43 ± 1.21 | 2.36 | |
| Fe | August | 2112.29 ± 3.84 | 282.35 ± 5.41 | 7.48 |
| September | 2398.43 ± 4.75 | 247.60 ± 5.62 | 9.69 | |
| October | 1668.21 ± 8.12 | 414.37 ± 0.98 | 4.03 | |
| Co | August | 2.12 ± 1.05 | 0.64 ± 0.05 | 3.31 |
| September | 3.98 ± 1.18 | 0.52 ± 0.12 | 7.65 | |
| October | 1.75 ± 0.46 | 0.39 ± 0.10 | 4.49 | |
| Ni | August | 6.18 ± 3.89 | 1.42 ± 0.25 | 4.35 |
| September | 7.27 ± 4.92 | 1.44 ± 0.04 | 5.05 | |
| October | 8.12 ± 6.53 | 3.18 ± 0.21 | 2.55 | |
| Cu | August | 89.23 ± 7.34 | 19.63 ± 2.82 | 4.55 |
| September | 33.86 ± 2.10 | 14.23 ± 2.18 | 2.38 | |
| October | 36.31 ± 1.23 | 13.50 ± 3.25 | 2.69 | |
| Zn | August | 12.10 ± 2.12 | 3.02 ± 0.57 | 4.01 |
| September | 25.50 ± 3.12 | 10.61 ± 2.01 | 2.40 | |
| October | 66.35 ± 1.29 | 12.82 ± 2.91 | 5.18 | |
| As | August | 0.96 ± 0.05 | 0.32 ± 0.06 | 3.00 |
| September | 0.84 ± 0.10 | 0.22 ± 0.09 | 3.82 | |
| October | 0.78 ± 0.12 | 0.31 ± 0.08 | 2.52 | |
| Se | August | 10.78 ± 1.21 | 2.31 ± 0.98 | 4.67 |
| September | 11.35 ± 2.12 | 3.89 ± 0.82 | 2.92 | |
| October | 9.86 ± 2.32 | 4.12 ± 0.58 | 2.39 | |
| Mo | August | 1.73 ± 0.87 | 0.22 ± 0.42 | 7.86 |
| September | 1.52 ± 0.21 | 0.97 ± 0.05 | 1.57 | |
| October | 1.44 ± 1.02 | 0.86 ± 0.08 | 1.67 | |
| Ag | August | 2.33 ± 0.07 | 0.78 ± 0.10 | 2.99 |
| September | 2.01 ± 0.97 | 0.99 ± 0.21 | 2.03 | |
| October | 1.98 ± 0.15 | 1.02 ± 0.92 | 1.94 | |
| Cd | August | 3.22 ± 0.21 | 0.89 ± 0.12 | 3.62 |
| September | 3.02 ± 1.01 | 0.95 ± 0.11 | 3.18 | |
| October | 2.88 ± 1.00 | 1.21 ± 0.77 | 2.38 | |
| Pb | August | 14.12 ± 2.78 | 2.89 ± 1.21 | 4.89 |
| September | 10.12 ± 0.94 | 4.92 ± 4.10 | 2.06 | |
| October | 12.44 ± 2.91 | 2.98 ± 2.00 | 4.17 |
Table 1: The mean trace metal content of Melanoides tuberculata and sediment samples upstream (control sites) of the effluent outfall in Alaro stream (parts per million, ppm)
| Trace metal | Month | Snail shell | Sediment | tissue/ sediment ratio (BF) |
|---|---|---|---|---|
| V | August | 8.56 ± 2.12 | 1.08 ± 0.28 | 7.93 |
| September | 6.25 ± 1.72 | 3.12 ± 1.32 | 2.00 | |
| October | 7.68 ± 2.32 | 2.18 ± 0.21 | 3.52 | |
| Mn | August | 148.14 ± 10.22 | 25.20 ± 2.14 | 5.88 |
| September | 126.32 ± 6.76 | 34.34 ± 5.62 | 3.68 | |
| October | 142.33 ± 14.18 | 44.43 ± 12.21 | 3.20 | |
| Fe | August | 6337.12 ± 113.12 | 888.25 ± 15.62 | 7.13 |
| September | 5219.32 ± 104.12 | 1249.60 ± 15.23 | 4.18 | |
| October | 4012.01 ± 88.39 | 1192.21 ± 19.78 | 3.37 | |
| Co | August | 8.52 ± 2.15 | 2.23 ± 1.02 | 3.82 |
| September | 9.23 ± 2.14 | 2.12 ± 1.19 | 4.35 | |
| October | 5.63 ± 1.29 | 2.91 ± 0.40 | 1.93 | |
| Ni | August | 12.61 ± 3.20 | 3.23 ± 1.20 | 3.90 |
| September | 27.02 ± 5.11 | 6.12 ± 2.12 | 4.42 | |
| October | 16.82 ± 3.23 | 4.21 ± 1.76 | 4.00 | |
| Cu | August | 132.89 ± 9.71 | 20.62 ± 8.22 | 6.44 |
| September | 80.21 ± 12.31 | 21.43 ± 6.82 | 3.74 | |
| October | 96.12 ± 7.34 | 25.40 ± 6.12 | 3.78 | |
| Zn | August | 24.10 ± 3.32 | 4.21 ± 0.54 | 5.72 |
| September | 65.21 ± 9.24 | 15.06 ± 4.24 | 4.33 | |
| October | 86.64 ± 5.78 | 16.02 ± 5.23 | 5.41 | |
| As | August | 4.33 ± 0.75 | 1.11 ± 0.68 | 3.90 |
| September | 3.35 ± 1.09 | 1.05 ± 0.96 | 3.19 | |
| October | 2.97 ± 0.37 | 0.98 ± 0.12 | 3.03 | |
| Se | August | 19.10 ± 3.97 | 5.62 ± 1.82 | 3.40 |
| September | 23.10 ± 4.83 | 5.98 ± 1.42 | 3.86 | |
| October | 16.40 ± 3.27 | 5.13 ± 2.11 | 3.20 | |
| Mo | August | 4.32 ± 0.90 | 0.97 ± 0.32 | 4.45 |
| September | 3.99 ± 1.21 | 1.99 ± 0.45 | 2.01 | |
| October | 3.09 ± 0.74 | 1.93 ± 0.12 | 1.60 | |
| Ag | August | 6.88 ± 2.17 | 1.95 ± 0.84 | 3.53 |
| September | 4.89 ± 1.31 | 1.56 ± 0.43 | 3.13 | |
| October | 2.79 ± 0.25 | 1.37 ± 0.82 | 2.03 | |
| Cd | August | 13.73 ± 2.37 | 3.02 ± 0.78 | 4.55 |
| September | 13.28 ± 5.24 | 3.98 ± 1.10 | 3.34 | |
| October | 12.23 ± 3.98 | 3.46 ± 0.99 | 3.53 | |
| Pb | August | 24.30 ± 4.21 | 4.33 ± 1.86 | 5.61 |
| September | 21.65 ± 6.42 | 5.02 ± 2.89 | 4.31 | |
| October | 14.34 ± 3.88 | 4.01 ± 1.03 | 3.58 |
Table 2: The mean trace metal content of Melanoides tuberculata and sediment samples downstream of the effluent outfall in Alaro stream (parts per million, ppm)
Results of the mean trace metal bioconcentration and bioconcentration factors determined in the freshwater snail Melanoides tuberculata (Mollusca: Thiaridae ) and sediment samples from upstream and downstream of the effluents in Alaro stream ecosystem of Southwest Nigeria are shown in Table 1 and Table 2, while the mean trace metal concentration in the body tissues (visceral and shell) of Melanoides tuberculata upstream and downstream of the effluent outfall in Alaro stream are shown in Table 3 and 4, while Table 5 shows the significant correlation coefficient in the relationship between trace metals in the snail tissue and sediment samples at the six study stations.
| Trace metal | Month | Viscera | Shell |
|---|---|---|---|
| V | August | 1.98 ± 0.43 | 1.52 ± 0.73 |
| September | 2.05 ± 0.99 | 1.57 ± 0.98 | |
| October | 1.86 ± 0.54 | 0.87 ± 0.19 | |
| Mn | August | 20.64 ± 2.13 | 1.03 ± 0.35 |
| September | 10.60 ± 0.35 | 2.66 ± 1.08 | |
| October | 12.22 ± 3.98 | 1.06 ± 0.55 | |
| Fe | August | 824.49 ± 3.55 | 98.14 ± 5.90 |
| September | 685.43 ± 15.32 | 175.56 ± 7.86 | |
| October | 240.36 ± 43.12 | 77.64 ± 8.65 | |
| Co | August | 3.52 ± 1.21 | 1.22 ± 0.84 |
| September | 1.99 ± 0.57 | 1.31 ± 0.43 | |
| October | 1.80 ± 0.69 | 0.40 ± 0.62 | |
| Ni | August | 30.23 ± 4.43 | 10.49 ± 2.93 |
| September | 34.50 ± 6.12 | 12.64 ± 0.54 | |
| October | 23.34 ± 4.28 | 9.18 ± 1.17 | |
| Cu | August | 168.32 ± 5.66 | 75.78 ± 5.86 |
| September | 126.65 ± 8.29 | 83.93 ± 21.42 | |
| October | 146.90 ± 6.42 | 63.43 ± 9.64 | |
| Zn | August | 1.48 ± 0.84 | 0.89 ± 0.23 |
| September | 2.32 ± 0.75 | 2.10 ± 0.28 | |
| October | 2.01 ± 0.79 | 0.98 ± 0.64 | |
| As | August | 1.95 ± 0.68 | 0.96 ± 0.18 |
| September | 1.73 ± 0.24 | 1.24 ± 0.12 | |
| October | 1.28 ± 0.90 | 0.68 ± 0.22 | |
| Se | August | 10.87 ± 3.24 | 1.87 ± 0.15 |
| September | 11.32 ± 4.22 | 9.73 ± 2.45 | |
| October | 10.57 ± 4.97 | 2.76 ± 1.54 | |
| Mo | August | 1.50 ± 0.89 | 0.35 ± 0.08 |
| September | 1.25 ± 0.77 | 1.02 ± 0.87 | |
| October | 1.75 ± 0.72 | 1.23 ± 0.93 | |
| Ag | August | 2.30 ± 0.91 | 1.85 ± 0.77 |
| September | 2.13 ± 0.34 | 1.34 ± 0.98 | |
| October | 1.98 ± 0.96 | 1.55 ± 0.16 | |
| Cd | August | 3.18 ± 0.77 | 1.45 ± 0.21 |
| September | 2.01 ± 1.02 | 1.03 ± 0.88 | |
| October | 1.52 ± 0.54 | 0.37 ± 0.05 | |
| Pb | August | 7.99 ± 3.08 | 3.83 ± 1.77 |
| September | 1.02 ± 0.73 | 0.98 ± 0.12 | |
| October | 4.33 ± 1.67 | 2.36 ± 0.46 |
Table 3: Mean trace metal concentration in the body tissues of Melanoides tuberculata (ppm) upstream of the effluent outfall in Alaro stream
| Trace metal | Month | Viscera | Shell |
|---|---|---|---|
| V | August | 6,82 ± 4.12 | 4.75 ± 1.24 |
| September | 6.32 ± 2.12 | 3.54 ± 2.21 | |
| October | 5.87 ± 2.89 | 2.53 ± 0.78 | |
| Mn | August | 223.21 ± 9.03 | 102.43 ± 5.09 |
| September | 117.54 ± 10.82 | 63.76 ± 10.33 | |
| October | 328.66 ± 30.23 | 200.19 ± 12.86 | |
| Fe | August | 1762.11 ± 43.01 | 527.33 ± 84.12 |
| September | 2629.71 ± 31.93 | 1093.52 ± 10.88 | |
| October | 1964.76 ± 15.29 | 834.91 ± 16.23 | |
| Co | August | 5.86 ± 1.80 | 3.72 ± 2.02 |
| September | 4.87 ± 1.08 | 2.16 ± 1.06 | |
| October | 6.81 ± 2.82 | 3.22 ± 1.92 | |
| Ni | August | 63.09 ± 9.16 | 34.09 ± 7.82 |
| September | 67.45 ± 8.12 | 33.01 ± 4.92 | |
| October | 52.07 ± 7.89 | 44.83 ± 7.97 | |
| Cu | August | 225.21 ± 6.89 | 160.92 ± 12.98 |
| September | 252.59 ± 8.12 | 142.09 ± 6.55 | |
| October | 399.15 ± 9.44 | 187.29 ± 6.02 | |
| Zn | August | 10.78 ± 3.01 | 4.99 ± 1.07 |
| September | 9.43 ± 2.82 | 4.33 ± 1.26 | |
| October | 8.33 ± 2.01 | 3.52 ± 1.12 | |
| As | August | 2.15 ± 1.88 | 1.27 ± 0.86 |
| September | 2.36 ± 1.09 | 1.26 ± 0.75 | |
| October | 1.76 ± 0.34 | 1.52 ± 0.91 | |
| Se | August | 17.63 ± 3.76 | 5.33 ± 1.51 |
| September | 18.23 ± 2.10 | 9.33 ± 3.13 | |
| October | 17.85 ± 3.93 | 7.64 ± 2.81 | |
| Mo | August | 3.60 ± 0.21 | 2.03 ± 1.07 |
| September | 2.58 ± 1.01 | 1.97 ± 0.84 | |
| October | 3.12 ± 0.82 | 2.51 ± 0.87 | |
| Ag | August | 2.98 ± 0.87 | 1.93 ± 0.66 |
| September | 3.74 ± 0.49 | 1.34 ± 0.53 | |
| October | 4.52 ± 1.73 | 2.33 ± 0.72 | |
| Cd | August | 3.49 ± 0.75 | 2.15 ± 0.99 |
| September | 7.41 ± 1.33 | 3.01 ± 0.54 | |
| October | 5.26 ± 1.54 | 3.73 ± 1.67 | |
| Pb | August | 12.09 ± 2.92 | 8.39 ± 1.25 |
| September | 10.21 ± 3.98 | 3.09 ± 0.75 | |
| October | 13.02 ± 4.01 | 2.11 ± 1.03 |
Table 4: Mean trace metal concentration in the body tissues of Melanoides tuberculata (ppm) downstream of the effluent outfall in Alaro stream
| Trace metal | Upstream | Downstream |
|---|---|---|
| V | 0.9823 | 0.9023 |
| Mn | 0.2839 | 0.8083 |
| Fe | 0.9837 | 0.6927 |
| Co | 0.7291 | 0.9812 |
| Ni | 0.8926 | 0.8278 |
| Cu | 0.4302 | 0.8216 |
| Zn | 0.9287 | 0.3093 |
| As | 0.1927 | 0.8291 |
| Se | 0.9628 | 0.7382 |
| Mo | 0.6298 | 0.9278 |
| Ag | 0.8276 | 0.9287 |
| Cd | 0.9726 | 0.8298 |
| Pb | 0.8273 | 0.8192 |
Table 5: Significant correlation coefficient in the relationship between trace metals in the snail tissue and sediment samples
Results in Table 1 and 2 indicate that the mean trace metal level in the body tissue is higher than that of the sediment for all the metals analyzed, while the Bioconcentration Factors (BF) all exceeds 1. Trace metals in the upstream were lower than that of the downstream stations for both body tissue and sediments. In Table 3 and 4, trace metal concentration in viscera were higher than that of the shell with the downstream levels higher than that of the upstream stations. Table 5 shows a positive significant correlation between the snail tissue trace metal and that of the sediments.
The trace metal concentrations in the snail tissue were more in the viscera than the shell for all metals (Table 3)
The sampling stations-specific trace metal concentration varied in both the freshwater snail and sediment samples with the downstream area having more trace metal concentration. Concentration of trace metals in the freshwater snail, M. tuberculata varied in reflection of the amount in the sediment samples.
Trace metal concentration relationship between the sediments and freshwater snail tissue concentration showed a significant correlation for most of the trace metals as shown in Table 5.
Conclusion
Sediments are known to be a major sink of trace metals in aquatic ecosystems and serve as an indication of the trend and profile of pollution [1] as shown in Table 1 and 2.As shown in Table 3 and 4, the high concentration of trace metals observed downstream in the freshwater snail and sediment appears to be greatly enhanced by high level of effluent discharge into the Alaro stream. This is in addition to the municipal and industrial runoffs .The tissue concentration of the trace metals in the snail depends on the sampling stations as the upstream (control) stations recorded the least level while in the downstream had significantly higher concentration of trace metals (Table 3 and 4).This is attributable to the profile of the trace metal concentration in the sediments.
In this study, the observed higher levels of trace metals in the viscera of the freshwater snail is an indication that ingestion of both sediment and algae might be the primary source of uptake as observed by Herg et al. (2001).The regulatory capacity of the snail is accountable to high concentration of all the trace metals in the viscera and shell that is organ specific as discussed by Brusca and Brusca [12]. The shell of the freshwater snail Melanoides tuberculata contained lower tissue concentration of the trace metals in accordance with the observation of Herg [4].
The levels of all trace metals in the snails were positively correlated with the sediment samples as shown in Table 5, and also a strong correlation which may be attributed to the feeding habits of the snail whose food source is obtained from algae and sediment.
The variability in the levels of V, Mn, Fe, Co, Ni, Cu and Zn in the snail tissues is an indication of their metabolic demands as required trace nutrients and the regulatory capacity to control the other trace metals on an equilibrated scale of essentiality threshold which takes a large concentration to exceed [13].
There was bioconcentration of trace metals in the tissues of the freshwater snail upstream and downstream as shown in the variability of the Bioconcentration Factors (BF) (tissue/sediment ratio) in Table 1 and 2 that varied between 1.57(Mo) and 9.69 (Fe) upstream and 1.60 (Mo) and 7.93 (V) downstream.
This study shows that the freshwater snail Melanoides tuberculata is capable of bioconcentrating trace metals in its tissues which indicate that the main source of uptake is ingestion from the sediment and algae. Potential for bioconcentration is strongly dependent on the amount of trace metals in the sediments and the regulatory capacity of the snail. This shows that the freshwater snail Melanoides tuberculata is a useful bioindicators of trace metal pollution in the aquatic ecosystem. Since the Bioconcentration Factors (BFs) exceed 1, it shows that the freshwater snail Melanoides tuberculata can be used for clean-up of trace metal contaminated freshwater ecosystems. However, there are limitations to its use for clean-up exercises which include: vectorial capacity of Melanoides tuberculata to carry certain parasites which are harmful to humans [14] that includes Clonorchis sinensis (Chinese liver fluke), Paragonimus westermani (Oriental liver fluke),Metagonimus, and Opisthorchis sinensis; bioconcentration of the trace metals in the tissues of the snail only postpones the evil day, as the contaminants may still find their way back into the ecosystem when the snail dies; and the clean-up is not as rapid as compared to chemical and physical methods because it relies on the ecological physiology of the freshwater snail, Melanoides tuberculata.
Acknowledgements
This work benefited from the University of Ibadan Staff Development/McArthur Foundation Fellowship and a laboratory space offered by Professor Jerome Nriagu, Department of Environmental Health Sciences, University of Michigan, Ann Arbor.
References
- Luoma N, Rainbow PS (2008) Metal Contamination in Aquatic Environment: science and lateral management. Cambridge University Press, New York, USA.
- Divrikli U, Horzum N, Soylak M, Elci L (2006) Trace heavy metal contents of some spices and herbal plants from western Anatolia, Turkey. Int J Food Sci Tech 41: 712-716.
- Costa SC, Hartz SM (2009) Evaluation of trace metals (cadmium, chromium, copper and zinc) in tissues of a commercially important fish (Leporinus obtusidens) from Guaíba Lake, Southern Brazil. Braz arch biol technol 52.
- Heng LY, Mokhtar MB, Rusin S (2004) The bioaccumulation of trace essential metals by the freshwater snail, Turritella sp. found in the rivers of Borneo East Malaysia. J Biol Sci 4: 441-444.
- Pihan F, de Vaufleury A (2000) The snail as a target organism for the evaluation of industrial waste dump contamination and the efficiency of its remediation. Ecotox Environ Safe 46: 137-147.
- Ezemonye LIN, Enobakhare V, Ileche I (2006) Bioaccumulation of heavy metals (Cu, Zn, Fe) in freshwater snail (Pila ovata, Oliver 1804) from Ikpoba River of Southern Nigeria. J Aquat Sci 21: 23-28.
- Avelar WEP, Mantelatto FLM, Tomazelli AC, Solva DML, Shuhama T, et al. (2000) The marine mussel Perna perna (Mollusca, Bivalvia, Mytillidae) as an indicator of contamination by heavy metals in the Ubatuba Bay, Sao Paula, Brazil. Water Air Soil Poll 118: 65-72.
- Boon-Nierrmeger EK, Van den Berg A, Wikman A, Wiegant FAC (2000) Phyto-adaptogens protect against environmental stress-induced death of embryo from the freshwater snail Lymnea stagnalis. Phytomedicine 7: 389-399.
- Snyman RG, Reinecke SA, Reinecke AJ (2000) Hemocytic lysosome response in the snail Helix aspersa after exposure to the fungicide copper oxychloride. Arch Environ Contam Toxicol 39: 480-485.
- Duggan IC (2002) First record of a wild population of the tropical snail Melanoides tuberculata in New Zealand natural waters. New Zeal J Mar Fresh Res 36: 825-829.
- Mitchell AJ, Brandt TM (2005) Temperature Tolerance of Red-Rim Melania Melanoides tuberculatus, an Exotic Aquatic Snail Established in the United States. T Am Fish Soc 134: 126-131.
- Brusca RC, Brusca GJ (2003) Phylum Mollusca. Invertebrates. Sinauer Associates, Inc.
- Vijver M (2001) Uptake kinetics of essential and non-essential metals by soil invertebrates; Dynamic Energy and Mass Budgets underlying metals uptake. Ph.D Dissertation of Institute of Ecological Science Vrije University, Amsterdam.
- Pinto HA, Melo AL (2011) A checklist of trematodes (Platyhelminthes) transmitted by Melanoides tuberculata (Mollusca: Thiaridae). Zootaxa 2799: 15-28.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 15131
- [From(publication date):
June-2013 - Dec 22, 2025] - Breakdown by view type
- HTML page views : 10315
- PDF downloads : 4816
