Research Article Open Access
Clinal Variation in Flowering Time and Vernalisation Requirement across a 3000-M Altitudinal Range in Perennial Arabidopsis kamchatica Ssp.Kamchatica and Annual Lowland Subspecies Kawasakiana
Tanaka Kenta1*, Ayumu Yamada2 and Yoshihiko Onda11Sugadaira Montane Research Center, Univ. Tsukuba, Sugadaira-kogen, Ueda, 386-2204, Japan
2Department of Biology, Faculty of Science, Toho University, Funabashi, Chiba 274-8510, Japa
- *Corresponding Author:
- Tanaka Kenta
1278-294 Sugadaira-kogen Ueda
386-2204 Japan
Tel: +81 268 74 2002
Fax: +81 0268 74 2016
E-mail: kenta@sugadaira.tsukuba.ac.jp
Received date: July 18, 2010; Accepted date: August 22, 2011; Published date: August 25, 2011
Citation: Kenta T, Yamada A, Onda Y (2011) Clinal Variation in Flowering Time and Vernalisation Requirement across a 3000-M Altitudinal Range in Perennial Arabidopsis kamchatica Ssp. Kamchatica and Annual Lowland Subspecies Kawasakiana. J Ecosys Ecograph S6:001. doi:10.4172/2157-7625.S6-001
Copyright: © 2011 Kenta T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
Clines in ecologically important traits across environmental gradients provide evidence of historical natural selection. Among the many adaptive traits that are relevant in natural habitats, flowering timing is a primary determinant of a plant’s lifetime fitness. In a laboratory experiment, we studied the divergence in flowering time and vernalisation requirement among 38 populations of two Arabidopsis subspecies: perennial A. kamchatica ssp. kamchatica across an altitudinal gradient from 30 to nearly 3000 m in the Japanese Alps, and its annual lowland subspecies, kawasakiana. Flowering time with vernalisation was not different and flowering time without vernalisation was slightly different between the subspecies; however, the altitude of the source populations was the dominant determinant of the variation in these traits for the species as a whole. The flowering time increased linearly and the effect of vernalisation to shorten the flowering time increased non-linearly with increasing altitude of the source population. Low-altitude populations of ssp. kamchatica from laboratory-collected seeds showed a stronger response to vernalisation than field-collected seeds, suggesting an effect of the maternal environment. By replicating the altitudinal gradient and explicitly accounting for the maternal effect, our results clearly suggest the existence of genetically based clinal patterns that provide signs of adaptive evolution. Early flowering is probably advantageous to completing reproduction before the hot summer at low altitudes. In ssp. kamchatica, which is perennial and can reproduce even if it does not flower in its first year, the strong vernalisation requirement may delay the first reproduction at high altitudes, where the growing season is short.
Keywords
Local adaptation; Altitudinal cline; Elevational cline; Altitudinal gradient; Elevational gradient; Maternal effect; Vernalisation; Wild relatives of Arabidopsis; Climatic gradient; Japanese Alps
Introduction
The adaptation of species to environmental gradients has been a central issue in ecology, ecography, and evolutionary biology. Evidence of the action of natural selection over an environmental gradient can be provided by the existence of genetic clines in ecologically important traits along the gradient [1]. Among the many traits that are relevant to adaptation in natural environments, the timing of reproduction is a critical component of any organism's life cycle, and in nature, it can be a primary determinant of an individual's lifetime fitness [2]. Because the optimal timing of flowering may vary substantially along gradients related to latitude and altitude, which are both associated with the length of the growing season, the response of flowering to environmental cues is suspected to be a selection factor, leading to genetic divergence among populations. Life-history theory [3,4] assumes the existence of a trade-off between rapid reproductive development and maximum vegetative growth that results in increased future reproduction, and predicts that plants will flower earlier in environments with a shorter growing season. Indeed, many empirical studies have demonstrated the existence of latitudinal clines in which populations originating at higher latitudes flower earlier than those from lower latitudes when grown in a common environment [5-8]. On the other hand, the opposite pattern, in which populations from warmer locations flower earlier, have been shown for both latitudinal [9,10] and altitudinal [11] gradients. The adaptive significance of this opposite pattern can be explained by the relatively dry summer at warmer locations in these studies, because early flowering is often advantageous in habitats that experience drought in growing seasons [2,12-15].
The timing of flowering is often regulated by the existence of a winter season in strongly seasonal climates. Some plants require exposure to cold temperatures before they can flower after the winter; this well-known effect of the cold period is referred to as "vernalisation". In this paper, we refer to the extent by which vernalisation shortens the flowering time compared with flowering in the absence of vernalisation as the "vernalisation requirement" of a species. An intra-specific genetic variation in the response to vernalisation has been reported in many species, including Arabidopsis thaliana [16,17], Arabidopsis lyrata [18], and the wild beet Beta vulgaris [19]. However, the degree of clinal variation in vernalisation requirement has been documented much less than flowering timing. In the annual plant A. thaliana, the vernalisation requirement is stronger in populations from lower latitudes, which respond to a shorter period of vernalisation than populations from higher latitudes [20]. These authors suggested that the low-latitude population might have evolved a higher sensitivity to vernalisation to respond to the milder winter signals. In contrast, populations of the wild beet, a perennial plant, have a stronger vernalisation requirement at higher latitudes, which is shown by the lower proportion of flowering plants in the absence of strong vernalisation [19]. These authors pointed out that the vernalisation requirement would regulate the age of first reproduction, and its optimal strength would be associated with the optimal age of first reproduction under a given length of growing season. Although it is hard to base a strong conclusion on limited studies, the discrepancy between these two reports may be associated with differences in the life forms of these plants. Although vernalisation should control the flowering season of annual plants, which have only one year in which to reproduce, it should control the year of first flowering as well as the flowering season of perennial plants.
Despite the usefulness of clinal variation to understanding the process of biological adaptation, two points should be carefully considered before the existence of clines is interpreted as evidence of natural selection. First, when clinal patterns are derived from a single altitudinal gradient, this can sometimes be caused by historical artefacts such as populations at low and high ends of a gradient that were founded by distinct ancestral lineages; this would generate the clinal pattern without requiring any adaptive evolution [11]. The same problem potentially exists for both altitudinal and latitudinal clinal patterns. These problems can be overcome by replication of the environmental gradients at different sites, since this increases the likelihood that at least some of the observed variation will be due to the consequence of natural selection rather than the result of founder effects. Second, when clinal patterns are assessed by growing fieldcollected seeds in a common garden or a laboratory, the effects of the maternal environment (hereafter, the "maternal effect") may contribute to any among-population differences that are observed [7,21,22]. Thus, proper assessment of genetic differences in any traits requires the use of seeds collected from mothers that experienced a common environment, thereby eliminating the maternal effect. However, only a fraction of the studies of clinal variation [7,10,11] in flowering time and vernalisation requirement have been fully or partly validated using this approach.
Here, we investigated the flowering traits of Arabidopsis kamchatica (Fisch. ex DC.) K. Shimizu & Kudoh, which is an allopolyploid plant derived from A. lyrata and Arabidopsis halleri [23]. Wild relatives of A. thaliana, which are ecologically diverse and genetically tractable, can serve as powerful models in evolutionary biology [24-26], and their use has become increasingly popular in this field [27-36]. Arabidopsis kamchatica harbours two ecologically diverse subspecies: ssp. kamchatica is a perennial plant with remarkably wide altitudinal distribution, ranging from 30 to nearly 3000 m, within a narrow latitudinal range (35.2°N to 36.8°N) in central Japan, whereas ssp. kawasakiana is an endangered annual plant whose distribution is limited to lowland seaside and lakeside sites [37]. One of the main advantages of using altitude as the clinal parameter in ecological and evolutionary studies is that steep environmental gradients occur within short spatial distances, providing powerful natural experiments [38].
We investigated how flowering time and vernalisation requirement have diverged between these annual and perennial subspecies and across such a wide altitudinal gradient in ssp. kamchatica. To ensure replication of altitudinal gradients, we investigated 29 populations of ssp. kamchatica across five altitudinal gradients in different mountain systems of the Japanese Alps, as well as 9 populations of ssp. kamchatica from lowland populations. The Japanese Alps include six separate mountain chains or peaks with elevations around 3000 m, and therefore provide useful replication of altitudinal gradients that can be used in evolutionary studies. We used both field-collected and laboratory-generated seeds to explicitly account for the maternal effect. We grew plants in common laboratory environments with and without vernalisation. The divergence between the two subspecies can be examined by comparisons conducted at the same altitude. We predicted (i) that the annual subspecies would flower earlier, because rapid growth and early flowering would ensure reproduction of the annual plants but would decrease survival and reproductive output at later stages in the perennial plants [39-41]; and (ii) that the annual subspecies would have a weaker vernalisation requirement, because annual plants should be constrained to reproduce during their first year (i.e., their only year), irrespective of the strength and existence of a winter season, which are variable between years and depend on whey they germinate. Also, the altitudinal divergence within the subspecies can also be examined by comparing populations of ssp. kamchatica across altitudinal gradients. We predicted (iii) that populations from higher altitudes would flower earlier than those from lower altitudes, because the length of the growing season and duration of suitable environmental conditions would be shorter at higher altitudes in central Japan, where the summer is generally moist and the snowfall is much greater at higher altitude; and (iv) that populations from higher altitudes would have a stronger vernalisation requirement, because the optimal age of starting reproduction would be older for the perennial subspecies owing to the shorter growing season and the need to allocate resources to early survival and growth in a difficult environment rather than to reproduction.
Materials and Methods
Plant materials and climatic census
Arabidopsis kamchatica is self-compatible herb and capable of autogamy [37]. Ssp. kawasakiana is winter annlual that germinates in autumn or winter and flowers in spring. Ssp. kamchatica is perennial that flowers in spring at low altitude and in summer at high altitude. Newly established seedlings of ssp. kamchatica were observed both in autumn (from September to November) and in spring and summer (from May to August), suggesting that the seeds germinate both before and after winter in this subspecies (Y. Onda & T. Kenta, unpublished data). The variation in germination timing along altitude has not been known.
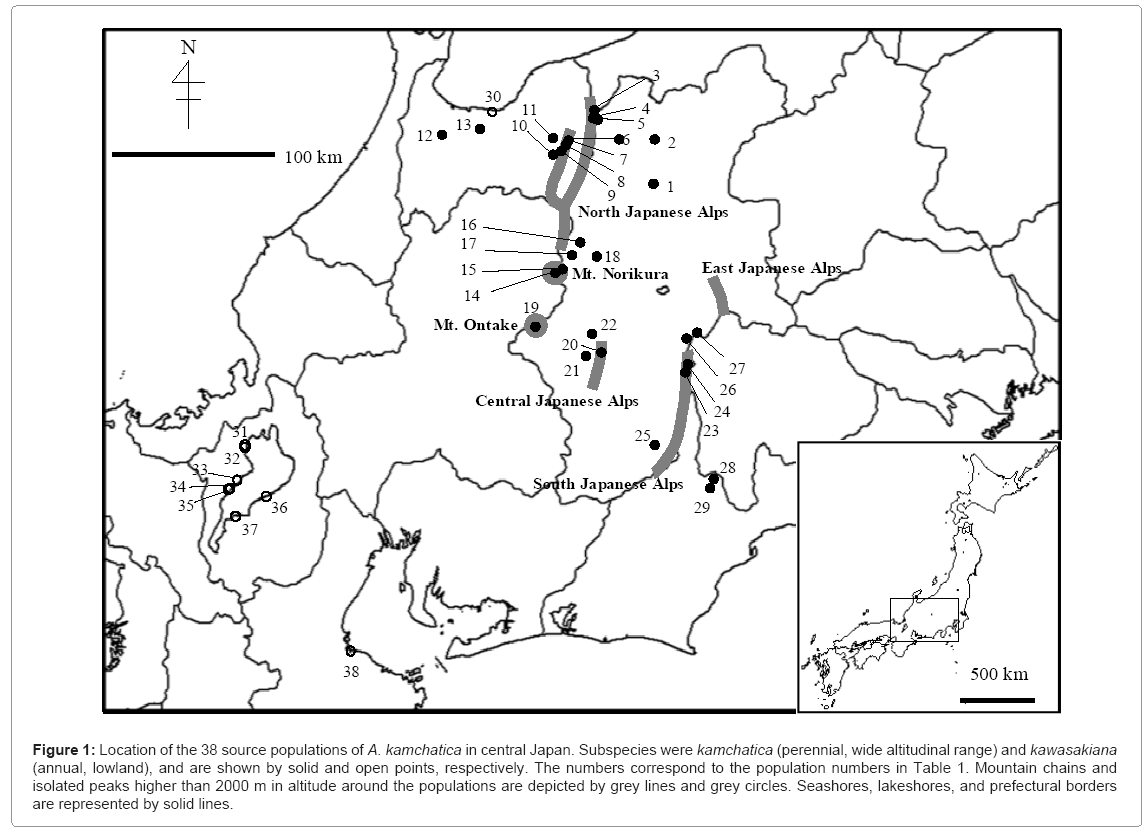
We explored natural populations of ssp. kamchatica by exploiting information on specimens that were available in museums and herbariums (Table 1). Using this information, we chose 29 populations that covered a wide range of altitudes (from 30 to 2949 m) around three mountain chains and two single-peak mountains in the Japanese Alps (Figure 1). Seeds of ssp. kamchatica were collected from these populations in both 2008 and 2009. We obtained all required permits for collection of seeds at these locations. Seeds of ssp. kawasakiana from the nine populations were kindly provided by researchers at Kyoto University (Table 1). All seeds of the both subspcies were preserved separately for each maternal plant. Part of those seeds were grown in 2009 in the same laboratory under growing conditions similar to those used in the flowering experiment described below, and the flowers were bagged to produce self-pollinated seeds. Seeds of each maternal plant were again preserved separately so that each lineage was descended from a single maternal plant from the field. Hereafter, plants from the field-collected seeds and those from the lab-derived seeds are referred to as the first and second laboratory generations, respectively. Maternal plants of the first laboratory generation experienced a variable field environment, whereas those from the second generation experienced a common laboratory environment. If flowering timing differed between these generations, the maternal effect could be involved.
| No. | Population | Subspecies | Latitude (°N) | Longitude (°E) | Altitude (m) | Specimen No.1 |
|---|---|---|---|---|---|---|
| 1 | Mt. Hiziri | kamchatica | 36.48248 | 138.01974 | 1399 | SHIN 20040 |
| 2 | Kinasa | kamchatica | 36.67268 | 138.02860 | 664 | SHIN 156387 |
| 3 | Mt. Sirouma | kamchatica | 36.75535 | 137.75693 | 2835 | SHIN 86241 |
| 4 | Mt. Hakubayari | kamchatica | 36.73358 | 137.75465 | 2821 | SHIN 20014 |
| 5 | Hakubayari-onsen | kamchatica | 36.72448 | 137.76720 | 1990 | Y. Onda 32 |
| 6 | Mt. Turugi | kamchatica | 36.62175 | 137.61507 | 2835 | Y. Onda 8 |
| 7 | Kenzan | kamchatica | 36.60931 | 137.61255 | 2469 | Y. Onda 31 |
| 8 | Turugigozen | kamchatica | 36.59641 | 137.60985 | 2745 | Y. Onda 7 |
| 9 | Murodô | kamchatica | 36.57913 | 137.59699 | 2421 | TOYA 67341 |
| 10 | Midagahara | kamchatica | 36.56646 | 137.55511 | 1915 | KYO 93-480 |
| 11 | Riv. Tateyama | kamchatica | 36.64639 | 137.55705 | 759 | TOYA 1818 |
| 12 | Ogamiôhasi | kamchatica | 36.61561 | 137.00118 | 67 | Y. Onda 2 |
| 13 | Toyama Airport | kamchatica | 36.64915 | 137.18937 | 30 | TOYA 45552 |
| 14 | Mt. Norikura | kamchatica | 36.11542 | 137.55321 | 2792 | SHIN 83776 |
| 15 | Kuraigahara | kamchatica | 36.11904 | 137.56980 | 2359 | Y. Onda 12 |
| 16 | Kamikôti | kamchatica | 36.25169 | 137.66347 | 1528 | TNS 903742 |
| 17 | Sakamaki-onsen | kamchatica | 36.19700 | 137.61507 | 1180 | SHIN 107993 |
| 18 | Simasimadani | kamchatica | 36.18933 | 137.77744 | 780 | SHIN 20019 |
| 19 | Mt. Ontake | kamchatica | 35.90261 | 137.48103 | 2908 | Y. Onda 28 |
| 20 | Mt. Kisokoma | kamchatica | 35.78966 | 137.80278 | 2902 | KYO 3982 |
| 21 | Riv. Name | kamchatica | 35.77931 | 137.74566 | 1173 | Y. Onda 15 |
| 22 | Iwahana | kamchatica | 35.85445 | 137.76059 | 976 | Y. Onda 14 |
| 23 | Mt. Senzyô | kamchatica | 35.71978 | 138.18284 | 2949 | SHIN 20147 |
| 24 | Ôdaira | kamchatica | 35.74268 | 138.20912 | 1959 | Y. Onda 19 |
| 25 | Sirabiso | kamchatica | 35.43417 | 138.02813 | 1900 | MAK 314823 |
| 26 | Ôgaya | kamchatica | 35.86312 | 138.21581 | 1309 | Y. Onda 18 |
| 27 | Kamanasi | kamchatica | 35.88184 | 138.23993 | 805 | SHIN 76025 |
| 28 | Riv. Abe | kamchatica | 35.29289 | 138.33650 | 757 | Y. Onda 23 |
| 29 | Magosazima | kamchatica | 35.26229 | 138.32737 | 543 | KYO 26 |
| 30 | Hamakurosaki | kawasakiana | 36.75807 | 137.28194 | 2 | TOYA 43194 |
| 31 | Nakasyo-hama | kawasakiana | 35.44031 | 136.04572 | 86 | ncu |
| 32 | Imazu-hama | kawasakiana | 35.41800 | 136.04622 | 86 | ncu |
| 33 | Sirahige-hama | kawasakiana | 35.27920 | 136.01839 | 87 | ncu |
| 34 | Ômimaiko | kawasakiana | 35.23461 | 135.96033 | 87 | ncu |
| 35 | Kitahira | kawasakiana | 35.22439 | 135.95817 | 87 | ncu |
| 36 | Satuma-tyô | kawasakiana | 35.23297 | 136.15967 | 85 | ncu |
| 37 | Maiami-hama | kawasakiana | 35.13921 | 135.99767 | 87 | ncu |
| 38 | Hukiinoura | kawasakiana | 34.60270 | 136.58644 | 2 | ncu |
1Specimen number from museum and herbarium collections that provided information on the locations of the natural populations from which we collected seeds. If we did
not use such information to find a population, our own specimen number (Y. Onda) is shown. Abbreviations: TOYA, Toyama Science Museum; KYO, Kyoto University
Museum; SHIN, Shinshu University Herbarium; TNS, National Museum of Nature and Science; MAK, Tokyo Metropolitan University; ncu, seeds were not collected by
us, and were instead provided by Dr. J. Sugisaka and Mr. M. Yamaguchi of Kyoto University.
Table 1: Location of the 38 A. kamchatica populations from which seeds were collected. The number of each population corresponds to the number in Figure 1.
Figure 1: Location of the 38 source populations of A. kamchatica in central Japan. Subspecies were kamchatica (perennial, wide altitudinal range) and kawasakiana (annual, lowland), and are shown by solid and open points, respectively. The numbers correspond to the population numbers in Table 1. Mountain chains and isolated peaks higher than 2000 m in altitude around the populations are depicted by grey lines and grey circles. Seashores, lakeshores, and prefectural borders are represented by solid lines.
In each population of ssp. kamchatica, we monitored soil temperatures at a depth of 5 cm using an iButton DS1921G temperature datalogger (Maxim, Sunnyvale, CA, USA) at 2-h intervals for 40 - 90 days from July to September of 2009. Because census duration differed between populations, we only analysed the maximum temperature in the duration. After eliminating datasets from dataloggers that broke or were moved, we obtained data for 22 of the populations.
Flowering experiment
For ssp. kamchatica, we sowed a total of 90 seeds obtained from 1 to 7 lineages per population from each of 14 and 18 populations selected to cover the full range of altitudes of the source populations from the first and second laboratory generations, respectively; 12 of these populations were the same in both generations. For ssp. kawasakiana, we sowed a total of 90 seeds obtained from 1 to 7 lineages from each of the 9 populations in the second laboratory generation (Table 2). Seeds of each generation of each population were uniformly divided into 3 treatments with different growing conditions: (1) a 22°C day temperature (22DT), (2) a 22°C day temperature with a constant 5°C vernalisation treatment for 4 weeks before return to the 22°C day temperature (22/5/22DT), and (3) a 15°C day temperature with a constant 5°C vernalisation treatment for 4 weeks before return to the 15°C day temperature (15/5/15DT). In all treatments, the light period was 20 h per day, with 4 h of night and a night temperature of 16°C.
| No. | Ssp. 1 | Sown seeds | Flowering plants | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 22DT2 | 22/5/22DT2 | 15/5/15DT2 | |||||||
| 1st gen.3 | 2nd gen.3 | 1st gen. | 2nd gen. | 1st gen. | 2nd gen. | 1st gen. | 2nd gen. | ||
| 1 | kam. | 3 (30-30) | - | 1 (1) | - | 3 (10-12) | - | 3 (5-6) | - |
| 2 | kam. | 6 (7-21) | 6 (15-15) | - | 4 (1-3) | - | 6 (2-5) | - | 3 (1-1) |
| 3 | kam. | 7 (4-18) | 7 (12-13) | 1 (1) | 4 (1-3) | 1 (1) | 4 (1-3) | 1 (1) | 2 (1-1) |
| 4 | kam. | 3 (30-30) | - | 1 (3) | - | 3 (1-6) | - | 3 (1-3) | - |
| 5 | kam. | 5 (9-30) | - | 4 (2-11) | - | 5 (1-10) | - | 4 (2-7) | - |
| 6 | kam. | 3 (30-30) | 3 (30-30) | 1 (1) | 3 (1-8) | - | 3 (3-7) | 1 (3) | 3 (1-3) |
| 7 | kam. | - | 3 (30-30) | - | 3 (7-10) | - | 3 (7-10) | - | 3 (3-6) |
| 8 | kam. | 5 (7-30) | 3 (30-30) | 2 (2-7) | 3 (2-3) | 2 (3-6) | 3 (1-4) | 2 (2-3) | 2 (1-3) |
| 9 | kam. | - | 3 (30-30) | - | 3 (6-12) | - | 3 (2-11) | - | 3 (2-6) |
| 10 | kam. | 3 (30-30) | 3 (30-32) | 2 (1-5) | - | 3 (8-10) | 2 (1-6) | 3 (5-6) | 1 (5) |
| 11 | kam. | 3 (30-30) | - | - | - | - | - | - | - |
| 12 | kam. | 4 (22-23) | 3 (30-30) | 2 (6-9) | 2 (1-2) | 2 (5-8) | 3 (1-2) | 2 (3-5) | - |
| 13 | kam. | 3 (30-30) | 3 (30-30) | - | 1 (1) | 1 (1) | - | - | - |
| 14 | kam. | 3 (30-30) | - | - | - | 3 (2-11) | - | 2 (5-6) | - |
| 15 | kam. | 3 (30-30) | 3 (30-30) | - | 1 (1) | 3 (6-12) | 2 (1-5) | 3 (2-4) | 1 (1) |
| 16 | kam. | 3 (30-30) | 3 (30-30) | 3 (2-5) | 3 (2-8) | 3 (2-7) | 3 (4-7) | 3 (1-3) | 2 (1-2) |
| 17 | kam. | - | 3 (30-30) | - | 2 (8-10) | - | 2 (7-11) | - | 2 (6-7) |
| 18 | kam. | 5 (7-30) | 5 (18-18) | 1 (2) | 5 (5-6) | - | 5 (3-6) | 1 (1) | 5 (3-4) |
| 19 | kam. | 3 (30-30) | - | 1 (1) | - | 3 (2-7) | - | 3 (2-5) | - |
| 20 | kam. | 3 (30-30) | - | 2 (2-3) | - | 2 (2-5) | - | 3 (1-3) | - |
| 21 | kam. | - | 2 (30-60) | - | 2 (4-11) | - | 2 (4-12) | - | 2 (1-5) |
| 22 | kam. | - | 1 (90) | - | 1 (8) | - | 1 (6) | - | 1 (6) |
| 23 | kam. | 3 (30-30) | - | - | - | - | - | - | - |
| 24 | kam. | 4 (14-28) | - | - | - | 3 (2-5) | - | 4 (1-3) | - |
| 25 | kam. | 3 (30-30) | 3 (30-30) | - | 3 (1-6) | - | 3 (1-6) | 1 (1) | 2 (2-3) |
| 26 | kam. | 5 (9-27) | 3 (30-30) | - | 2 (2-3) | - | 2 (2-3) | - | 3 (1-2) |
| 27 | kam. | - | 2 (30-60) | - | 2 (2-13) | - | 2 (4-14) | - | 1 (4) |
| 28 | kam. | 3 (30-30) | - | 3 (1-9) | - | 3 (1-10) | - | 2 (2-4) | - |
| 29 | kam. | 3 (30-30) | - | 3 (6-7) | - | 3 (6-8) | - | 3 (2-5) | - |
| 30 | kaw. | - | 3 (30-30) | - | 1 (3) | - | 2 (3-4) | - | 2 (1-5) |
| 31 | kaw. | - | 3 (30-30) | - | 3 (7-7) | - | 3 (3-6) | - | 3 (1-6) |
| 32 | kaw. | - | 3 (30-30) | - | 3 (1-4) | - | 3 (1-7) | - | 3 (1-4) |
| 33 | kaw. | - | 3 (30-30) | - | 3 (2-12) | - | 3 (6-10) | - | 3 (4-6) |
| 34 | kaw. | - | 3 (30-30) | - | 3 (5-11) | - | 3 (5-12) | - | 3 (6-6) |
| 35 | kaw. | - | 3 (30-30) | - | 3 (5-12) | - | 3 (9-11) | - | 3 (5-6) |
| 36 | kaw. | - | 2 (30-60) | - | 2 (1-9) | - | 2 (1-9) | - | 2 (2-7) |
| 37 | kaw. | - | 3 (30-30) | - | 3 (2-10) | - | 3 (1-8) | - | 2 (3-6) |
| 38 | kaw. | - | 4 (15-30) | - | 2 (1-1) | - | 3 (1-4) | - | 3 (1-5) |
1Subspecies kamchatica (kam) or ssp. kawasakiana (kaw). 2Growing conditions: 22DT, 22-°C day temperature; 22/5/22DT, 22-°C day temperature with a 5-°C vernalisation
treatment (4 weeks) followed by a return to the 22-°C day temperature; 15/5/15DT, 15-°C day temperature with a 5-°C vernalisation treatment (4 weeks) followed by
a return to the 15-°C day temperature. See the Methods section for details. 3 Laboratory generations: 1st, plants from seeds collected in the field; 2nd, plants from seeds
of laboratory-grown plants.
Table 2: Numbers of lineages and of plants per lineage (in parentheses) used in this study. Population numbers are the same ones shown in Table 1.
For the 22/5/22DT and 22DT treatments, fluorescent lamps suitable for plant growth (FL40SBR-A and FL20SBR-A; NEC, Tokyo, Japan) were used to provide photosynthetically active radiation (PAR) of approximately 120 µmol/m2/s in average. The 15/5/15DT treatment was conducted in another growth chamber with a uniform mixture of FL40SBR-A fluorescent lamps, FLR40S-EX-N fluorescent lamps (from Toshiba, Tokyo, and Panasonic, Kadoma, Japan), and FL40SD fluorescent lamps (NEC, Tokyo). PAR was approximately 60 µmol/m2/s in average. Plant trays were moved and rotated twice each week so that they experienced more uniform light and other microenvironmental conditions.
Each seed was sown on a 3-cm cube of rockwool (Nittobo, Tokyo) on a depth of approximately 1 cm of vermiculite (Nittai, Osaka). Plants were watered with the mixture of 1/2000 Hyponex 6-10-5 (containing 1.0 mM ammonium-N, 0.4 mM nitrate-N, 0.7 mM other N, 0.5 mM phosphoric acid, 0.6 mM K, and micronutrients; Hyponex Japan, Osaka) and 0.75 mM magnesium sulphate twice a week. Every time of watering, the fertiliser solution reached a height of approximately 3 cm from the bottom of the trays. The solution included 5 g/L acephate, which was provided once every 2 weeks to control flies. When plants reached at least the six-leaf stage (~1 month after germination), plants in the 22/5/22DT and 15/5/15DT treatments were vernalised at 5°C for 4 weeks, followed by transfer to 125-cm3 cubical pots filled with vermiculite (Nittai, Osaka) under the same temperature conditions as used before vernalisation. Plants in the 20DT treatment were directly transferred into pots at the same growth stage as the other plants, but with no vernalisation treatment. We observed plant germination and flowering weekly and used the number of days from germination to flowering as the flowering time.
Statistical analyses
The relationship between soil temperature and altitude was fitted using a non-linear model, the nls() function, provided by version 2.13.0 of the R statistical software [42]:
MT = β0I + β1A + β2A2
where MT is the summer maximum of the daily minimum, mean, and maximum soil temperatures (in separate regressions); I is the temperature intercept; A is the altitude; and ßi (for i = 0 to 2) is the regression coefficient for each explanatory variable. Variable selection was conducted by calculating the value of Akaike's Information Criterion (AIC) for all possible combinations of variables. The model with the lowest AIC was selected as the best model.
Flowering time in the 22DT and 22/5/22DT treatments was analysed simultaneously because these treatments were identical apart from the presence of vernalisation in the latter treatment. The effect of vernalisation was explicitly examined. We then analysed the 15/5/15DT treatment separately because it was conducted in a different growth cabinet, so the growth conditions may have been different from those in the first growth cabinet. Flowering time in the 22DT and 22/5/22DT treatments was fitted to altitude, vernalisation, laboratory generation, and subspecies by using a non-linear mixed-effects model, the lme() function, provided by the R software [42]:
F = I|P/L + β0I + β1A + β2A2M + β3 + β4G + β5S + interactions
where F is the number of days to flowering, I|P /L is the random effect on the intercept of population and lineage within the population, A is altitude, V is vernalisation (0 without, 1 with vernalisation), G is the laboratory generation (0 for first, 1 for second laboratory generation), S is the subspecies (0 for kamchatica, 1 for kawasakiana), and ßi (for i = 0 to 5) is the coefficient of each explanatory variable. In addition, we included all possible interactions between variables in the model except those between A and A2M (to avoid autocorrelation), between A and S and between A2 and S (because ssp. kawasakiana was limited to low-altitude sites), and between G and S (because ssp. kawasakiana was composed entirely of the second laboratory generation). Variable selection was conducted by calculating AIC for all the remaining combinations of main and interactive effects of variables, and the model with the lowest AIC was selected as the best model.
Flowering time in the 15/5/15DT treatment was fitted to the same model as that in the 22DT and 22/5/22DT treatments, with all the same variables except for V (vernalisation), because there was no corresponding control treatment without vernalisation.
Results
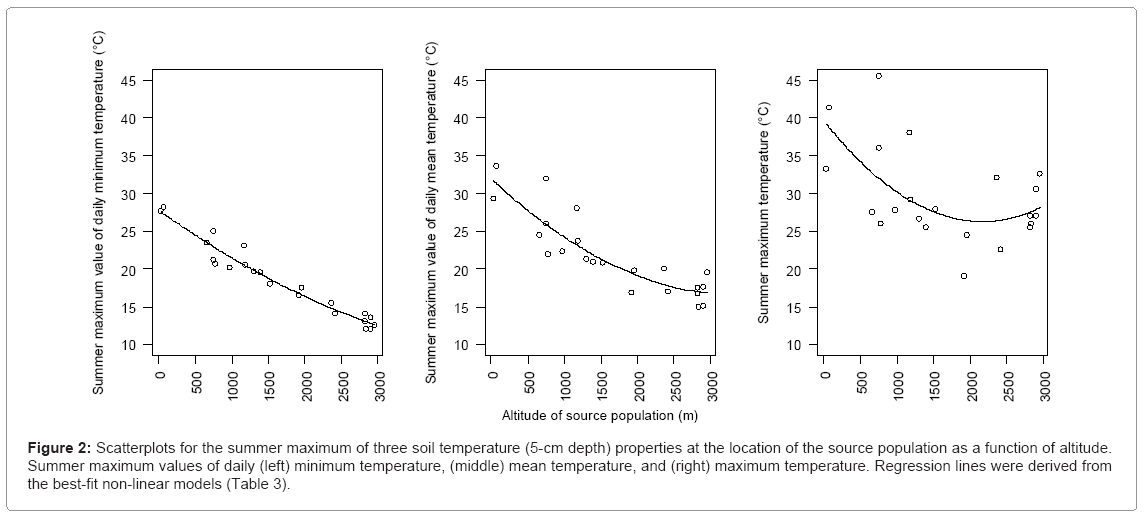
Three measures of the summer maximum soil temperature (minimum, mean, and maximum) were quadratically fitted to altitude (Table 3). The AICs of the quadratic models were slightly (1.4 to 1.8) lower than those of the linear model and greatly (4.9 to 60.8) lower than those of the null model. The estimated coefficient of the square of the altitude (A2) was smallest for the daily minimum temperature, followed by those of the daily mean and maximum temperatures. The quadratic regression lines with smaller coefficients of A2 were closer to linear (Figure 2). The three measures of the summer maximum soil temperature decreased with increasing altitude, but the daily maximum temperature increased slightly above an altitude of 2000 m.
Figure 2: Scatterplots for the summer maximum of three soil temperature (5-cm depth) properties at the location of the source population as a function of altitude. Summer maximum values of daily (left) minimum temperature, (middle) mean temperature, and (right) maximum temperature. Regression lines were derived from the best-fit non-linear models (Table 3).
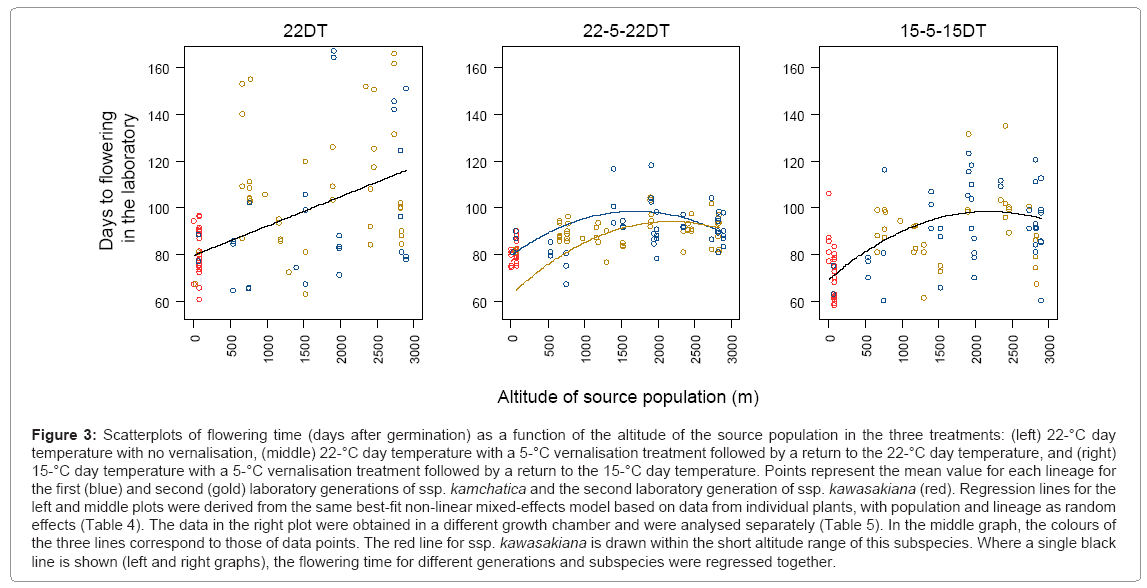
The best model indicates that flowering time was affected by altitude (A) and interactions between vernalisation and each of altitude (V × A), square altitude (V × A2), laboratory generation (V × G), subspecies (V × S) and both the altitude and generation (V × A × G) (Table 4). All of these variables were highly significant, and AIC was improved to a large degree by the inclusion of each of those variables (Table 4). This means that in the absence of vernalisation (Figure 3, left), flowering time was fitted linearly to altitude, whereas in the vernalised treatment (Figure 3, middle), it was fitted quadratically to altitude with the effects of laboratory generation and subspecies. The V × A × G interaction indicates that the effect of the laboratory generation changes with altitude only when the plants have been vernalised. In the 15/5/15DT treatment, flowering time was fitted quadratically to altitude without the effects of laboratory generation and subspecies (Table 5; Figure 3, right). The effects of altitude was highly significant (P < 0.001), and inclusion of the variable largely improved AIC. The effects of A2 was significant (P =0.018), and inclusion of the variable considerably improved AIC.
Figure 3: Scatterplots of flowering time (days after germination) as a function of the altitude of the source population in the three treatments: (left) 22-°C day temperature with no vernalisation, (middle) 22-°C day temperature with a 5-°C vernalisation treatment followed by a return to the 22-°C day temperature, and (right) 15-°C day temperature with a 5-°C vernalisation treatment followed by a return to the 15-°C day temperature. Points represent the mean value for each lineage for the first (blue) and second (gold) laboratory generations of ssp. kamchatica and the second laboratory generation of ssp. kawasakiana (red). Regression lines for the left and middle plots were derived from the same best-fit non-linear mixed-effects model based on data from individual plants, with population and lineage as random effects (Table 4). The data in the right plot were obtained in a different growth chamber and were analysed separately (Table 5). In the middle graph, the colours of the three lines correspond to those of data points. The red line for ssp. kawasakiana is drawn within the short altitude range of this subspecies. Where a single black line is shown (left and right graphs), the flowering time for different generations and subspecies were regressed together.
Discussion
The divergence between subspecies and the maternal effect
Based on the best model, flowering time did not differ between the two subspecies when they were not vernalised, and populations of both subspecies could therefore be regressed using the same linear equation as a function of altitude (Figure 3, left). This suggests that the flowering time has not diverged between the two subspecies, and that altitude is the main determinant of flowering time for the species as a whole when the plants have not been vernalised. This contradicts our prediction that the annual subspecies would flower earlier than the perennial subspecies under the same growing conditions; the reason is discussed later in this section, along with the effect of vernalisation.
When the plants were vernalised, the model that the second laboratory population of ssp. kamchatica shortened flowering time at the lower end of the range in source altitude was selected (Figure 3 middle, yellow line), and V × G and V × A × G interactions were highly significant (Table 4). However, because our dataset lacked low-altitude populations of ssp. kamchatica in the second laboratory generation, further study will be required to confirm the difference between the laboratory generations. Important point is, the effect of altitude and vernalisation found by this study is not violated by the maternal effect because those effects were detected independently from laboratory generations.
In contrast to the second laboratory generation of ssp. kamchatica, which exhibited shorter flowering time after vernalisation at the low altitude, the flowering time of the same generation of ssp. kawasakiana was not greatly changed by the vernalisation. The difference between subspecies in an environment with vernalisation (i.e., the V × S interaction; Table 4) was statistically significant. This result is consistent with our prediction that the perennial subspecies would have a stronger vernalisation requirement. However, we again lacked data for the low-altitude ssp. kamchatica populations in the second laboratory generation, which would have permitted a direct comparison between the subspecies. The estimated difference between subspecies was also small (14 days), and ssp. kawasakiana did not show a large deviation from the overall relationship between flowering time and the altitude of the source population that the second laboratory generation of ssp. kamchatica showed. This overall pattern suggests that the flowering time of the whole species (both subspecies combined) after vernalisation is determined more strongly by the altitude of the source population than by the difference between the subspecies.
We recently found that the annual field survival rate of the perennial ssp. kamchatica was much lower at lower altitudes, mainly owing to higher summer mortality (Y. Onda & T. Kenta, unpublished data). This result suggests that the subspecies has a life history that resembles that of an annual plant at low altitudes; however, because ssp. kawasakiana is a typical annual plant, its annual survival rate is zero, which is still lower than that of the low-altitude populations of ssp. kamchatica. The absence of divergence in flowering time between the subspecies when the plants are not vernalised and the relatively weak subspecies divergence in vernalisation requirement are consistent with the small difference in life history between the subspecies at low altitudes. Further studies will be required to understand the extent of the ecological divergence between these subspecies.
Altitudinal cline in ssp. Kamchatica
Flowering time increased linearly with increasing altitude of the source population when the plants were not vernalised (Table 4; Figure 3, left). The effect of vernalisation also differed as a function of altitude, as indicated by the significant V × A and V × A2 interactions (Table 4), and the flowering time with the vernalisation treatment increased quadratically with increasing altitude of the source population (Figure 3, middle). The magnitude of the dispersion the observed data from the regression line decreased greatly when the plants had been vernalised, suggesting that genetic variation that is not determined by the altitude of the source population is exposed in the absence of vernalisation, although the reason for this is unclear.
The difference in regressed flowering time between the 22DT and 22/5/22DT treatments increased quadratically with increasing altitude of the source population (Figure 3, left and middle) owing to the negative coefficient of the V × A2 interaction, suggesting that plants from high altitudes have a stronger vernalisation requirement for flowering. Although the flowering time in the 15/5/15DT treatment was more variable than that in the 22/5/22DT treatment, possibly owing to the difference in growth chambers between these treatments, the single regression curve for the 15/5/15DT treatment was intermediate between the two regression curves for the two laboratory generations in the 22/5/22DT treatment (Figure 3, middle and right). Because the relationship between flowering time after vernalisation and the altitude of the source population was reproduced in two treatments under different growing conditions, this strongly supports our prediction that plants from higher altitudes would have a stronger vernalisation requirement for flowering. Because plants experienced the same environment in each treatment, and because we explicitly incorporated the maternal effect, these differences in flowering traits along an altitudinal gradient can be attributed to genetic differences. Also, because we replicated the altitudinal gradients by selecting gradients from five different locations, we can exclude the possibility of historical artefacts [11], i.e. the effects of a genetic structure associated with colonisation history. Thus, our results strongly suggest that the observed clinal variation is a sign of evolutionary adaptation of A. kamchatica to certain environments that are generally associated with altitude per se.
Interestingly, the finding that populations from a higher altitude flowered earlier contradicts our prediction and the results of many previous studies [5-8]. The adaptive significance of this result can be easily explained if lower locations are at greater risk of drought, because early flowering has been repeatedly found to be advantageous in habitats that experience drought during the growing season [2,12-15]. Although more snowfall and a longer period of snow cover could provide snowmelt water for longer periods at higher altitudes, the summer precipitation is generally high in Japan and we cannot confirm whether drought is more severe at lower altitudes at our study sites.
Another factor that may favour early flowering at low altitude is the hot summer temperature. At our study sites, the summer soil temperature tended to be higher at lower altitude, with a maximum that sometimes exceeds 35 or 40°C (Figure 2). In A. thaliana, exposure to a daily maximum air temperature of 40°C decreases seed set to less than half of that of control plants, and to only a few per cent of the control level in particularly sensitive accessions, partly on account of the failure of pollen production [43]. In addition, summer mortality of ssp. kamchatica in the field has been found to increase at lower altitudes (Y. Onda & T. Kenta, unpublished data). Because central Japan is located near the southern limit of the distribution of this subspecies, which ranges from ca. 33°N to 65°N across East Asia, Russia, and North America [44], the hot summer temperature probably represents a major environmental challenge for it at low altitudes in central Japan. This can explain the advantage of early flowering, which would let the plants complete reproduction before the severe summer temperatures begin. A similar clinal pattern, in which plants from populations at warmer locations flower earlier, has been found in A. thaliana [10,11] and A. lyrata [9], in contrast to many other plants, which show the opposite pattern. The addition of our results to the literature raises the question of whether avoiding hot summer temperatures are a common and significant life history strategy in this genus.
Our prediction that the vernalisation requirement of ssp. kamchatica would be stronger at higher altitudes was supported by our results. Our study sites at high altitude are covered with snow for as long as 9 to 10 months, whereas some sites at low altitude have snow cover for as short as 1 to 2 months, and the length of the plant's growing season therefore differs drastically as a function of altitude. In the field, newly established seedlings of ssp. kamchatica were observed both in autumn and in winter, suggesting that the seeds germinate both before and after winter (Y. Onda & T. Kenta, unpublished data). The growing season at high altitude is probably not sufficiently long to let plants that germinate after winter complete their reproduction, and a strong vernalisation requirement would prevent those plants from flowering during their first year. In addition, the optimal age for starting reproduction in perennial plants should be associated with the length of the growing season, and the vernalisation requirement may exert some control on this age. These interpretations are congruent with a similar clinal pattern in which the vernalisation requirement increased with the latitude of the source population in the perennial wild beet [19] and showed the opposite pattern in the annual A. thaliana [20]. Further studies will be required to evaluate whether stronger vernalisation at colder locations would be a common pattern in perennial plants.
Another possible explanation for the clinal variation in the vernalisation requirement is the genetic association between the flowering time without vernalisation and the vernalisation requirement. The flowering pathway of A. thaliana is one of the best-characterized genetic networks in plants, and genes involved in the autonomous, photoperiod, vernalisation, and gibberellin pathways are known to regulate, in an integrated manner, the expression of downstream genes that trigger flowering [45]. The flowering pathway is expected to be strongly conserved in related species. For example, sequence differences in FRI are responsible for variation in the vernalisation requirement in both A. thaliana [46] and A. lyrata [18]. In A. thaliana, accessions that flower later, when not vernalised, tend to have stronger vernalisation sensitivity, and a genetic correlation between these two phenotypes is suspected [20]. If the same genetic regulation is conserved in A. kamchatica, selections that favour later flowering at higher altitude might lead to the evolution of a stronger vernalisation requirement, or vice versa.
The flowering time of the vernalised plants showed a convexupward non-linear curve (Figure 3, middle and right). This pattern may be associated with non-linearity in some environmental conditions for the source populations along the altitudinal gradient. For example, daily maximum and mean temperatures showed a stronger non-linear trend as a function of altitude than the daily minimum temperature showed (Figure 2). Non-linearity in the summer maximum temperature can be explained by the fact that the study sites at the highest altitudes are on mountain ridges that receive strong direct sunlight for most of the day, whereas study sites at middle altitudes are on slopes that are shaded by the ridges and trees for a longer portion of the day. The snow-cover season is also probably longer at mid-altitude sites (snowbed slopes) than at the highest altitudes (fellfield ridges). The distribution of ssp. kamchatica is sporadic, and populations on ridges and those on slopes are usually not continuous, suggesting that these populations may have experienced different suites of environmental conditions for a long time.
Acknowledgements
We thank Mr. M. Yamaguchi, Dr. J. Sugisaka, and Prof. K. Kudoh for providing seeds of ssp. kawasakiana and advice on its growth and on the design of our experiment; Dr. G. Takimoto for advice on our research plan and on earlier versions of the manuscript; Mr. I. Sakaguti and Mr. K. Yamaura for collecting seeds of ssp. kamchatica; Ms. M. Suzuki, and Ms. N. Tanaka for technical assistance in growing and measuring the plants; Mr. R. Kanai for preparation and maintenance of the plant growing cabinet; and to Dr. A. Hirao for statistical advice. This work was supported by the Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government (MEXT), an Inamori Foundation research grant, a Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (Young Researchers B, 2277023), and a JSPS research exchange program between Japan and UK (10037611-000065).
References
- Endler JA (1977) Geographic variation, speciation, and clines. Princeton: Princeton University Press.
- Hall MC, Willis JH (2006) Divergent selection on flowering time contributes to local adaptation in Mimulus guttatus populations. Evolution 60: 2466-2477.
- Cohen D (1976) Optimal timing of reproduction. Am Nat 110: 801-807.
- Kozlowski J (1992) Optimal allocation of resources to growth and reproduction - implications for age and size at maturity. Trends Ecol Evol 7: 15-19.
- Rathcke B, Lacey EP (1985) Phenological patterns of terrestrial plants. Annu Rev Ecol Syst 16: 179-214.
- Weber E, Schmid B (1998) Latitudinal population differentiation in two species of Solidago (Asteraceae) introduced into Europe. Am J Bot 85: 1110-1121.
- Olsson K, Agren J (2002) Latitudinal population differentiation in phenology, life history and flower morphology in the perennial herb Lythrum salicaria. J Evol Biol 15: 983-996.
- Winn AA, Gross KL (1993) Latitudinal variation in seed weight and flower number in Prunella vulgaris. Oecologia 93: 55-62.
- Riihimaki M, Savolainen O (2004) Environmental and genetic effects on flowering differences between northern and southern populations of Arabidopsis lyrata (Brassicaceae). Am J Bot 91: 1036-1045.
- Stinchcombe JR, Weinig C, Ungerer M, Olsen KM, Mays C, et al. (2004) A latitudinal cline in flowering time in Arabidopsis thaliana modulated by the flowering time gene FRIGIDA. Proc Natl Acad Sci U S A 101: 4712-4717.
- Montesinos-Navarro A, Wig J, Pico FX, Tonsor SJ (2010) Arabidopsis thaliana populations show clinal variation in a climatic gradient associated with altitude. New Phytol 189: 282-294.
- Franke DM, Ellis AG, Dharjwa M, Freshwater M, Fujikawa M, et al. (2006) A steep cline in flowering time for Brassica rapa in southern California: Populationlevel variation in the field and the greenhouse. Int J Plant Sci 167: 83-92.
- Franks SJ, Sim S, Weis AE (2007) Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc Natl Acad Sci U S A 104: 1278-1282.
- Gimenez-Benavides L, Escudero A, Iriondo JM (2007) Reproductive limits of a late-flowering high-mountain Mediterranean plant along an elevational climate gradient. New Phytol 173: 367-382.
- Sherrard ME, Maherali H (2006) The adaptive significance of drought escape in Avena barbata, an annual grass. Evolution 60: 2478-2489.
- Karlsson BH, Sills GR, Nienhuis J (1993) Effects of photoperiod and vernalization on the number of leaves at flowering in 32 Arabidopsis thaliana (Brassicaceae) ecotypes. Am J Bot 80: 646-648.
- Nordborg M, Bergelson J (1999) The effect of seed and rosette cold treatment on germination and flowering time in some Arabidopsis thaliana (Brassicaceae) ecotypes. Am J Bot 86: 470-475.
- Kuittinen H, Niittyvuopio A, Rinne P, Savolainen O (2008) Natural variation in Arabidopsis lyrata vernalization requirement conferred by a FRIGIDA indel polymorphism. Mol Biol Evol 25: 319-329.
- Boudry P, McCombie H, Van Dijk H (2002) Vernalization requirement of wild beet Beta vulgaris ssp. maritima: among population variation and its adaptive significance. J Ecol 90: 693-703.
- Stinchcombe JR, Caicedo AL, Hopkins R, Mays C, Boyd EW, et al. (2005) Vernalization sensitivity in Arabidopsis thaliana (Brassicaceae): The effects of latitude and FLC variation. Am J Bot 92: 1701-1707.
- Roach DA, Wulff RD (1987) Maternal effects in plants. Annu Rev Ecol Syst 18: 209-235.
- Rossiter MC (1996) Incidence and consequences of inherited environmental effects. Annu Rev Ecol Syst 27: 451-476.
- Shimizu KK, Fujii S, Marhold K, Watanabe K, Kudoh H (2005) Arabidopsis kamchatica (Fisch. ex DC.) K. Shimizu & Kudoh and A. kamchatica subsp. kawasakiana (Makino) K. Shimizu & Kudoh, new combinations. APG: Acta phytotaxonomica et geobotanica 56: 163-172.
- Pigliucci M (1998) Ecological and evolutionary genetics of Arabidopsis. Trends Plant Sci 3: 485-489.
- Mitchell-Olds T (2001) Arabidopsis thaliana and its wild relatives: a model system for ecology and evolution. Trends Ecol Evol 16: 693-700.
- Shimizu KK (2002) Ecology meets molecular genetics in Arabidopsis. Popul Ecol 44: 221-233.
- Schmickl R, Jorgensen MH, Brysting AK, Koch MA (2010) The evolutionary history of the Arabidopsis lyrata complex: a hybrid in the amphi-Beringian area closes a large distribution gap and builds up a genetic barrier. BMC Evol Biol 10: 98.
- Davey MP, Woodward FI, Quick WP (2009) Intraspecfic variation in coldtemperature metabolic phenotypes of Arabidopsis lyrata ssp. petraea. Metabolomics 5: 138-149.
- Shimizu-Inatsugi RIE, Lihov J, Iwanaga H, Kudoh H, Marhold K, et al. (2009) The allopolyploid Arabidopsis kamchatica originated from multiple individuals of Arabidopsis lyrata and Arabidopsis halleri. Mol Ecol 18: 4024-4048.
- Vergeer P, van den Berg LLJ, Bulling MT, Ashmore MR, Kunin WE (2008) Geographical variation in the response to nitrogen deposition in Arabidopsis lyrata petraea. New Phytol 179: 129-141.
- Mable BK, Adam A (2007) Patterns of genetic diversity in outcrossing and selfing populations of Arabidopsis lyrata. Mol Ecol 16: 3565-3580.
- Kawagoe T, Kudoh H (2010) Escape from floral herbivory by early flowering in Arabidopsis halleri subsp. gemmifera. Oecologia: 1-8.
- Schierup MH, Bechsgaard JS, Nielsen LH, Christiansen FB (2006) Selection at work in self-incompatible Arabidopsis lyrata: Mating patterns in a natural population. Genetics 172: 477-484.
- Kunin WE, Vergeer P, Kenta T, Davey MP, Burke T, et al. (2009) Variation at range margins across multiple spatial scales: environmental temperature, population genetics and metabolomic phenotype. Proceedings of the Royal Society B-Biological Sciences 276: 1495-1506.
- Aikawa S, Kobayashi MJ, Satake A, Shimizu KK, Kudoh H (2010) Robust control of the seasonal expression of the Arabidopsis FLC gene in a fluctuating environment. Proceedings of the National Academy of Sciences 107: 11632- 11637.
- Sandring S, Agren J (2009) Pollinator-mediated selection on floral display and flowering time in the perennial herb Arabidopsis lyrata. Evolution 63: 1292- 1300.
- Sugisaka J, Kudoh H (2008) Breeding system of the annual Cruciferae, Arabidopsis kamchatica subsp. kawasakiana. J Plant Res 121: 65-68.
- Körner C (2007) The use of 'altitude' in ecological research. Trends Ecol Evol 22: 569-574.
- Snow AA, Whigham DF (1989) Costs of flower and fruit production in Tipularia discolor (orchidaceae). Ecology 70: 1286-1293.
- Reekie EG, Bazzaz FA (1992) Cost of reproduction as reduced growth in genotypes of 2 congeneric species with contrasting life histories. Oecologia 90: 21-26.
- Primack RB, Hall P (1990) Costs of reproduction in the pink lady's slipper orchid: a 4-year experimental-study. Am Nat 136: 638-656.
- R Development Core Team (2011) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- Zinn KE, Tunc-Ozdemir M, Harper JF (2010) Temperature stress and plant sexual reproduction: uncovering the weakest links. J Exp Bot 61: 1959-1968.
- Hoffmann MH (2005) Evolution of the realized climatic niche in the genus Arabidopsis (Brassicaceae). Evolution 59: 1425-1436.
- Ehrenreich IM, Hanzawa Y, Chou L, Roe JL, Kover PX, et al. (2009) Candidate gene association mapping of Arabidopsis flowering time. Genetics 183: 325.
- Johanson U, West J, Lister C, Michaels S, Amasino R, et al. (2000) Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290: 344-347.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 16288
- [From(publication date):
June-2013 - Dec 07, 2025] - Breakdown by view type
- HTML page views : 11579
- PDF downloads : 4709