Review Article Open Access
Caffeinated Alcoholic Beverages - An Emerging Trend in Alcohol Abuse
Kelle M Franklin*, Sheketha R Hauser, Richard L. Bell and Eric A EnglemanInstitute of Psychiatric Research, Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN 46202, USA
- *Corresponding Author:
- Kelle M. Franklin
Indiana University School of Medicine
Institute of Psychiatric Research
791 Union Drive, Indianapolis, IN 46202, USA
Tel: 1-317-278-4938
Fax: 1-317-274-1365
E-mail: kmfrankl@iupui.edu
Received June 27, 2013; Accepted August 16, 2013; Published August 22, 2013
Citation: Franklin KM, Hauser SR, Bell RL, Engleman EA (2013) Caffeinated Alcoholic Beverages – An Emerging Trend in Alcohol Abuse. J Addict Res Ther S4:012. doi:10.4172/2155-6105.S4-012
Copyright: © 2013 Franklin KM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Alcohol use disorders are pervasive in society and their impact affects quality of life, morbidity and mortality, as well as individual productivity. Alcohol has detrimental effects on an individual’s physiology and nervous system, and is associated with disorders of many organ and endocrine systems impacting an individual’s health, behavior, and ability to interact with others. Youth are particularly affected. Unfortunately, adolescent usage also increases the probability for a progression to dependence. Several areas of research indicate that the deleterious effects of alcohol abuse may be exacerbated by mixing caffeine with alcohol. Some behavioral evidence suggests that caffeine increases alcohol drinking and binge drinking episodes, which in turn can foster the development of alcohol dependence. As a relatively new public health concern, the epidemiological focus has been to establish a need for investigating the effects of caffeinated alcohol. While the trend of co-consuming these substances is growing, knowledge of the central mechanisms associated with caffeinated ethanol has been lacking. Research suggests that caffeine and ethanol can have additive or synergistic pharmacological actions and neuroadaptations, with the adenosine and dopamine systems in particular implicated. However, the limited literature on the central effects of caffeinated ethanol provides an impetus to increase our knowledge of the neuroadaptive effects of this combination and their impact on cognition and behavior. Research from our laboratories indicates that an established rodent animal model of alcoholism can be extended to investigate the acute and chronic effects of caffeinated ethanol.
Keywords
Alcohol; Caffeine; Caffeinated alcohol; Energy drink; Adenosine; Dopamine; Alcohol-preferring rat
Abbreviations
MCL: Mesocorticolimbic; DA: Dopamine; VTA: Ventral Tegmental Area; mPFC: Medial Prefrontal Cortex; NAc: Nucleus Accumbens; GABA: γ-aminobutyric Acid; EtOH: Ethanol; TH: Tyrosine Hydroxylase; BDZ: Benzodiazepine; NBTI: Nitrobenzylthioinosine
Prevalence and Impact of Alcohol use Disorders
Extensive evidence indicates that alcohol abuse has widespread social, economic, behavioral, and physiological consequences [1]. The Centers for Disease Control and Prevention (CDC) rank alcohol abuse as the third leading cause of preventable death [2]. For example, a causal relationship has been suggested between alcohol abuse and at least 50 different medical conditions [3]; also see [4] for a discussion on the role of genetics). According to the National Highway Traffic Safety Administration [5], nearly one-third of traffic fatalities in the United States involve at least one vehicle operator with a blood alcohol level (BAL) of at least 0.08 gram %, which is the legal BAL threshold for driving while impaired in the United States [6]. Direct and indirect costs of alcohol abuse have been estimated to approximate $500 billion annually [7]. Over half of adult Americans have a close relative with an alcohol use disorder (AUD), and a subset of these individuals have this trait across multiple generations.
The gap between men and women in the prevalence of AUDs and episodes of intoxication has been decreasing among youth and the elderly [8,9]. Regardless of gender, recent years have witnessed an increase in alcohol-related problems, particularly in young drinkers [10-12]. This is a serious concern, as early onset of alcohol use is a significant risk factor in the development and time-course of alcohol dependence [13,14].
Binge drinking similarly has been associated with future alcohol dependence problems and with younger drinkers [15-17]. More than 70% of surveyed college students report having engaged in alcohol binge drinking during their high school years [17]. Alcohol drinking in underage drinkers aged 12-20 years accounts for 11% of alcohol consumption in the United States, with a majority of this intake occurring during binge drinking episodes [18]. Regarding post-collegeage binge-drinking, 45% of Americans aged 21-25 report that they had engaged in alcohol binge drinking in the previous month [19]. Nearly half of all individuals meeting life-time diagnostic criteria for alcohol dependence do so by the age of 21 and this increases to approximately two-thirds by the age of 25 [20]. In summary, the association of binge drinking with the development of alcohol dependence is magnified by the propensity of young people to participate in this behavior.
Caffeine in the Context of Alcohol Abuse
Further complicating this issue is the common occurrence of consuming others drugs with alcohol. Polydrug use may introduce unique, complex cognitive and behavioral interactions that cannot be addressed by assessing the effects of each drug individually. Alcoholic populations exhibit an extremely high rate of poly-drug use [21]. One substance that is increasingly paired with alcohol is caffeine [22].
Caffeine (1,3-trimethylxanthine) is the United States’ most used psychoactive substance [23,24]. Caffeine largely has been eschewed as a psychoactive drug, despite, or perhaps because of, its widespread use. The U.S. Food and Drug Administration (FDA) have categorized caffeine as an ingredient “generally recognized as safe” [22]. However, it is a mild psychostimulant, with motor effects similar to more potent drugs of abuse, such as cocaine and amphetamine [25,26]. Despite basic and clinical evidence that caffeine is reinforcing [tolerance can develop to some of its effects, and cravings and mild withdrawal symptoms can ensue after cessation of consumption [25,27-30], it is rarely considered a drug of abuse. Nevertheless, if dependence criteria, in their broadest sense, from the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders Text Revision (DSM-IV-TR) [31] are used, a significant portion of the population would be considered dependent upon caffeine intake [32,33]. What is especially troubling is that, similar to alcohol, adolescents and young adults are especially prone to abuse caffeinated beverages [34-36].
Behavioral and genetic associations indicate that there is a significant link between caffeine and alcohol intake [37-42]. Regarding caffeine abuse by alcoholics, Zeiner et al. [43] reported that alcoholdependent individuals consume approximately 30% more caffeine daily, compared to their non-alcoholic counterparts. In addition, reports suggest that detoxified alcoholics consume twice as much coffee following cessation of alcohol drinking, compared to their intake prior to treatment [44]. This could be a serious concern for treatmentseeking alcoholics. For example, using caffeine intake as a substitute stimulus for alcohol consumption could interfere with psychological and physiological efforts to overcome addiction-related behaviors. Further, it is unclear what impact, if any, a history of heavy alcohol drinking could have on caffeine’s pharmacological profile, and whether this could affect the caffeine levels consumed by actively drinking and detoxified alcoholics. Assuming that caffeine and ethanol share at least some mechanisms of action, it is possible that physiological adaptations could generalize between the two substances. In addition, this elevated caffeine intake by alcoholics could be indicative of a more generalized physiological or genetic vulnerability to substance abuse and could mandate specialized treatment strategies.
In support of this latter suggestion, an assessment of adult twins indicates that ethanol and caffeine use disorders reflect approximately 50% heritability, although genetic loading for caffeine dependence may be lower than that for alcoholism [45-47]. In addition, poly-drug twin study analyses have reported a significant heritability effect on the couse of ethanol and caffeine [48-50]. Similarly, Svikis et al. [51] reported that caffeine-dependent women who were family history positive (FHP) for alcoholism were less successful in reducing caffeine intake during pregnancy, relative to those who were family-history negative (FHN) for alcoholism. These authors [51] concluded that FHP individuals may require more intensive dependence nterventions, due to a generalized vulnerability to heavy substance use. Within the context of dual-diagnosis, it is important to recognize that Bergin and Kendler [52] reported significant correlations (genetic and/or environmental) between caffeine use, tolerance and/or withdrawal and generalized anxiety, panic, phobic as well as major depressive disorders, conditions which also have been associated with heavy alcohol use.
Behavioral genetic reports indicating that a family history of alcoholism affects caffeine intake coincide with some pre-clinical findings in our laboratory. We performed experiments to investigate whether rats selectively bred for high alcohol preference also would display greater caffeine intake, relative to their progenitor stock. Alcohol-preferring (P) rats satisfy the criteria proposed for animal models of alcoholism, including consuming alcohol for its pharmacological effects and exhibiting signs of alcohol dependence and tolerance [53,54]. The genetically selected P rat shares some of the characteristics of FHP individuals, including early onset of excessive alcohol intake, lower sensitivity to the high-dose effects of alcohol and greater sensitivity to the behavioral and autonomic stimulating effects of low-dose alcohol, compared with NP (FHN) and outbred Wistar rats [55,56]. These rats exhibit neural and behavioral differences, as compared to nonpreferring NP counterparts and non-selected rats [57,58], such as performance of binge-like alcohol drinking behaviors [59-61]. P rats obtain pharmacologically relevant blood alcohol levels under several alcohol access conditions [57,59,61,62], and evidence supports the use of these animals to model human binge drinking [63].
Also similar to human alcoholics, P rats and other rodent models of alcoholism exhibit a greater general preference for some rewarding stimuli, such as illicit drugs, including nicotine [64] 3,4-methylenedioxymethamphetamine (ecstasy) [65], sucrose, and novelty, relative to non-preferring and non-selected rodent lines [e.g., 66-68]. Previous studies have reported that subjects selectively bred for high alcohol intake exhibit greater behavioral and neurochemical responses to psychostimulants, compared to low alcohol drinking or non-selected lines. Hyyatiä and Sinclair [69] reported that high alcoholpreferring Alko Alcohol (AA) rats consume more cocaine orally than do their alcohol non-preferring Alko, non-Alcohol (ANA) counterparts. Similarly, AA and Wistar rats behaviorally selected for high alcohol consumption exhibit increased conditioned place preference for cocaine [70,71], compared to their low drinking counterparts. Further, greater increases in cocaine-induced extracellular DA release in striatal compartments have been reported in Sardinian alcohol-preferring (sP) and AA rats, compared to alcohol non-preferring subjects [72,73]. Taken in conjunction with findings that high alcohol-preferring rat lines exhibit differential consumption of and neurobehavioral responses to other drugs of abuse beyond alcohol, such as cocaine and nicotine, compared to nonpreferring and nonselected counterparts, it is likely that these animals also could provide a useful model to examine caffeine intake.
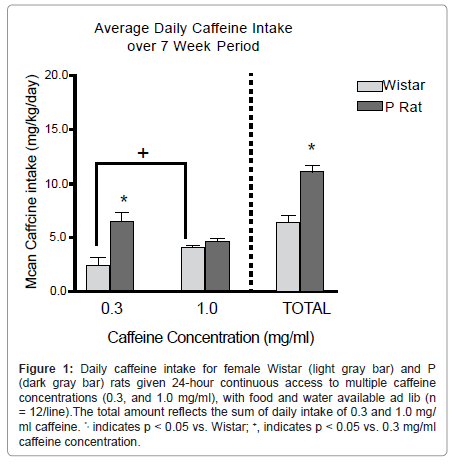
Regarding the association of a genetic background for high alcohol preference with caffeine intake, we assessed female P rats and outbred Wistar rats for free-choice 24-hr intake of multiple concentrations (0.3 and 1.0 mg/ml) of caffeine and water over a 7-week period. These concentrations were selected from those used in a previous caffeine intake study [74]. From the human perspective, these concentrations amount to the difference between a 16-oz. serving of coffee from McDonald’s® or Starbucks®, respectively [75]. We hypothesized that P rats would consume more total caffeine per day, vs. Wistar rats, and that the greatest differences would occur at the 1.0 mg/ml caffeine concentration.
At no time in the course of this study did either P or Wistar groups exhibit a preference for caffeine solution over water. It has been shown previously that a period of forced access to caffeine is needed to observe caffeine preference over water [76]. The results indicated that P rats consumed more total caffeine (mg/kg) per 24-hr period than Wistar rats, such that consumption levels were approximately 10.5 vs. 6.5 mg/ kg/24 hr, respectively (Figure 1). Clinically, reports indicate that nondependent humans consume about 4-5 mg caffeine/70 g body weight. In contrast to our a priori hypothesis, the majority of this difference was associated with the lower (0.3 mg/ml) caffeine concentration, whereas intake of the higher (1.0 mg/ml) concentration was approximately equal in the two rat lines. One possible explanation for this finding is that intake of the higher concentration may have been limited by its flavor profile in both rat lines. In contrast, the two rat lines may have exhibited differential intake of the lower caffeine concentration due to the pharmacological properties of the compounds. Similar line differences for concentration-dependent differences in response to alcohol have been reported previously [77]. Overall, P rats consumed more caffeine than did outbred Wistar rats. This difference suggests that genetic selection for high alcohol preference also may have generated a propensity for elevated caffeine intake. These findings may provide additional validity for the use of the P animal model of alcoholism to examine ethanol co-abuse with other substances. Furthermore, this study provides basic research support for clinical findings indicating an association between alcohol and caffeine intake, which may be mediated by common reward neurocircuitry.
Figure 1:Daily caffeine intake for female Wistar (light gray bar) and P (dark gray bar) rats given 24-hour continuous access to multiple caffeine concentrations (0.3, and 1.0 mg/ml), with food and water available ad lib (n = 12/line).The total amount reflects the sum of daily intake of 0.3 and 1.0 mg/ ml caffeine. *, indicates p < 0.05 vs. Wistar; +, indicates p < 0.05 vs. 0.3 mg/ml caffeine concentration.
Caffeinated Alcoholic Beverages
The introduction of Red Bull® energy drink in the United States in 1997 sparked a surge in consumption of “energy drinks,” as well as energy drinks mixed with alcohol [78]. These products essentially combine purported performance-enhancing ingredients (e.g., guarana, taurine, ginseng, green tea extract, and/or B-complex vitamins) with high doses of caffeine (55 to 505 mg/serving) [78]. In comparison, 12- oz cans of Coca-Cola and Mountain Dew soft drinks contain 34 and 54 mg caffeine, respectively [79,80]. As a parallel to binge alcoholdrinking, approximately one-third of 12-24 year-olds consume energy drinks on a regular basis [81]. A reported decline in soft drink consumers between 2003 and 2008 likely reflects, in part, a doubling in the number of energy drink consumers during that same time frame [82].
As indicated above, an alarming trend is the addition of alcohol to these energy drinks. In general, marketing strategies for energy drinks have targeted young males [78], a group which also exhibits the greatest prevalence and frequency of alcohol binge drinking [83]. This is particularly of concern due to the lack of available information that addresses whether a history of heavy caffeine intake affects alcoholrelated pharmacology and toxicology. With the wide acceptance of energy drinks, more and more drinking establishments and manufacturers have increased the caffeine content of their alcoholic beverages. The growing availability of high-caffeine energy drinks and pre-caffeinated alcoholic drinks has translated into high levels of caffeine and ethanol co-consumption. Young drinkers are the target market for many of these cocktails [84] and have contributed to their popularity [85]. Particularly concerning is that caffeinated alcoholic beverages may initiate earlier and more extreme caffeine and alcohol intake in younger populations. A 2006 web-based survey of ten colleges in North Carolina found that 24% of students who reported alcohol intake in the prior month had mixed alcohol with an energy drink [86]. Another sample of U.S. college students indicated that nearly half of survey respondents had consumed alcohol mixed with energy drink [84]. In Brazil, more than three-quarters of survey participants reported regular consumption of energy drinks mixed with alcohol [87], whereas in Turkey, the incidence in college students has been reported at 40% [88].
The popularity of these beverages has encouraged researchers to question what motivates their consumption. A standardized assessment of the impact of expectancies on motivation to consume these beverages indicated that caffeine was believed to enhance alcoholrelated intoxication, a factor also associated with increased intake of the mixtures [89]. Caffeine and energy drinks have been reported to reduce ethanol-induced sedation or perception of sedation in humans and animals [87,90-92], although these findings have been mixed [93]. Consumption of caffeinated alcoholic beverages also increases reports of happiness and euphoria, behavioral disinhibition, and physical vigor, relative to alcohol alone [87,94].
However, just as individuals who consume large amounts of alcohol often underestimate how much alcohol affects them [95], increasing evidence indicates this may be compounded when these individuals consume caffeinated alcoholic beverages. Despite indications that young consumers of these drinks do not discount the risks of alcoholrelated negative consequences when caffeine is co-consumed [89], some evidence in the U.S. and Canada indicates that adding caffeine to alcohol actually may increase these risks. Several reports indicate that caffeine co-administration increases alcohol intake and hazardous alcohol drinking [86,94,96,97]. Co-consumption of energy drinks and alcohol has been reported to triple the likelihood of binge alcohol drinking, relative to drinking alcohol alone [96]. O’Brien et al. [86] found that students who mixed alcohol and caffeine reported more heavy alcohol drinking episodes and twice as many episodes of weekly intoxication. A recent self-report study found that high-frequency energy drink consumers (>1/week) were heavier alcohol drinkers, drank alcohol more often, had greater risk for alcohol-related problems, and exhibited a higher risk of meeting DSM-IV criteria for alcohol dependence, relative to those with low or no energy drink consumption [97], but see comment by Skeen and Glenn [98]. Several reports indicate that consumers of caffeinated alcoholic beverages engage in more violent and risky behaviors, and experience more negative consequences, compared to those drinking alcohol alone. Particularly concerning are reports that consumers of caffeinated alcoholic beverages evidence more assaults (as perpetrators or victims), automobile incidents, and planned and actual alcohol-impaired driving episodes, relative to those drinking alcohol alone [86,99-101]. These negative consequences are particularly evident in adolescent populations [102].
It has been postulated that caffeine reduces perceived ethanol intoxication with little or no change to the cognitive impairing effects of ethanol [103,104], although this decrease may be taskdependent [105,106]. Expectations also could be involved in these findings. Evidence indicates that psychomotor tolerance to ethanol is increased in humans with a prior history of combining caffeine and ethanol, compared to individuals who have experienced either drug alone [107]. Moreover, this tolerance likely is, in part, an unconscious cognitive construct. Fillmore et al. [107] reported that informing subjects that caffeine would interfere with ethanol-induced sedation diminished caffeine’s ability to do so, and Fillmore [108] suggested that the expectation that caffeine would interfere with ethanol-induced sedation could trigger compensatory physiological mechanisms to maintain the disruptive influence of ethanol. These results emphasize the role of cognitive processes in the physiological response to ethanol in human subjects.
With regard to motor functioning, Marczinski and Fillmore [109] and Marczinski et al. [110] presented evidence that caffeine attenuates ethanol-induced motor skill disturbances. n a cued go/no go task, consuming an energy drink along with ethanol was reported to improve reaction time for identifying and providing a keyboard response for a “go” visual target (i.e. a green rectangle), but failed to attenuate ethanol-induced reductions in inhibitory control, in response to a “nogo” target (i.e. a blue rectangle) [109,110].
Marczinski et al. [111] recently reported that the co-administration of the energy drink Red Bull® with alcohol did not alter the ethanolinduced impairment on objective measures such as dual-task information and motor coordination but reduced perceptions of mental fatigue and enhanced feelings of stimulation compared to alcohol alone. These results may suggest that the combination of ethanol with caffeine could lead to inaccurate perceptions of performance abilities [111]. This finding is particularly disturbing in light of the increased propensity to engage in alcohol-impaired driving following caffeinated alcohol intake, relative to alcohol alone [96,101], as well as findings which indicate that caffeine does not attenuate alcohol-related impairment in simulated driving or sustained attention/reaction time [112]. Overall, caffeine likely exerts stimulatory effects to counter ethanol’s sedative properties, but does not alter ethanol-induced behavioral disinhibition, which could contribute to increased risk-taking during impaired driving. Similarly, Ferreira et al. [87] reported that subjective reports of physiological states and behavioral abilities following consumption of Red Bull® energy drink and 37.5% v/v vodka co-administration did not correspond to objective behavioral measures of intoxication. Participants in this study reported reduced headache, weakness, dry mouth, and motor impairment following the co-administration of an energy drink and alcohol. However, measurements of motor coordination and visual reaction time indicated no differences between individuals consuming alcohol with or without the addition of the energy drink [87].
While some behavioral data have been used to conclude that caffeinated alcohol is pharmacologically differentiable from alcohol alone, existing behavioral support for this stance is correlational. These reports could be overlooking intervening variables that link caffeinated alcoholic beverage intake with high levels of alcohol consumption, such as high sensation-seeking personality [32] or age. It also is noteworthy that many studies that report detrimental effects of mixing alcohol and caffeine were reported in the U.S. or Canada, and could reflect social or cultural norms. As indicated above, a survey of young adults in Brazil and Turkey found 75% vs. 40% engaged in caffeinated alcohol beverage consumption, respectively [87,88]. Recent reports from The Netherlands and Australia suggest that energy drink consumption actually reduces alcohol drinking [113] and/or negative alcohol-related consequences [94,113]. Thus, it currently is unclear whether caffeine’s apparent exacerbation of heavy alcohol drinking reflects a physiological effect or a loading of social and demographic risk factors such as race, age, gender, culture, religion, and involvement in the college Greek community, which can influence the propensity to consume large amounts of caffeinated alcoholic beverages [86]. Regardless of whether caffeine-facilitated elevations in alcohol intake are social or biologica phenomena, the long-term consequences of this behavior are cause for concern [22].
Besides the growing concern from the scientific research detailing risks associated with consumption of caffeinated alcoholic beverages reports of several deaths and hospitalizations following consumption of this drug combination have increased the focus on the potential dangers. In response to evidence that caffeinated alcohol intake may be harmful, the FDA proclaimed caffeine to be an “unsafe food additive” when combined with alcohol [22]. The primary explanation for this characterization was due to caffeine’s ability to mask some indicators of alcohol intoxication, without exhibiting clear effects on alcohol metabolism [22,114]. Warning letters from the FDA to 4 caffeinated alcoholic beverage manufacturers questioned the safety of caffeinated alcoholic beverages, and prompted several distributors to withdraw their products from the market [22]. However, it is unclear what impact this move will have on caffeinated alcohol intake in general, as reports suggest that the majority of these beverages are mixed by consumers, rather than being caffeinated prior to retail sale [89]. Further, an overall dearth in the body of research investigating the neurobehavioral effects of caffeinated alcoholic beverages weakened this warning and precluded a definitive statement regarding the relative safety of these mixtures.
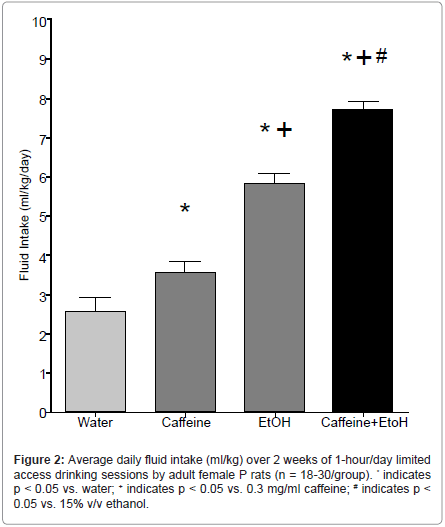
Findings that alcoholics and individuals with increased vulnerability for developing AUDs also consume more caffeine confirm a need to investigate whether alcohol and caffeine co-consumption increases alcohol intake and/or increases the likelihood of developing AUDs (e.g. [97]). Young people, in particular, have exhibited an attraction for consuming caffeinated alcoholic beverages, and often engage in binge drinking this combination. An extensive literature highlights the deleterious effects, both peripherally and centrally, of binge alcoholdrinking [86]. Given that the addition of caffeine to alcoholic beverages appears to exacerbate binge-drinking, it is absolutely necessary that research into the acute and long-term effects of this combination be undertaken immediately. Following our previous findings that P rats consume more caffeine than do Wistar rats (Figure 1), as well as existing research detailing differences in ethanol intake between the two rat lines [58], our laboratory sought to examine levels of caffeinated ethanol intake in a controlled basic research environment. To that end, female P rats were assessed for intake of a caffeinated alcoholic solution, or one of its component ingredients: water, 0.3 mg/ml caffeine, or 15% ethanol over a 14-day 1-hr/day scheduled access period. In line with our a priori hypotheses, the results indicated that caffeinated ethanol intake was significantly greater than intake of water, caffeine, or ethanol alone (Figure 2). In addition, only the rats consuming caffeinated ethanol evidenced progressive increases in alcohol intake over the 14- day access period, which may signal a transition from casual drinking behaviors to dependence (data not shown). These findings may exhibit sub-additive or synergistic interactions of the two compounds, which may alter their rewarding or behavioral properties. However, without further investigation, it is unclear what neurobehavioral alterations are reflected by the increased intake of the combined compounds. In addition, these experiments were performed in female rats; however, sex-specific differences in self-administration are an important consideration. Future directions should look toward repeating these experiments in males to ascertain whether they would consume similar amounts of the solutions.
Overall, the findings indicate that caffeine elevates ethanol intake and, conversely, ethanol increases caffeine intake, even in the absence of human cultural biases. Generalized increases in activity due to the activating effects of caffeine and/or ethanol [115-119] could contribute to the increase in alcohol intake when caffeine is co-consumed. Elevations in drinking as a result of locomotor stimulation may support the common assumption that caffeine attenuates or masks the sedating effects of alcohol [91], which may enable individuals to consume more caffeinated alcohol drinks over a longer period of time [120].
Some animal research supports findings that caffeine attenuates the behavioral sedation associated with ethanol. El Yacoubi et al. [121] reported that 25 mg/kg caffeine decreased the duration of ethanolinduced loss of righting reflex in mice. Ferreira and colleagues [92] presented evidence that caffeinated energy drinks attenuate the locomotor-depressing effects of ethanol, such that 10.71 ml/kg Red Bull® significantly decreased the sedating effects of 2.5 g/kg ethanol in Swiss mice. It is possible that combined mechanisms of caffeine and alcohol are involved in these findings. For example, caffeine can inhibit benzodiazepine binding to GABAA receptors [122]. This is especially noteworthy/concerning because benzodiazepines are commonly used during alcohol detoxification [123], despite their high abuse/co-abuse potential [124]. These effects result primarily from caffeine blockade of adenosine receptors on striatal GABA neurons [119]. Blockade of VTA GABAA receptor activation previously was posited to increase ethanol intake by reversing the drug’s GABA-mediated sedative-hypnotic effects [125].
In line with this research, Sudakov et al. [126] reported that chronic caffeine drinking increases sensitivity to the locomotor stimulating properties of ethanol, particularly in ethanol-insensitive subjects. As motor stimulation previously has been associated with low-dose ethanol preference and reinforcement [118], this suggestion may indicate that caffeine facilitates ethanol drinking by increasing sensitivity to rewardinducing psychomotor effects associated with ethanol intake.
Pharmacokinetic Interactions of Alcohol and Caffeine
The possibility that caffeine increases alcohol intake due to changes in ethanol’s pharmacokinetic properties has prompted several laboratories, including our own, to pursue this line of research. Caffeine increases metabolic rate, both in active and sedentary individuals [127]. It is plausible that the thermogenic or diuretic properties of caffeine [127-129] could facilitate ethanol elimination through increases in transdermal evaporation or water and sodium excretion. However, according to Ferreira et al. [87,92] and Kunin et al. [130], caffeine exposure does not alter ethanol pharmacokinetics in humans or rodents to a significant degree.
Ferreira et al. [87] reported that the addition of Red Bull® energy drink did not alter breath alcohol concentration levels or alcohol elimination rates associated with 0.6 or 1.0 g/kg 37.5% v/v vodka, over a 150-min time course. Similarly, intragastric administration of a Red Bull®/vodka mixture did not reduce 30-min BALs in mice [92]. Kunin et al. [130] reported similar findings using male Wistar rats. These researchers [130] reported that caffeine pretreatment (i.p.) delivered 30 min prior to systemic ethanol injections (i.p.), did not alter BALs at 15 or 30 min following alcohol exposure [130].
Our laboratory has observed similar outcomes to those presented by Kunin et al. [130] and Ferreira et al. [87,92] with regard to peak ethanol levels. We utilized subcutaneous microdialysis [131] to assess the ability of caffeine to alter alcohol pharmacokinetics in female P rats. The time-course of interstitial ethanol levels was assessed, beginning immediately after acute exposure to 1 mg/kg caffeine dissolved in 0.5 g/kg 15% w/v ethanol, or 30 mg/kg caffeine dissolved in 2.0 g/kg 15% w/v ethanol, or equivalent doses of 15% w/v ethanol alone. In line with previous evidence, we hypothesized that co-administration of caffeine would not alter the BALs of P rats. In support of this hypothesis, peak ethanol levels were not altered by caffeine co-administration. However, we did observe some evidence that caffeine increases ethanol clearance at low doses (1 mg/kg caffeine; 0.5 g/kg ethanol; Table 1). Due to methodological differences, it is impossible to make direct comparisons with the results presented by Ferreira et al. [87], who did not observe caffeine-induced differences in ethanol clearance. Furthermore, this effect was not present with the higher dose combination, indicating that this alteration was low-dose specific. The apparent lack of effects at higher alcohol and caffeine doses does not support extrapolation of these data to explain some of the behavioral differences that have been reported following heavy consumption of caffeinated alcoholic beverages. In contrast, these data likely support previous reports that exclude differences in ethanol-related pharmacokinetics as a potential explanation for caffeine’s ability to alter the effects of ethanol.
With the exception of assessing alcohol levels, few studies deliver a concentrated effort to identify the neural and physiological mechanisms that might underlie purported behavioral and cognitive differences between alcohol drinking and caffeinated alcohol drinking. To that end, research that examines neural effects of caffeine or ethanol individually often is the only available tool to extrapolate the effects of these substances when consumed in combination.
| Treatment Condition | Ethanol Peak Level (mg%) | Ethanol Elimination(mg%/min) |
|---|---|---|
| Ethanol√?¬†√?¬† Low | 48√?¬† ±√?¬†√?¬† 5 | -0.70 ± 0.05 |
| Ethanol/Caffeine Low | 68√?¬† ± √?¬†6 | -0.92 √?¬Ī 0.14* |
| Ethanol√?¬†√?¬† High | 235√?¬† ± √?¬†10 | -0.78√?¬† ± 0.17 |
| Ethanol/Caffeine High | 222 ±√?¬† 19 | -0.68√?¬†√?¬† ± 0.02 |
Table 1: Peak ethanol levels (mg%) and ethanol elimination rate (mg%/min) in female P rats (n = 5-7/group) following acute i.p. exposure to 1 mg/kg caffeine dissolved in 0.5 g/kg 15% w/v ethanol (low) , or 30 mg/kg caffeine dissolved in 2.0 g/kg 15% w/v ethanol (high), or equivalent doses of15% w/v ethanol alone. *, indicates p < 0.05 vs. ethanol alone group.
Overlapping Systems Associated with Caffeine and Ethanol: Adenosine and Dopamine
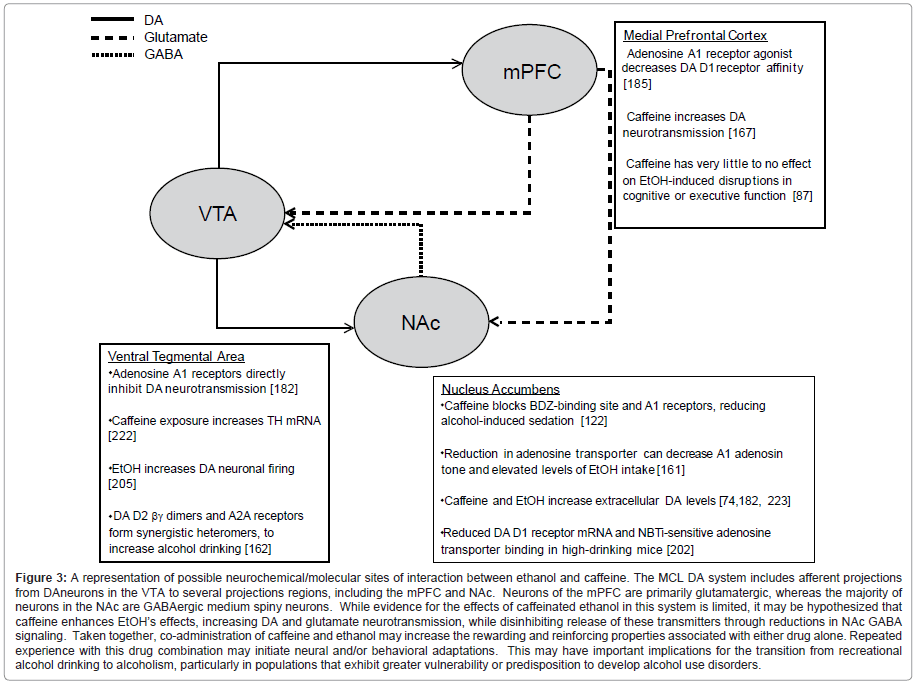
Due to the apparent behavioral and genetic interactions of AUDs and caffeine intake, an important area of research is to identify neural systems that underlie some of these interactions. Both caffeine and ethanol affect dopaminergic and adenosinergic neurotransmission, which are thought to contribute to their neurobiological and reinforcing properties. Figure 3 shows a schematic of dopamine, glutamate, and GABA neurotransmission in the MCL system, as well as providing selected evidence for the manner in which caffeine and ethanol exposure could affect these signals.
Adenosine
Adenosine is a purine neurotransmitter/neuromodulator with many (largely inhibitory) central and peripheral sites of action mediated through four identified G-protein coupled receptors, A1, A2A, A2B, and A3 [132,133]. Adenosine receptors exhibit widespread central distribution and modulate the majority of neurotransmitter systems, either directly or through indirect modulation of amino acid neurotransmitters [134,135]. Adenosine A1- and A3-type receptors are cyclic adenosine 5’monophosphate (cAMP)-inhibiting, while A2A- and A2B-type receptors are cAMP-stimulating [136]. The majority of research involving adenosine receptors has targeted the A1 and A2A receptors, which are sufficiently sensitive for activation through tonic adenosine signaling.
A1 receptors are the most densely populated adenosine receptor subtype within the central nervous system, and are present in the hippocampus, cerebral and cerebellar cortices, hypothalamus, and some areas of the thalamus [137,138]. A1 receptors have the highest affinity for adenosine binding (70 nM concentration) [139]. They have been associated with physiological-behavioral functions, such as sleep, arousal, and anxiety [139]. These receptors modulate the actions of nearly all other neurotransmitters, including dopamine.
Adenosine A2 receptors have been subdivided into A2A and A2B receptor types. A2A receptors have an adenosine binding affinity of 150 nM. These receptors are less widespread in the CNS, relative to A1 or A2B receptors. A2A receptors primarily are localized in the striatum, olfactory tubercle, nucleus accumbens (NAc), and other areas receiving significant dopamine innervation [139-141]. A2 receptors are linked to adenylyl cyclase stimulation; their primary function is to regulate neurotransmitter release [142]. For further reviews on adenosine, see [143,144].
Figure 3:A representation of possible neurochemical/molecular sites of interaction between ethanol and caffeine. The MCL DA system includes afferent projections from DAneurons in the VTA to several projections regions, including the mPFC and NAc. Neurons of the mPFC are primarily glutamatergic, whereas the majority of neurons in the NAc are GABAergic medium spiny neurons. While evidence for the effects of caffeinated ethanol in this system is limited, it may be hypothesized that caffeine enhances EtOH√ʬ?¬?s effects, increasing DA and glutamate neurotransmission, while disinhibiting release of these transmitters through reductions in NAc GABA signaling. Taken together, co-administration of caffeine and ethanol may increase the rewarding and reinforcing properties associated with either drug alone. Repeated experience with this drug combination may initiate neural and/or behavioral adaptations. This may have important implications for the transition from recreational alcohol drinking to alcoholism, particularly in populations that exhibit greater vulnerability or predisposition to develop alcohol use disorders.
Adenosine and caffeine: Caffeine is a relatively nonspecific competitive antagonist of adenosine receptors. Adenosine reports indicate that chronic caffeine exposure may reduce the compound’s ability to block A1 receptors, due to conformational changes in the receptors or endogenous ligand activity [74,145,146].
There has been a great deal of inconsistency in reports associated with the effects of caffeine intake on adenosine A2A receptors. Some reports indicate that caffeine exposure does not have a lasting effect on adenosine A2A receptors or their pharmacological modulators [147-149]. However, others have indicated that chronic caffeine experience up-regulates [150,151] or down-regulates [152] A2A receptors. These results largely depend on the tested species and the employed caffeine exposure procedures.
Adenosine and ethanol: While the availability of research that examines caffeinated alcohol is limited, caffeine’s primary pharmacological mechanism of action occurs through direct antagonism of adenosine receptors. Therefore, evidence for direct interactions of adenosine and ethanol potentially could provide useful information regarding interactions of caffeine and ethanol.
Ethanol drinking has been reported to increase A1 receptor number [153], although chronic exposure also might desensitize these receptors in a manner similar to caffeine [74,145]. Adenosine neurotransmission has been associated with some effects of acute and prolonged ethanol exposure, as well as ethanol withdrawal [154,155]. Chronic ethanol also increases extracellular CNS adenosine levels with brain region specificity [156]. Adenosine A1 receptors have been implicated in mediating the anxiolytic and motor impairing effects of ethanol [157,158], as well as withdrawal-induced seizures [154].
Ethanol’s effects also are associated with adenosine A2A receptor signaling. Adenosine A2A receptors contribute to ethanol-induced motor effects. For example, El Yacoubi et al. [121] found that A2A knockout mice exhibit a shorter latency to regain righting reflex following acute ethanol exposure, relative to wild-type mice. Some pre-clinical research indicates that ethanol-induced reinforcement is reduced in subjects lacking or having diminished A2A receptor functioning [159,160]. It has been reported that systemic administration of 3 and 10 [159] or 10 and 20 [160] mg/kg doses of the partially selective A2A receptor antagonist 3,7-dimethyl-1-propargylxanthine (DMPX) dose-dependently reduced ethanol-reinforced operant responding. In contrast, a lower (1 mg/kg) dose of DMPX has been shown to increase ethanol-reinforced operant responding [160].
Evidence indicates that sedative and ataxic responses to ethanol also are reduced in mice lacking the equilibrative nucleoside transporter 1 [161]. This transporter is responsible for facilitating adenosine diffusion across cellular membranes. Acute ethanol exposure blocks these transporters [162-164], increasing extracellular adenosine levels, facilitating extracellular adenosine receptor activation, and inducing some of ethanol’s sedative-hypnotic effects. In contrast, chronic ethanol exposure likely desensitizes these transporters to ethanol blockade and contributes to desensitization of adenosine receptors [162-164]. The widespread central effects of ethanol and caffeine are not localized to adenosine systems only. They are manifested in multiple other neural circuits, including the mesocorticolimbic (MCL) dopamine system.
Mesocorticolimbic dopamine system
The MCL dopamine system is comprised of dopamine cell bodies in the ventral tegmental area (VTA), and their projections to several forebrain areas, including the basolateral amygdala, hippocampus, lateral septum, olfactory tubercle, NAc, and the medial prefrontal cortex (mPFC). For a review on MCL dopamine activity, see [165].
Exposure to either caffeine or ethanol increases extracellular dopamine levels in MCL terminal regions [74,166-169], and stimulates feedback to the VTA to terminate dopamine output from this region [170-172]. Together, these findings suggest that caffeine and ethanol activate both inhibitory and excitatory neurocircuitry within the MCL system which may have implications for drug reward and abuse. From these and other reports, researchers have proposed that the role of adenosine receptors to modulate ethanol or caffeine reward likely is related, in part, to interactions with central dopaminergic systems.
Dopamine and adenosine in the mesocorticolimbic reward circuit: Adenosine signaling directly affects dopamine systems. Adenosine and dopamine interactions likely play integral roles in caffeine reinforcement and psychomotor stimulation. Adenosine A1 and A2A receptors are found in many dopamine-rich areas of the central nervous system, including the MCL dopamine system [162,167,168]. Research indicates that adenosine and dopamine receptors form functionally interactive, primarily antagonistic heteromeric receptor complexes [173-179]. Similarly, caffeine-induced adenosine receptor antagonism alters dopamine neurotransmission. Acute high (10 mg/ kg+) caffeine doses increase extracellular NAc dopamine levels [180], while lower doses [181] or prolonged exposure [74] have no such effect (but see [167]).
Quarta et al. [74] reported that prolonged (14 day) exposure to caffeine in the drinking water results in tolerance to the ability of caffeine or an A1 antagonist to increase extracellular NAc dopamine and glutamate levels [74]. In contrast, Borycz et al. [182] did not find that acute treatment with an A1 receptor antagonist altered extracellular mPFC dopamine levels. In line with reports from Quarta et al. [74], chronic modulation of adenosine receptors may be necessary to observe these neuroadaptations in the A1 receptor.
A1 receptors likely block some of the behavioral and neurochemical actions of D1 receptors. For example, pharmacological modulation of NAc and mPFC A1 receptors alters motor responses to D1 agonists and antagonists [183,184], as well as some D1-dependent behaviors [175,183]. In line with these findings, activation of A1 receptors desensitizes D1 receptors in striatal and limbic regions [178,185]. Similarly, adenosine A1 receptor blockade increases dopamine neurotransmission [145,177,186,187]. Evidence from cell lines and striatal regions indicates that A1 receptors drive the uncoupling of dopamine D1 receptors from stimulatory G-proteins and alter the conformation and binding affinity of MCL dopamine D1 receptors [175,177,185,186], which could account for some of the antagonistic influence that A1 receptors exert upon D1 receptors. Quarta et al. [145] reported that local perfusion with A1 receptor antagonist CPT (300 uM or 1 mM) increases extracellular NAc dopamine levels, although the range for CPT-induced alterations of dopamine neurotransmission may be concentration- and brain region-specific [182,188]. Overall, these findings suggest that A1 receptors play an inhibitory neuromodulatory role to diminish functional kinetics associated with dopamine D1 receptors. These effects likely represent some of the mechanisms through which adenosine receptor agonists induce sedation, and antagonists, such as caffeine, stimulate behavior [175].
Striatal A2 receptors largely are co-expressed with postsynaptic dopamine D2 receptors [189-191]. Research suggests that low density A2 receptor populations are sufficient to diminish the binding affinity of dense D2 receptor populations [192]. Constitutive populations of A2-D2 heteromeric complexes have been identified [173,193]. Conformational changes resulting from A2 receptor activation are transmitted to D2 receptors [194], and may present a mechanism through which A2 receptor activation diminishes inhibitory D2 receptor signaling [179]. Moreover, A2A receptor antagonists decrease dopamine tissue levels [195]. Previous studies have reported that a single injection of an A2 antagonist can decrease extracellular dopamine levels both in vivo and in vitro [145,196]. Similarly, Dassesse et al. [197] reported that mutant mice with diminished striatal A2 activity exhibit a concomitant decrease in extracellular dopamine levels, while mice lacking striatal D2 receptors exhibit impairments in A2 receptormediated functions [197]. In addition, A2A receptor antagonists have been reported to counteract the cataleptic and tremor effects associated with D2 receptor blockade [195,198]. These effects may be mediated through A2A receptor antagonist-induced conformational changes in D2 receptors and associated reductions in the binding affinity of D2- like receptor antagonists. Ultimately, these pharmacological alterations may result in desensitization of extracellular dopamine binding to excitatory neurons and decrease dopamine-modulated behavioral pathologies [195,198]. Taken together, these findings suggest that A2 and D2 receptors interact to regulate adenosine and dopamine neurotransmission, as well as their resulting behavioral outputs.
A few initiatives have examined A1-D2 and A2A-D1 receptor interactions. Karcz-Kubicha [199] reported that co-activation of A1 receptors is necessary for A2A-mediated increases in c-fos immunoreactivity in the mPFC anterior cingulate region [199]. These researchers [199] suggest that this permissive role for A1 receptors is related to their ability to block tonic dopamine neurotransmission and D2 receptor activation. A2A and D1 receptor subtypes are not largely co-localized [200]; however, there apparently are some functional and behavioral interactions of the two receptor subtypes. For example, blockade of A2 receptors has been reported to potentiate the excitatory neurotransmission associated with dopamine D1 receptors [201]. In addition, A2A and D1 receptor interactions have been reported to promote alcohol consumption [202]. However, overall, evidence indicates that A2 antagonists have greater ability to block D2 receptormediated effects, relative to those modulated by D1 receptors [193].
Dopamine and ethanol in the mesocorticolimbic reward circuit: There is substantial evidence for the involvement of the MCL dopamine system in ethanol drinking and reward. For example, neuroimaging experiments indicate that alcohol increases striatal dopamine neurotransmission in young adult males [203,204]. Similar pre-clinical findings have been reported in laboratory rodents. Pharmacologically relevant levels of ethanol have been reported to increase firing of VTA dopamine neurons [205,206]. In line with this, the VTA, NAc, and mPFC have been implicated in operant oral ethanol self-administration [207,208]. Rats will self-infuse ethanol directly into the VTA [209]. Local, peripheral and oral ethanol exposure potentiates extracellular dopamine levels in the NAc, likely leading to neuroadaptations in dopamine D2autoreceptor regulation of the circuit [169,210-214]. Similarly, naïve rats bred for high alcohol preference exhibit lower NAc tissue dopamine levels, compared to their alcohol non-preferring counterparts [169,215-217]. These findings suggest that differential dopamine neurotransmission in the MCL circuit could alter the reinforcing responses to ethanol exposure, and may represent a predisposing factor toward high ethanol intake and, by extension, possibly could increase the intake of caffeine, as well.
Dopamine and ethanol and adenosine in the mesocorticolimbic reward circuit: Adenosine modulation of MCL dopamine neurotransmission likely is involved in ethanol consumption. Despite the relatively low co-localization of A2A and D1 receptors [200], evidence suggests that communication between these networks may be involved in alcohol drinking. Short et al. [202] selectively deleted A2A and D1 receptors from mice to examine the involvement of these receptors in ethanol intake. Subjects with dual A2A and D1 receptor knock-out significantly reduced their ethanol intake, relative to those with singledeletion or no alteration in the receptors [218]. In contrast, Naassila et al. [219] reported that A2A receptor knock-out alone increases ethanol intake (but see, [220]). Differences in these studies may suggest that dopamine D1 receptors compensate for absent A2A receptors and may implicate both of these receptor types in neural processing associated with alcohol drinking.
Yao et al. [162] found that A2 and D2 receptors synergize in the presence of ethanol to increase gene transcription via potentiation of PKACα translocation to the cell nucleus. These researchers [162] reported that ethanol- and dopamine-induced increases in PKACα translocation could be blocked by adenosine A1 and A2 receptor antagonists, in a manner mediated primarily by A2 receptors [162]. This synergy likely sensitizes the system to subsequent ethanol exposure, as well as endogenous dopamine D2 receptor signaling [162] and adenosinemediated desensitization of Gαs-coupled receptors [164]. With prolonged elevation of extracellular adenosine levels (including that associated with chronic ethanol drinking [162]), A2 and D2 receptors uncouple, such that the inhibitory effects of D2 receptors predominate [221]. Chronic ethanol exposure has been reported to desensitize A2A receptor response to agonists, as well as reduce the dopamine response to A2A antagonism [162]. These reports present one mechanism through which A2 receptors may alter gene expression in a D2 receptor dependent manner. These effects may be potentiated by ethanol exposure. In line with this suggestion, Short et al. [202] reported differences in the NAc dopamine and adenosine systems of ethanol-naïve high alcoholdrinking C57Bl/6J (B6) mice and low alcohol-drinking CD-1 mice, which correlated with subsequent measures of ethanol intake. They reported decreases in D1 receptor mRNA and increases in D2 receptor binding in B6 vs. CD-1 mice. Short et al. [202] also reported that nucleoside transporter blocker nitrobenzylthioinosine (NBTI) binding was reduced in B6 mice, compared to the CD-1 line. This dysfunction would be expected to increase extracellular adenosine concentrations [162] and, under chronic conditions, may cause neuroadaptations, affecting adenosine receptor activity. These findings in rodent models suggest that a predisposition for high alcohol consumption may be correlated with decreases in intracellular adenosine transport.
Conclusions
The negative impact of heavy alcohol use continues to be a public health concern. Binge alcohol drinking and early onset of alcohol use, in particular, appear to be risk factors for future alcohol dependence. Existing evidence suggests that the increased availability of caffeinated alcoholic beverages exacerbates both of these predispositions for developing alcohol dependence. This underscores the need for research examining the interactions of these two substances, on cognitive, behavioral, and neuronal/physiological function. The impact of caffeinated alcohol consumption has not been characterized fully; clinical evidence is mixed regarding whether caffeine increases alcohol intake and negative alcohol-related consequences in young drinkers, relative to alcohol alone. As with most substances of abuse, the interplay between abuse and factors such as gender, age, culture, social strata and religion is complex. However, pre-clinical evidence supports the contention that caffeine increases alcohol consumption, and animal models may provide new insights into central mechanisms that underlie the rewarding and reinforcing properties of this combination. The experimental data that we have presented from our laboratory were collected in a single genetic model of heavy and binge [60] alcohol intake, the P rat, during adulthood. However, future experiments should seek to evaluate systematically whether neural and behavioral evidence associated with intake of caffeinated alcohol solutions generalizes across different genetic populations. Findings from studies using different rodent models of alcoholism (e.g. B6 mice, P rats, AA rats, sP rats, etc.) and genetic mutant animal models can be expected to reveal important information on the central effects of this often abused drug combination. Given the increasing consumption of caffeinated alcoholic beverages in high-risk populations (e.g. adolescent bingedrinkers), future research should occur at multiple developmental time-points. Knowledge of the neurocircuitry and adaptations that are associated with acute and chronic caffeinated alcohol intake, as well as their long-range alterations following such consumption, will provide useful information regarding the intervention and treatment strategies for those who abuse this combination.
Acknowledgments
The preparation of this manuscript was supported in part by AA13522, AA010717, and AA020396 from the National Institute on Alcohol Abuse and Alcoholism.
References
- NIAAA (2013) Alcohol's Effects on the Body.
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL (2004) Actual causes of death in the United States, 2000. JAMA 291: 1238-1245.
- Rehm J, Room R, Monteiro M, Gmel G, Graham K, et al. (2003) Alcohol as a risk factor for global burden of disease. Eur Addict Res 9: 157-164.
- Reed T, Page WF, Viken RJ, Christian JC (1996) Genetic predisposition to organ-specific endpoints of alcoholism. Alcohol Clin Exp Res 20: 1528-1533.
- National Highway Traffic Safety Administration (2008) 2007 Traffic Safety Annual Assessment – Alcohol-Impaired Driving Fatalities.
- Mercer SL, Sleet DA, Elder RW, Cole KH, Shults RA, et al. (2010) Translating evidence into policy: lessons learned from the case of lowering the legal blood alcohol limit for drivers. Ann Epidemiol 20: 412-420.
- McKenzie JF, RR Pinger, JE Koteki (2008) “Alcohol, Tobacco, and Other Drugs: A Community Concern.” An Introduction to Community Health. (J.a. Bartlett edn), Sudbury.
- Brienza RS, Stein MD (2002) Alcohol use disorders in primary care: do gender-specific differences exist? J Gen Intern Med 17: 387-397.
- Nelson CB, Wittchen HU (1998) DSM-IV alcohol disorders in a general population sample of adolescents and young adults. Addiction 93: 1065-1077.
- Bava S, Tapert SF (2010) Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychol Rev 20: 398-413.
- Pitkänen T, Lyyra AL, Pulkkinen L (2005) Age of onset of drinking and the use of alcohol in adulthood: a follow-up study from age 8-42 for females and males. Addiction 100: 652-661.
- Johnson PB, Glassman M (1999) The moderating effects of gender and ethnicity on the relationship between effect expectancies and alcohol problems. J Stud Alcohol 60: 64-69.
- Chou SP, Pickering RP (1992) Early onset of drinking as a risk factor for lifetime alcohol-related problems. Br J Addict 87: 1199-1204.
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, et al. (1997) Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. J Stud Alcohol 58: 280-290.
- Dawson DA, Grant BF, Stinson FS, Chou PS (2004) Another look at heavy episodic drinking and alcohol use disorders among college and noncollege youth. J Stud Alcohol 65: 477-488.
- Presley CA, Karmos JS (1994) An instrument to assess adolescent alcohol involvement. Addict Behav 19: 655-665.
- Wechsler H, Kuo M, Lee H, Dowdall GW (2000) Environmental correlates of underage alcohol use and related problems of college students. Am J Prev Med 19: 24-29.
- NIAAA (2012) Underage Drinking.
- Substance Abuse and Mental Health Services Administration (2008) Results from the 2007 National Survey on Drug Use and Health: National Findings.
- Hingson RW, Heeren T, Winter MR (2006) Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med 160: 739-746.
- Martin CS, Clifford PR, Maisto SA, Earleywine M, Kirisci L, et al. (1996) Polydrug use in an inpatient treatment sample of problem drinkers. Alcohol Clin Exp Res 20: 413-417.
- FDA (2010) Serious Concerns Over Alcoholic Beverages with Added Caffeine.
- James JE (1991) Caffeine and Health. Academic Press, New York.
- Gilbert RM (1984) Caffeine consumption. Prog Clin Biol Res 158: 185-213.
- Griffiths RR, LM Juliano, A Chausmer (2003) Caffeine: pharmacology and clinical effects. Addiction Medicine.
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE (1999) Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51: 83-133.
- Dingle RN, Dreumont-Boudreau SE, Lolordo VM (2008) Caffeine dependence in rats: effects of exposure duration and concentration. Physiol Behav 95: 252-257.
- Griffiths RR, AL Chausmer (2000) Caffeine as a model drug of dependence: recent developments in understanding caffeine withdrawal, the caffeine dependence syndrome, and caffeine negative reinforcement. Nihon Shinkei Seishin Yakurigaku Zasshi 20: 223-231.
- Griffiths RR, Woodson PP (1988) Reinforcing effects of caffeine in humans. J Pharmacol Exp Ther 246: 21-29.
- West O, Roderique-Davies G (2008) Development and initial validation of a caffeine craving questionnaire. J Psychopharmacol 22: 80-91.
- American Psychiatric Association (2004) Diagnostic and Statistical Manual of Mental Disorders. (4th edn), text rev, Washington, DC.
- Jones HA, Lejuez CW (2005) Personality correlates of caffeine dependence: the role of sensation seeking, impulsivity, and risk taking. Exp Clin Psychopharmacol 13: 259-266.
- Ogawa N, Ueki H (2007) Clinical importance of caffeine dependence and abuse. Psychiatry Clin Neurosci 61: 263-268.
- Bernstein GA, Carroll ME, Thuras PD, Cosgrove KP, Roth ME (2002) Caffeine dependence in teenagers. Drug Alcohol Depend 66: 1-6.
- Oberstar JV, Bernstein GA, Thuras PD (2002) Caffeine use and dependence in adolescents: one-year follow-up. J Child Adolesc Psychopharmacol 12: 127-135.
- Young C, Oladipo O, Frasier S, Putko R, Chronister S, et al. (2012) Hemorrhagic stroke in young healthy male following use of sports supplement Jack3d. Mil Med 177: 1450-1454.
- Kendler KS, Prescott CA (1999) Caffeine intake, tolerance, and withdrawal in women: a population-based twin study. Am J Psychiatry 156: 223-228.
- Kendler KS, Prescott CA, Myers J, Neale MC (2003) The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry 60: 929-937.
- Bierut LJ, SH Dinwiddie, H Begleiter, RR Crowe, V Hesselbrock, et al. (1998) Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the Collaborative Study on the Genetics of Alcoholism. Arch Gen Psychiatry 55: 982-988.
- Goldman D, Bergen A (1998) General and specific inheritance of substance abuse and alcoholism. Arch Gen Psychiatry 55: 964-965.
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, et al. (1998) Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry 55: 967-972.
- Swan GE, Carmelli D, Cardon LR (1996) The consumption of tobacco, alcohol, and coffee in Caucasian male twins: a multivariate genetic analysis. J Subst Abuse 8: 19-31.
- Zeiner AR, Stanitis T, Spurgeon M, Nichols N (1985) Treatment of alcoholism and concomitant drugs of abuse. Alcohol 2: 555-559.
- Aubin HJ, Laureaux C, Tilikete S, Barrucand D (1999) Changes in cigarette smoking and coffee drinking after alcohol detoxification in alcoholics. Addiction 94: 411-416.
- Kendler KS, Aggen SH, Prescott CA, Crabbe J, Neale MC (2012) Evidence for multiple genetic factors underlying the DSM-IV criteria for alcohol dependence. Mol Psychiatry 17: 1306-1315.
- Kendler KS, Sundquist K, Ohlsson H, Palmér K, Maes H, et al. (2012) Genetic and familial environmental influences on the risk for drug abuse: a national Swedish adoption study. Arch Gen Psychiatry 69: 690-697.
- Bevilacqua L, Goldman D (2009) Genes and addictions. Clin Pharmacol Ther 85: 359-361.
- Hughes JR, Oliveto AH, MacLaughlin M (2000) Is dependence on one drug associated with dependence on other drugs? The cases of alcohol, caffeine and nicotine. Am J Addict 9: 196-201.
- Strain EC, Mumford GK, Silverman K, Griffiths RR (1994) Caffeine dependence syndrome. Evidence from case histories and experimental evaluations. JAMA 272: 1043-1048.
- Istvan J, Matarazzo JD (1984) Tobacco, alcohol, and caffeine use: a review of their interrelationships. Psychol Bull 95: 301-326.
- Svikis DS, Berger N, Haug NA, Griffiths RR (2005) Caffeine dependence in combination with a family history of alcoholism as a predictor of continued use of caffeine during pregnancy. Am J Psychiatry 162: 2344-2351.
- Bergin JE, Kendler KS (2012) Common psychiatric disorders and caffeine use, tolerance, and withdrawal: an examination of shared genetic and environmental effects. Twin Res Hum Genet 15: 473-482.
- Lester D, Freed EX (1973) Criteria for an animal model of alcoholism. Pharmacol Biochem Behav 1: 103-107.
- McBride WJ, Li TK (1998) Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol 12: 339-369.
- Crabbe JC, Phillips TJ, Belknap JK (2010) The complexity of alcohol drinking: studies in rodent genetic models. Behav Genet 40: 737-750.
- Bell RL, HJ Sable, G Colombo, P Hyytia, ZA Rodd, L Lumeng (2012) Animal models for medications development targeting alcohol abuse using selectively bred rat lines: neurobiological and pharmacological validity. Pharmacol Biochem Behav 103: 119-155.
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ (2006) The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol 11: 270-288.
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, et al. (2002) Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet 32: 363-388.
- Bell RL, Kimpel MW, Rodd ZA, Strother WN, Bai F, et al. (2006) Protein expression changes in the nucleus accumbens and amygdala of inbred alcohol-preferring rats given either continuous or scheduled access to ethanol. Alcohol 40: 3-17.
- Bell RL, Rodd ZA, Smith RJ, Toalston JE, Franklin KM, et al. (2011) Modeling binge-like ethanol drinking by peri-adolescent and adult P rats. Pharmacol Biochem Behav 100: 90-97.
- Dhaher R, McConnell KK, Rodd ZA, McBride WJ, Bell RL (2012) Daily patterns of ethanol drinking in adolescent and adult, male and female, high alcohol drinking (HAD) replicate lines of rats. Pharmacol Biochem Behav 102: 540-548.
- Bell RL, Rodd-Henricks ZA, Webster AA, Lumeng L, Li TK, et al. (2002) Heart rate and motor-activating effects of orally self-administered ethanol in alcohol-preferring (P) rats. Alcohol Clin Exp Res 26: 1162-1170.
- Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, et al. (2006) Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav 83: 35-46.
- Gordon TL, Meehan SM, Schechter MD (1993) P and NP rats respond differently to the discriminative stimulus effects of nicotine. Pharmacol Biochem Behav 45: 305-308.
- Meehan SM, Gordon TL, Schechter MD (1995) MDMA (Ecstasy) substitutes for the ethanol discriminative cue in HAD but not LAD rats. Alcohol 12: 569-572.
- Katner SN, SM Oster, ZM Ding, GA Deehan, Jr., JE Toalston, et al. (2011) Alcohol-preferring (P) rats are more sensitive than Wistar rats to the reinforcing effects of cocaine self-administered directly into the nucleus accumbens shell. Pharmacol Biochem Behav 99: 688-695.
- Eiler WJ 2nd, Woods JE 2nd, Masters J, McKay PF, Hardy L 3rd, et al. (2005) Brain stimulation reward performance and sucrose maintained behaviors in alcohol-preferring and -nonpreferring rats. Alcohol Clin Exp Res 29: 571-583.
- Nowak KL, Ingraham CM, Mckinzie DL, Mcbride WJ, Lumeng L, et al. (2000) An assessment of novelty-seeking behavior in alcohol-preferring and nonpreferring rats. Pharmacol Biochem Behav 66: 113-121.
- Hyyatiä P, Sinclair JD (1993) Oral etonitazene and cocaine consumption by AA, ANA and Wistar rats. Psychopharmacology (Berl) 111: 409-414.
- Stromberg MF, Mackler SA (2005) The effect of cocaine on the expression of motor activity and conditioned place preference in high and low alcohol-preferring Wistar rats. Pharmacol Biochem Behav 82: 314-319.
- Marttila K, Petteri Piepponen T, Kiianmaa K, Ahtee L (2007) Accumbal FosB/DeltaFosB immunoreactivity and conditioned place preference in alcohol-preferring AA rats and alcohol-avoiding ANA rats treated repeatedly with cocaine. Brain Res 1160: 82-90.
- Leggio B, Masi F, Grappi S, Nanni G, Gambarana C, et al. (2003) Sardinian alcohol-preferring and non-preferring rats show different reactivity to aversive stimuli and a similar response to a natural reward. Brain Res 973: 275-284.
- Mikkola JA, Honkanen A, Piepponen TP, Kiianmaa K, Ahtee L (2001) Effects of repeated cocaine treatment on striatal dopamine release in alcohol-preferring AA and alcohol-avoiding ANA rats. Naunyn Schmiedebergs Arch Pharmacol 363: 209-214.
- Quarta D, J Borycz, M Solinas, K Patkar, J Hockemeyer, et al. (2004) Adenosine receptor-mediated modulation of dopamine release in the nucleus accumbens depends on glutamate neurotransmission and N-methyl-D-aspartate receptor stimulation. J Neurochem 91: 873-880.
- Mayo Clinic (2012) Caffeine content for coffee, tea, soda and more.
- Vitiello MV, Woods SC (1975) Caffeine: preferential consumption by rats. Pharmacol Biochem Behav 3: 147-149.
- Bell RL, Stewart RB, Woods JE 2nd, Lumeng L, Li TK, et al. (2001) Responsivity and development of tolerance to the motor impairing effects of moderate doses of ethanol in alcohol-preferring (P) and -nonpreferring (NP) rat lines. Alcohol Clin Exp Res 25: 644-650.
- Reissig CJ, Strain EC, Griffiths RR (2009) Caffeinated energy drinks--a growing problem. Drug Alcohol Depend 99: 1-10.
- The Coca-Cola Company.
- PepsiCo Inc. (2011).
- The Mintel Group Ltd (2007) Energy Drinks.
- The Mintel Group (2009) Energy Drinks and Energy Shots.
- Centers for Disease Control (2012) Binge Drinking.
- Marczinski CA (2011) Alcohol mixed with energy drinks: consumption patterns and motivations for use in U.S. college students. Int J Environ Res Public Health 8: 3232-3245.
- Mart SM (2011) Alcohol marketing in the 21st century: new methods, old problems. Subst Use Misuse 46: 889-892.
- O'Brien MC, McCoy TP, Rhodes SD, Wagoner A, Wolfson M (2008) Caffeinated cocktails: energy drink consumption, high-risk drinking, and alcohol-related consequences among college students. Acad Emerg Med 15: 453-460.
- Ferreira SE, de Mello MT, Pompéia S, de Souza-Formigoni ML (2006) Effects of energy drink ingestion on alcohol intoxication. Alcohol Clin Exp Res 30: 598-605.
- Attila S, Çakir B (2011) Energy-drink consumption in college students and associated factors. Nutrition 27: 316-322.
- MacKillop J, Howland J, Rohsenow DJ, Few LR, Amlung MT, et al. (2012) Initial development of a measure of expectancies for combinations of alcohol and caffeine: the Caffeine + Alcohol Combined Effects Questionnaire (CACEQ). Exp Clin Psychopharmacol 20: 466-472.
- Smith AP (2013) Effects of caffeine and alcohol on mood and performance changes following consumption of lager. Psychopharmacology (Berl) 227: 595-604.
- Marczinski CA, Fillmore MT (2006) Clubgoers and their trendy cocktails: implications of mixing caffeine into alcohol on information processing and subjective reports of intoxication. Exp Clin Psychopharmacol 14: 450-458.
- Ferreira SE, Hartmann Quadros IM, Trindade AA, Takahashi S, Koyama RG, et al. (2004) Can energy drinks reduce the depressor effect of ethanol? An experimental study in mice. Physiol Behav 82: 841-847.
- Alford C, Hamilton-Morris J, Verster JC (2012) The effects of energy drink in combination with alcohol on performance and subjective awareness. Psychopharmacology (Berl) 222: 519-532.
- Peacock A, R Bruno, FH Martin (2012) The subjective physiological, psychological, and behavioral risk-taking consequences of alcohol and energy drink co-ingestion. Alcohol Clin Exp Res 36: 2008-2015.
- Peacock A, Bruno R, Martin FH (2013) Patterns of use and motivations for consuming alcohol mixed with energy drinks. Psychol Addict Behav 27: 202-206.
- Thombs D, Rossheim M, Barnett TE, Weiler RM, Moorhouse MD, et al. (2011) Is there a misplaced focus on AmED? Associations between caffeine mixers and bar patron intoxication. Drug Alcohol Depend 116: 31-36.
- Arria AM, Caldeira KM, Kasperski SJ, Vincent KB, Griffiths RR, et al. (2011) Energy drink consumption and increased risk for alcohol dependence. Alcohol Clin Exp Res 35: 365-375.
- Skeen MP, Glenn LL (2011) Imaginary link between alcoholism and energy drinks. Alcohol Clin Exp Res 35: 1375-1376.
- Brache K, Stockwell T (2011) Drinking patterns and risk behaviors associated with combined alcohol and energy drink consumption in college drinkers. Addict Behav 36: 1133-1140.
- Riesselmann B, Rosenbaum F, Schneider V (1996) [Alcohol and energy drink--can combined consumption of both beverages modify automobile driving fitness?]. Blutalkohol 33: 201-208.
- Thombs DL, O'Mara RJ, Tsukamoto M, Rossheim ME, Weiler RM, et al. (2010) Event-level analyses of energy drink consumption and alcohol intoxication in bar patrons. Addict Behav 35: 325-330.
- Wolk BJ, Ganetsky M, Babu KM (2012) Toxicity of energy drinks. Curr Opin Pediatr 24: 243-251.
- Weldy DL (2010) Risks of alcoholic energy drinks for youth. J Am Board Fam Med 23: 555-558.
- Curry K, Stasio MJ (2009) The effects of energy drinks alone and with alcohol on neuropsychological functioning. Hum Psychopharmacol 24: 473-481.
- Mackay M, Tiplady B, Scholey AB (2002) Interactions between alcohol and caffeine in relation to psychomotor speed and accuracy. Hum Psychopharmacol 17: 151-156.
- Grattan-Miscio KE, Vogel-Sprott M (2005) Alcohol, intentional control, and inappropriate behavior: regulation by caffeine or an incentive. Exp Clin Psychopharmacol 13: 48-55.
- Fillmore MT, Roach EL, Rice JT (2002) Does caffeine counteract alcohol-induced impairment? The ironic effects of expectancy. J Stud Alcohol 63: 745-754.
- Fillmore MT (2003) Alcohol tolerance in humans is enhanced by prior caffeine antagonism of alcohol-induced impairment. Exp Clin Psychopharmacol 11: 9-17.
- Marczinski CA, Fillmore MT (2003) Dissociative antagonistic effects of caffeine on alcohol-induced impairment of behavioral control. Exp Clin Psychopharmacol 11: 228-236.
- Marczinski CA, Fillmore MT, Bardgett ME, Howard MA (2011) Effects of energy drinks mixed with alcohol on behavioral control: risks for college students consuming trendy cocktails. Alcohol Clin Exp Res 35: 1282-1292.
- Marczinski CA, Fillmore MT, Henges AL, Ramsey MA, Young CR (2012) Effects of energy drinks mixed with alcohol on information processing, motor coordination and subjective reports of intoxication. Exp Clin Psychopharmacol 20: 129-138.
- Howland J, Rohsenow DJ, Arnedt JT, Bliss CA, Hunt SK, et al. (2011) The acute effects of caffeinated versus non-caffeinated alcoholic beverage on driving performance and attention/reaction time. Addiction 106: 335-341.
- de Haan L, de Haan HA, van der Palen J, Olivier B, Verster JC (2012) Effects of consuming alcohol mixed with energy drinks versus consuming alcohol only on overall alcohol consumption and negative alcohol-related consequences. Int J Gen Med 5: 953-960.
- Benac N (2011) United States Food and Drug Administration signals crackdown on caffeinated alcohol drinks. CMAJ 183: E47-48.
- Hsu CW, Chen CY, Wang CS, Chiu TH (2009) Caffeine and a selective adenosine A2A receptor antagonist induce reward and sensitization behavior associated with increased phospho-Thr75-DARPP-32 in mice. Psychopharmacology (Berl) 204: 313-325.
- Agabio R, Carai MA, Lobina C, Pani M, Reali R, et al. (2001) Alcohol stimulates motor activity in selectively bred Sardinian alcohol-preferring (sP), but not in Sardinian alcohol-nonpreferring (sNP), rats. Alcohol 23: 123-126.
- El Yacoubi M, Ledent C, Ménard JF, Parmentier M, Costentin J, et al. (2000) The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A(2A) receptors. Br J Pharmacol 129: 1465-1473.
- Waller MB, Murphy JM, McBride WJ, Lumeng L, Li TK (1986) Effect of low dose ethanol on spontaneous motor activity in alcohol-preferring and -nonpreferring lines of rats. Pharmacol Biochem Behav 24: 617-623.
- Fisone G, Borgkvist A, Usiello A (2004) Caffeine as a psychomotor stimulant: mechanism of action. Cell Mol Life Sci 61: 857-872.
- Howland J, Rohsenow DJ, Greece JA, Littlefield CA, Almeida A, et al. (2010) The effects of binge drinking on college students' next-day academic test-taking performance and mood state. Addiction 105: 655-665.
- El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM (2003) Caffeine reduces hypnotic effects of alcohol through adenosine A2A receptor blockade. Neuropharmacology 45: 977-985.
- Marangos PJ, Paul SM, Parma AM, Goodwin FK, Syapin P, et al. (1979) Purinergic inhibition of diazepam binding to rat brain (in vitro). Life Sci 24: 851-857.
- Amato L, S Minozzi, S Vecchi, M Davoli (2010) Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev CD005063.
- Griffiths RR, Weerts EM (1997) Benzodiazepine self-administration in humans and laboratory animals--implications for problems of long-term use and abuse. Psychopharmacology (Berl) 134: 1-37.
- Nowak KL, McBride WJ, Lumeng L, Li TK, Murphy JM (1998) Blocking GABA(A) receptors in the anterior ventral tegmental area attenuates ethanol intake of the alcohol-preferring P rat. Psychopharmacology (Berl) 139: 108-116.
- Sudakov SK, Rusakova IV, Medvedeva OF (2003) Effect of chronic caffeine consumption on changes in locomotor activity of WAG/G and Fischer-344 rats induced by nicotine, ethanol, and morphine. Bull Exp Biol Med 136: 563-565.
- Armstrong LE (2002) Caffeine, body fluid-electrolyte balance, and exercise performance. Int J Sport Nutr Exerc Metab 12: 189-206.
- Belza A, Toubro S, Astrup A (2009) The effect of caffeine, green tea and tyrosine on thermogenesis and energy intake. Eur J Clin Nutr 63: 57-64.
- Riesenhuber A, Boehm M, Posch M, Aufricht C (2006) Diuretic potential of energy drinks. Amino Acids 31: 81-83.
- Kunin D, Gaskin S, Rogan F, Smith BR, Amit Z (2000) Caffeine promotes ethanol drinking in rats. Examination using a limited-access free choice paradigm. Alcohol 21: 271-277.
- Engleman EA, Ingraham CM, Franklin KM, Keith CM, McClaren JA, et al. (2008) In vivo time-course changes in ethanol levels sampled with subcutaneous microdialysis. Alcohol Clin Exp Res 32: 435-442.
- Williams M, Jarvis MF (2000) Purinergic and pyrimidinergic receptors as potential drug targets. Biochem Pharmacol 59: 1173-1185.
- Dunwiddie TV (1985) The physiological role of adenosine in the central nervous system. Int Rev Neurobiol 27: 63-139.
- Martín R, Ladera C, Bartolomé-Martín D, Torres M, Sánchez-Prieto J (2008) The inhibition of release by mGlu7 receptors is independent of the Ca2+ channel type but associated to GABAB and adenosine A1 receptors. Neuropharmacology 55: 464-473.
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferré S (2007) Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol 83: 277-292.
- Olah ME, Stiles GL (1995) Adenosine receptor subtypes: characterization and therapeutic regulation. Annu Rev Pharmacol Toxicol 35: 581-606.
- Fastbom J, Pazos A, Palacios JM (1987) The distribution of adenosine A1 receptors and 5'-nucleotidase in the brain of some commonly used experimental animals. Neuroscience 22: 813-826.
- Rivkees SA, ME Lasbury, GS Stiles, O Henegariu, C Curtis, et al. (1995) The human A1 adenosine receptor: ligand binding properties, sites of somatic expression and chromosomal localization. Endocrine 3: 623-629.
- Dunwiddie TV, Masino SA (2001) The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 24: 31-55.
- Parkinson FE, Fredholm BB (1990) Autoradiographic evidence for G-protein coupled A2-receptors in rat neostriatum using [3H]-CGS 21680 as a ligand. Naunyn Schmiedebergs Arch Pharmacol 342: 85-89.
- Wan W, Sutherland GR, Geiger JD (1990) Binding of the adenosine A2 receptor ligand [3H]CGS 21680 to human and rat brain: evidence for multiple affinity sites. J Neurochem 55: 1763-1771.
- Golembiowska K, Zylewska A (1997) Adenosine receptors--the role in modulation of dopamine and glutamate release in the rat striatum. Pol J Pharmacol 49: 317-322.
- Burnstock G (2013) Introduction to purinergic signalling in the brain. Adv Exp Med Biol 986: 1-12.
- Chen JF, Eltzschig HK, Fredholm BB (2013) Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Discov 12: 265-286.
- Quarta D, S Ferre, M Solinas, ZB You, J Hockemeyer, et al. (2004) Opposite modulatory roles for adenosine A1 and A2A receptors on glutamate and dopamine release in the shell of the nucleus accumbens. Effects of chronic caffeine exposure. J Neurochem 88:1151-1158.
- Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, et al. (2003) Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology 28: 1281-1291.
- Kaplan GB, Greenblatt DJ, Ehrenberg BL, Goddard JE, Cotreau MM, et al. (1997) Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J Clin Pharmacol 37: 693-703.
- Shi D, Nikodijević O, Jacobson KA, Daly JW (1994) Effects of chronic caffeine on adenosine, dopamine and acetylcholine systems in mice. Arch Int Pharmacodyn Ther 328: 261-287.
- Popoli P, Reggio R, Pèzzola A (2000) Effects of SCH 58261, an adenosine A(2A) receptor antagonist, on quinpirole-induced turning in 6-hydroxydopamine-lesioned rats. Lack of tolerance after chronic caffeine intake. Neuropsychopharmacology 22: 522-529.
- Traversa U, Rosati AM, Florio C, Vertua R (1994) Effects of chronic administration of adenosine antagonists on adenosine A1 and A2a receptors in mouse brain. In Vivo 8: 1073-1078.
- Hawkins M, Dugich MM, Porter NM, Urbancic M, Radulovacki M (1988) Effects of chronic administration of caffeine on adenosine A1 and A2 receptors in rat brain. Brain Res Bull 21: 479-482.
- Svenningsson P, Nomikos GG, Fredholm BB (1999) The stimulatory action and the development of tolerance to caffeine is associated with alterations in gene expression in specific brain regions. J Neurosci 19: 4011-4022.
- Daly JW, Shi D, Wong V, Nikodijevic O (1994) Chronic effects of ethanol on central adenosine function of mice. Brain Res 650: 153-156.
- Jarvis MF, Becker HC (1998) Single and repeated episodes of ethanol withdrawal increase adenosine A1, but not A2A, receptor density in mouse brain. Brain Res 786: 80-88.
- Butler TR, Prendergast MA (2012) Neuroadaptations in adenosine receptor signaling following long-term ethanol exposure and withdrawal. Alcohol Clin Exp Res 36: 4-13.
- Sharma R, Engemann SC, Sahota P, Thakkar MM (2010) Effects of ethanol on extracellular levels of adenosine in the basal forebrain: an in vivo microdialysis study in freely behaving rats. Alcohol Clin Exp Res 34: 813-818.
- Batista LC, Prediger RD, Morato GS, Takahashi RN (2005) Blockade of adenosine and dopamine receptors inhibits the development of rapid tolerance to ethanol in mice. Psychopharmacology (Berl) 181: 714-721.
- Prediger RD, Batista LC, Takahashi RN (2004) Adenosine A1 receptors modulate the anxiolytic-like effect of ethanol in the elevated plus-maze in mice. Eur J Pharmacol 499: 147-154.
- Thorsell A, Johnson J, Heilig M (2007) Effect of the adenosine A2a receptor antagonist 3,7-dimethyl-propargylxanthine on anxiety-like and depression-like behavior and alcohol consumption in Wistar Rats. Alcohol Clin Exp Res 31: 1302-1307.
- Arolfo MP, Yao L, Gordon AS, Diamond I, Janak PH (2004) Ethanol operant self-administration in rats is regulated by adenosine A2 receptors. Alcohol Clin Exp Res 28: 1308-1316.
- Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, et al. (2004) The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci 7: 855-861.
- Yao L, Arolfo MP, Dohrman DP, Jiang Z, Fan P, et al. (2002) betagamma Dimers mediate synergy of dopamine D2 and adenosine A2 receptor-stimulated PKA signaling and regulate ethanol consumption. Cell 109: 733-743.
- Sapru MK, Diamond I, Gordon AS (1994) Adenosine receptors mediate cellular adaptation to ethanol in NG108-15 cells. J Pharmacol Exp Ther 271: 542-548.
- Nagy LE, Diamond I, Collier K, Lopez L, Ullman B, et al. (1989) Adenosine is required for ethanol-induced heterologous desensitization. Mol Pharmacol 36: 744-748.
- Van den Heuvel DM, Pasterkamp RJ (2008) Getting connected in the dopamine system. Prog Neurobiol 85: 75-93.
- Tu Y, Kroener S, Abernathy K, Lapish C, Seamans J, et al. (2007) Ethanol inhibits persistent activity in prefrontal cortical neurons. J Neurosci 27: 4765-4775.
- De Luca MA, Bassareo V, Bauer A, Di Chiara G (2007) Caffeine and accumbens shell dopamine. J Neurochem 103: 157-163.
- Acquas E, Tanda G, Di Chiara G (2002) Differential effects of caffeine on dopamine and acetylcholine transmission in brain areas of drug-naive and caffeine-pretreated rats. Neuropsychopharmacology 27: 182-193.
- Smith AD, Weiss F (1999) Ethanol exposure differentially alters central monoamine neurotransmission in alcohol-preferring versus -nonpreferring rats. J Pharmacol Exp Ther 288: 1223-1228.
- Rahman S, McBride WJ (2001) D1-D2 dopamine receptor interaction within the nucleus accumbens mediates long-loop negative feedback to the ventral tegmental area (VTA). J Neurochem 77: 1248-1255.
- Kohl RR, Katner JS, Chernet E, McBride WJ (1998) Ethanol and negative feedback regulation of mesolimbic dopamine release in rats. Psychopharmacology (Berl) 139: 79-85.
- Stoner GR, Skirboll LR, Werkman S, Hommer DW (1988) Preferential effects of caffeine on limbic and cortical dopamine systems. Biol Psychiatry 23: 761-768.
- Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, et al. (2003) Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem 278: 46741-46749.
- Ginés S, Hillion J, Torvinen M, Le Crom S, Casadó V, et al. (2000) Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc Natl Acad Sci U S A 97: 8606-8611.
- Ferré S, Popoli P, Giménez-Llort L, Finnman UB, Martínez E, et al. (1994) Postsynaptic antagonistic interaction between adenosine A1 and dopamine D1 receptors. Neuroreport 6: 73-76.
- Ferré S, O'Connor WT, Snaprud P, Ungerstedt U, Fuxe K (1994) Antagonistic interaction between adenosine A2A receptors and dopamine D2 receptors in the ventral striopallidal system. Implications for the treatment of schizophrenia. Neuroscience 63: 765-773.
- Ferré S, Torvinen M, Antoniou K, Irenius E, Civelli O, et al. (1998) Adenosine A1 receptor-mediated modulation of dopamine D1 receptors in stably cotransfected fibroblast cells. J Biol Chem 273: 4718-4724.
- O'Neill C, Nolan BJ, Macari A, O'Boyle KM, O'Connor JJ (2007) Adenosine A1 receptor-mediated inhibition of dopamine release from rat striatal slices is modulated by D1 dopamine receptors. Eur J Neurosci 26: 3421-3428.
- Fuxe K, Ferré S, Genedani S, Franco R, Agnati LF (2007) Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav 92: 210-217.
- Nehlig A, Daval JL, Boyet S, Vert P (1986) Comparative effects of acute and chronic administration of caffeine on local cerebral glucose utilization in the conscious rat. Eur J Pharmacol 129: 93-103.
- Nehlig A, Boyet S (2000) Dose-response study of caffeine effects on cerebral functional activity with a specific focus on dependence. Brain Res 858: 71-77.
- Borycz J, MF Pereira, A Melani, RJ Rodrigues, A Kofalvi, et al. (2007) Differential glutamate-dependent and glutamate-independent adenosine A1 receptor-mediated modulation of dopamine release in different striatal compartments. J Neurochem 101: 355-363.
- Rimondini R, Ferré S, Giménez-Llort L, Ogren SO, Fuxe K (1998) Differential effects of selective adenosine A1 and A2A receptor agonists on dopamine receptor agonist-induced behavioural responses in rats. Eur J Pharmacol 347: 153-158.
- Popoli P, Giménez-Llort L, Pezzola A, Reggio R, Martínez E, et al. (1996) Adenosine A1 receptor blockade selectively potentiates the motor effects induced by dopamine D1 receptor stimulation in rodents. Neurosci Lett 218: 209-213.
- Ferre S, Popoli P, Tinner-Staines B, Fuxe K (1996) Adenosine A1 receptor-dopamine D1 receptor interaction in the rat limbic system: modulation of dopamine D1 receptor antagonist binding sites. Neurosci Lett 208: 109-112.
- Cao Y, Xie KQ, Zhu XZ (2007) The enhancement of dopamine D1 receptor desensitization by adenosine A1 receptor activation. Eur J Pharmacol 562: 34-38.
- Okada M, Mizuno K, Kaneko S (1996) Adenosine A1 and A2 receptors modulate extracellular dopamine levels in rat striatum. Neurosci Lett 212: 53-56.
- Zetterström T, Fillenz M (1990) Adenosine agonists can both inhibit and enhance in vivo striatal dopamine release. Eur J Pharmacol 180: 137-143.
- Ferré S, Ciruela F, Quiroz C, Luján R, Popoli P, et al. (2007) Adenosine receptor heteromers and their integrative role in striatal function. ScientificWorldJournal 7: 74-85.
- Leonard SK, P Ferry-Leeper, RB Mailman (2006) Low affinity binding of the classical D1 antagonist SCH23390 in rodent brain: potential interaction with A2A and D2-like receptors. Brain Res 1117: 25-37.
- Chen JF, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, et al. (2001) The role of the D(2) dopamine receptor (D(2)R) in A(2A) adenosine receptor (A(2A)R)-mediated behavioral and cellular responses as revealed by A(2A) and D(2) receptor knockout mice. Proc Natl Acad Sci U S A 98: 1970-1975.
- Torvinen M, Marcellino D, Canals M, Agnati LF, Lluis C, et al. (2005) Adenosine A2A receptor and dopamine D3 receptor interactions: evidence of functional A2A/D3 heteromeric complexes. Mol Pharmacol 67: 400-407.
- Worden LT, Shahriari M, Farrar AM, Sink KS, Hockemeyer J, et al. (2009) The adenosine A2A antagonist MSX-3 reverses the effort-related effects of dopamine blockade: differential interaction with D1 and D2 family antagonists. Psychopharmacology (Berl) 203: 489-499.
- Agnati LF, S Ferre, C Lluis, R Franco, K Fuxe (2003) Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol Rev 55: 509-550.
- Luthra PM, Prakash A, Barodia SK, Kumari R, Mishra CB, et al. (2009) In silico study of naphtha [1, 2-d] thiazol-2-amine with adenosine A 2A receptor and its role in antagonism of haloperidol-induced motor impairments in mice. Neurosci Lett 463: 215-218.
- Koizumi S, Watano T, Nakazawa K, Inoue K (1994) Potentiation by adenosine of ATP-evoked dopamine release via a pertussis toxin-sensitive mechanism in rat phaeochromocytoma PC12 cells. Br J Pharmacol 112: 992-997.
- Dassesse D, Massie A, Ferrari R, Ledent C, Parmentier M, et al. (2001) Functional striatal hypodopaminergic activity in mice lacking adenosine A(2A) receptors. J Neurochem 78: 183-198.
- Ishiwari K, Madson LJ, Farrar AM, Mingote SM, Valenta JP, et al. (2007) Injections of the selective adenosine A2A antagonist MSX-3 into the nucleus accumbens core attenuate the locomotor suppression induced by haloperidol in rats. Behav Brain Res 178: 190-199.
- Karcz-Kubicha M, Quarta D, Hope BT, Antoniou K, Müller CE, et al. (2003) Enabling role of adenosine A1 receptors in adenosine A2A receptor-mediated striatal expression of c-fos. Eur J Neurosci 18: 296-302.
- Svenningsson P, Le Moine C, Kull B, Sunahara R, Bloch B, et al. (1997) Cellular expression of adenosine A2A receptor messenger RNA in the rat central nervous system with special reference to dopamine innervated areas. Neuroscience 80: 1171-1185.
- Cauli O, Morelli M (2005) Caffeine and the dopaminergic system. Behav Pharmacol 16: 63-77.
- Short JL, Ledent C, Drago J, Lawrence AJ (2006) Receptor crosstalk: characterization of mice deficient in dopamine D1 and adenosine A2A receptors. Neuropsychopharmacology 31: 525-534.
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, et al. (2003) Alcohol promotes dopamine release in the human nucleus accumbens. Synapse 49: 226-231.
- Urban NB, Kegeles LS, Slifstein M, Xu X, Martinez D, et al. (2010) Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [¹¹C]raclopride. Biol Psychiatry 68: 689-696.
- Brodie MS, Shefner SA, Dunwiddie TV (1990) Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res 508: 65-69.
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G (1985) Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res 348: 201-203.
- Gonzales RA, Weiss F (1998) Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci 18: 10663-10671.
- Melendez RI, Rodd-Henricks ZA, Engleman EA, Li TK, McBride WJ, et al. (2002) Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res 26: 318-325.
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK (1994) Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol 11: 557-564.
- Engleman EA, Ingraham CM, McBride WJ, Lumeng L, Murphy JM (2006) Extracellular dopamine levels are lower in the medial prefrontal cortex of alcohol-preferring rats compared to Wistar rats. Alcohol 38: 5-12.
- Franklin KM, Engleman EA, Ingraham CM, McClaren JA, Keith CM, et al. (2009) A single, moderate ethanol exposure alters extracellular dopamine levels and dopamine d receptor function in the nucleus accumbens of wistar rats. Alcohol Clin Exp Res 33: 1721-1730.
- Sahr AE, Thielen RJ, Lumeng L, Li TK, McBride WJ (2004) Long-lasting alterations of the mesolimbic dopamine system after periadolescent ethanol drinking by alcohol-preferring rats. Alcohol Clin Exp Res 28: 702-711.
- Thielen RJ, Engleman EA, Rodd ZA, Murphy JM, Lumeng L, et al. (2004) Ethanol drinking and deprivation alter dopaminergic and serotonergic function in the nucleus accumbens of alcohol-preferring rats. J Pharmacol Exp Ther 309: 216-225.
- Wozniak KM, Pert A, Mele A, Linnoila M (1991) Focal application of alcohols elevates extracellular dopamine in rat brain: a microdialysis study. Brain Res 540: 31-40.
- Murphy JM, McBride WJ, Lumeng L, Li TK (1982) Regional brain levels of monoamines in alcohol-preferring and -nonpreferring lines of rats. Pharmacol Biochem Behav 16: 145-149.
- Murphy JM, McBride WJ, Lumeng L, Li TK (1987) Alcohol preference and regional brain monoamine contents of N/Nih heterogeneous stock rats. Alcohol Drug Res 7: 33-39.
- Quintanilla ME, Bustamante D, Tampier L, Israel Y, Herrera-Marschitz M (2007) Dopamine release in the nucleus accumbens (shell) of two lines of rats selectively bred to prefer or avoid ethanol. Eur J Pharmacol 573: 84-92.
- Short JL, Drago J, Lawrence AJ (2006) Comparison of ethanol preference and neurochemical measures of mesolimbic dopamine and adenosine systems across different strains of mice. Alcohol Clin Exp Res 30: 606-620.
- Naassila M, Ledent C, Daoust M (2002) Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. J Neurosci 22: 10487-10493.
- El Yacoubi M, Costentin J, Vaugeois JM (2003) Adenosine A2A receptors and depression. Neurology 61: S82-87.
- Zahniser NR, Simosky JK, Mayfield RD, Negri CA, Hanania T, et al. (2000) Functional uncoupling of adenosine A(2A) receptors and reduced responseto caffeine in mice lacking dopamine D2 receptors. J Neurosci 20: 5949-5957.
- Datta U, Noailles PA, Rodriguez M, Kraft M, Zhang Y, et al. (1996) Accumulation of tyrosine hydroxylase messenger RNA molecules in the rat mesencephalon by chronic caffeine treatment. Neurosci Lett 220: 77-80.
- Imperato A, Di Chiara G (1986) Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther 239: 219-228.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 18158
- [From(publication date):
specialissue-2013 - Oct 07, 2025] - Breakdown by view type
- HTML page views : 13305
- PDF downloads : 4853