Distribution and Abundance of Finfish Eggs from Muthupettai, South East Coast of India
Received: 03-Aug-2013 / Accepted Date: 07-Oct-2013 / Published Date: 16-Oct-2013 DOI: 10.4172/2157-7617.1000166
Abstract
Finfish eggs were collected monthly during January 2011 to December 2012 at Muthupettai coast. Finfish eggs abundance data is an important for patterns of distribution, an areas providing information for nursery ground and a range of adult and spawning trends. Environmental parameters such as rainfall, atmospheric temperature, water temperature, salinity, pH and dissolved oxygen were recorded and correlated with the distribution of fish eggs. The density of fin fish eggs at all stations showed a seasonal variation. The maximum number of eggs were recorded during post monsoon followed by pre monsoon, summer and monsoon seasons. The seasonal occurrence of finfish eggs did not follow a similar pattern during the two-year period of study. This might be due to the fluctuation in the environmental parameters. It is evident from the present study that the water temperature and salinity appear to play a significant role in determining the distribution of fin fish eggs in the study area.
Keywords: Identified; Abundance; Fin fish; Eggs; Physico-chemical; Fact
5437Introduction
Estimation of abundance of fish eggs and larvae helps to evaluate marine fishery resources. Most of the marine fishes spawn in the open sea and produce pelagic eggs and larvae [1,2]. By regular collection of plankton, it is possible to map the marine area with respect to the breeding of fish and the relative abundance of ichthyoplankton of commercial fish stock, and use the information as an index of fish abundance or for the prediction of year class strength. Generally fishes spawn during a definite time of the day and this has been found to be true in marine fishes of Porto-Novo region [3,4]. Investigation on the occurrence and distribution of finfish eggs and larvae is an integral part of a fishery research programme. Most of the eggs and almost the larvae are pelagic and it is easy to sample several species over a wide area with simple plankton nets. Regular sampling of ichthyoplankton is essential for locating shoals of adult fishes and their spawning grounds. Ichthyoplankton studies are extensively useful in fishery investigation. Information on fish eggs and larvae of a particular region is useful in understanding the spawning season of fishes of commercial importance. Studies on the early developmental stages of fish allow us to comprehend the biology of the species besides determining their spawning seasons and to estimate spawning stock abundance. Such a study is also an essential prerequisite in undertaking the spawning biomass of target species monitoring, changes in exploitable stocks and yields, forecasting trends of production etc. [3-5]. Distribution of the early developmental stages, in space and time is known with considerable precision so that sampling effort can be efficiently concentrated in areas and time periods when they will be most effective [6]. Generally, fishes spawn during a definite time, hence, studies on the seasonal occurrence of fish eggs and larvae are useful in locating shoals of fish and their breeding grounds. Till recently studies on the quantitative aspects of fish eggs and larvae in Indian seas were limited to studies on their taxonomy, seasonal abundance based on material from the inshore plankton and post larval fish collections from restricted localities. However, in the present study the distribution and abundance of finfish eggs were conducted from Muthupettai coastal waters.
Materials and Methods
The study was conducted at Muthupettai coast during January 2011 to December 2012. Finfish eggs were collected every month in the early hours of the day during high tide, with the help of plankton net of diameter 0.5 m made of bolting silk (No: 10 mesh size, 158 μm). Volume of water filtered was quantified with the help of a calibrated flow meter (General Oceanics, INC model) attached to it. The net was towed horizontally along the surface water at a constant speed of 1.0 km/hr for about 15-20 minutes in each station by adopting the method of [7,8]. Samples from all the stations were preserved onboard in 5% buffered formalin-seawater and sorted in the laboratory [9]. Fin fish eggs were sorted out from this sample and their abundance was expressed as number of eggs/100 m3.
Description of the study area
Station I (Sethukuda): This station is situated at 10º20’49.68’ N Lat. and 79º32’13.23’ E Long. The average depth of the station is about 1 metre. Avicinnia sp. is dominant in this station (Plate 1).
Station II (Lagoon): This station is situated between 10º20’19.71’ N Lat. and 79º31’52.64’ E Long. The Lagoon is shallow with an average of 1m depth. Avicennia sp. borders the Lagoon (Plate 1).
Station III (Chellimunai): This station is situated at 10º19’42.56’ N Lat. and 79º33’35.10’ E Long. This station is shallow with an average depth of 1.5 m. The station is dominated by Avicennia sp (Plate 1).
Station IV (River Mouth): This station is situated at 10º18’20.76’ N Lat. and 79º31’08.02’ E Long (Plate 1).
Results and Discussion
Seasonal abundance and species/genus wise density of finfish eggs in the Muthupettai waters
Family: Ophichthidae
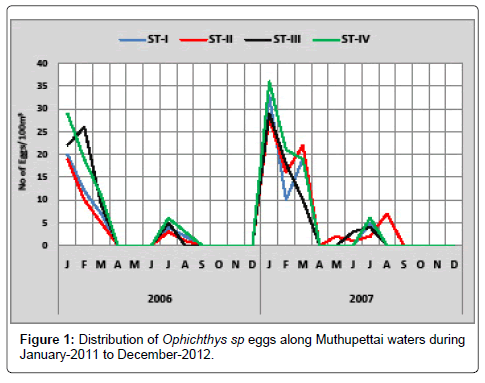
Ophichthys sp.:Single egg was observed during April -2011 at station II and maximum (29 eggs/100m3) observed during January-2011 at station IV. During second year also a single egg was noticed in the station II during July-2012 and Maximum 36 eggs/100 m3 observed during January -2012 at station IV (Figure 1).
Considerable numbers of Ophichthys sp. eggs were observed during pre monsoon, early month of monsoon and late month of post monsoon seasons. Present observation gives the picture about lengthened spawning period of these fishes. However, Ganapathi [10] observed only in post monsoon season along Waltair coast. Manickasundaram [11] observed in early month of monsoon and pre monsoon season at Coleroon estuary complex along the south east coast of India.
Family: Engraulidae
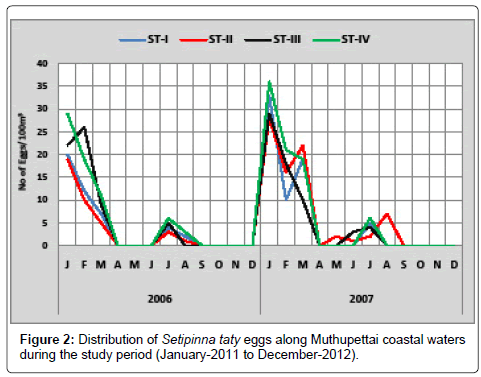
Setipinna taty: Minimum density (3 eggs/100 m3) was observed in May 2011 at station II and maximum (30 eggs/100 m3) in February 2011 at station III. In 2012 only single egg observed in the station III during December-2012 and maximum (32 eggs/100 m3) at station III during the month of January (Figure 2). Thangaraja [12] observed the occurrence of this species eggs during post monsoon season in the Parangipettai coastal waters. The present observation in the Muthupettai waters confirms that the spawning activity of these fishes is during the post monsoon season.
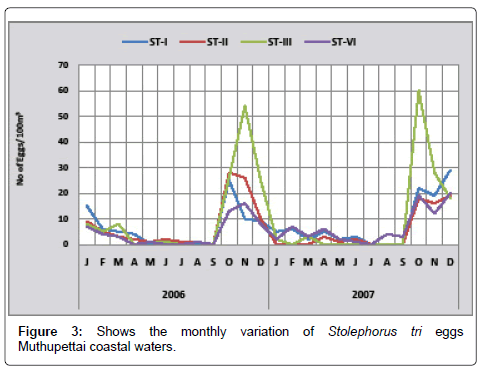
Stolephorus tri: Stolephorus tri 3 eggs/100 m3 of were collected during May 2011 at station II and (54 eggs/100 m3) in November at station IV. In 2012, the minimum density of 2 eggs/100 m3 were observed in January-2012 at station I and maximum of 60 eggs/100 m3 in October -2012 at station III (Figure 3).
Occurrences of the eggs of Stolephorus tri was observed almost throughout the year in Muthupettai waters. Bensam [8] recorded the eggs of this species during summer months from Parangipettai waters, Nair [13] observed December to January in the Madras coastal waters. Siraimeetan [14] observed biannual spawning season of this species along the Tuticorin coast, Ramaiyan [15] collected these eggs throughout the year from Parangipettai coast, these observations are in support of the present findings.
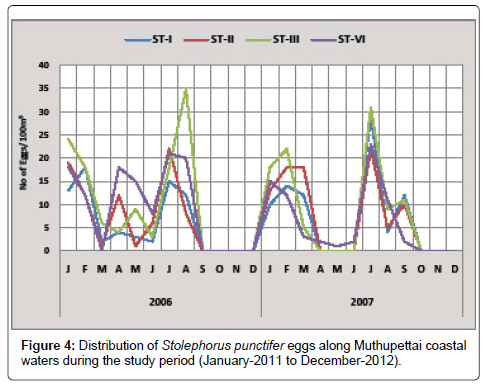
Stolephorus punctifer: Minimum number (1 egg/100 m3) was observed in July 2011 at station I and maximum (35 eggs/100 m3) in August 2011 at station III. During the second year 2012, the minimum density (1 egg/100 m3) was observed during May -2012 at station III and maximum density (31 eggs/100 m3) in July at station III (Figure 4).
Eggs of Stolephorus punctifer were collected during the early month of pre monsoon (July-September), post monsoon (January-March) and summer (October-December) during the present investigation. Observations made by Nair [13] during post monsoon and summer from Madras and Manickasundaram [11] along Coleroon estuary and Ramaiyan [15] observed this eggs in Porto Novo waters during both pre and post monsoon and also in summer are in support of the present investigation.
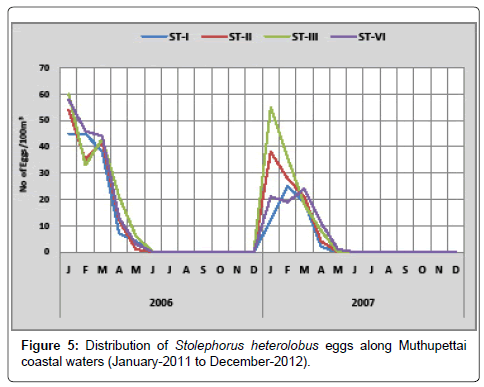
Stolephorus heterolobus: Minimum number (1 egg/100 m3) was observed in April at station IV and maximum (60 eggs/100 m3) in January at station III. In the second year these eggs were observed minimum density in the station IV (1 egg/100 m3) during May 2012 and maximum (55 eggs/100 m3) in station III during March 2012 (Figure 5).
In the present investigation, Stolephorus heterolobus eggs was observed only during post monsoon and summer seasons and is suggesting that the breeding of this species may be takes place only in these seasons. This observation coraboram works are made by Manickasundaram [11] in the Coleroon estuarine complex and Ramaiyan [15] along the Parangipettai coastal waters.
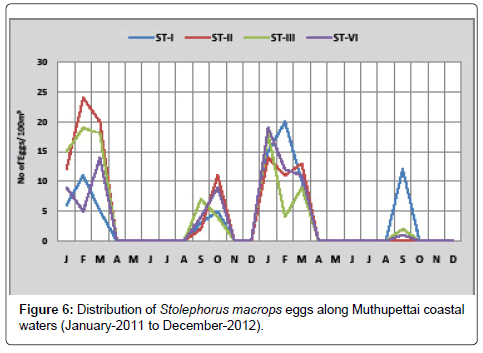
Stolephorus macrops: Figure 6 shows the monthly variation of eggs of Stolephorus macrops along Muthupettai coastal waters. Minimum density (2 eggs/100 m3) was observed in September at station II and maximum (24 eggs/100 m3) in February at station II during the year 2011. In 2012, a single egg was observed in October at station IV and maximum density (20 eggs/100 m3) was observed during February at station I.
Stolephorus macrops eggs were found to occur during post monsoon and pre monsoon, in the present investigation. In Parangipettai coastal waters, Thangaraja [12] found these eggs during February to March and Manickasundaram [11] observed during January to March with peak abundance in March along Coleroon estuary Ramaiyan [15] observed this eggs during post monsoon and summer season in Parangipettai waters.
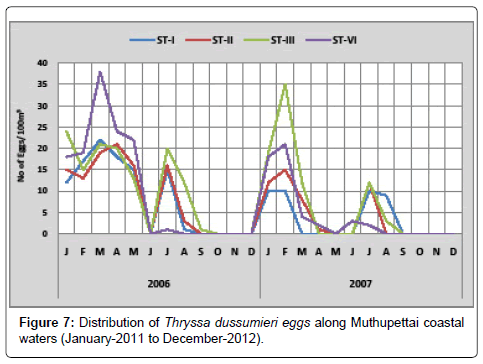
Thryssa dussumieri: Figure 7 shows the distribution of the eggs of Thryssa dussumieri along Muthupettai waters. During the study period, the minimum density (1 egg/100 m3) was observed during September at station III and maximum (38 eggs/100 m3) in March at station IV. In 2012, the minimum density (1 egg/100 m3) was observed in April at station II and maximum (35 eggs/100 m3) in August at station III.
Thryssa dussumieri eggs were found in pre monsoon, post monsoon and summer seasons during the present investigation. The present observations are in agreement with the earlier ones made by [7,8,12,15] along Parangipettai coastal waters.
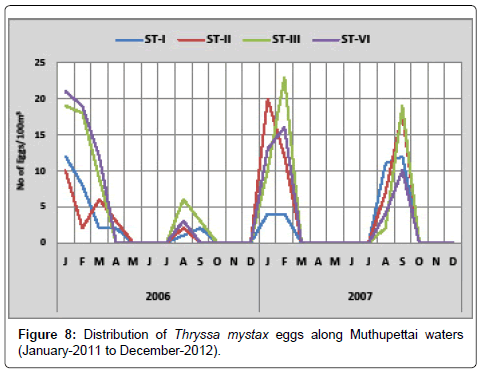
Thryssa mystax: Figure 8 shows the monthly variation of eggs of Thryssa mystax along Muthupettai waters. In the year 2011, minimum density was observed during August (1 egg/100 m3) at station I and the maximum (21 eggs/100 m3) during January at station IV. In year 2012, the minimum density of these eggs (2 eggs/100 m3) was noticed in August at station I and maximum (23 eggs/100 m3) in February at station III.
Thryssa mystax eggs were found in this investigation during post monsoon, early months of summer and premonsoon seasons in Muthupettai waters. Similar observations were made by Manickasundaram [11] from Coleroon estuarine, [7,15] from Parangipettai coastal waters and [16] in Madras the coast.
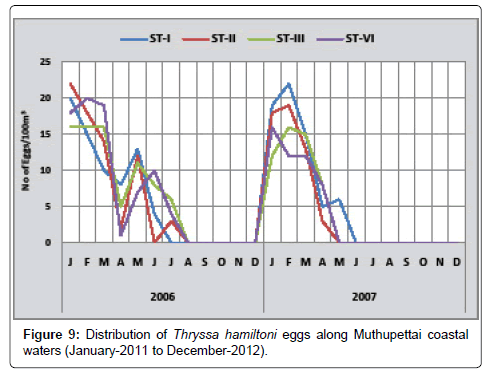
Thryssa hamiltoni: Monthly variation in the distribution of Thryssa hamiltoni eggs along Muthupettai coastal waters is shown in Figure 9. The minimum density of these eggs (1 egg/100 m3) were observed in April at station IV and the maximum density of (22 eggs/100 m3) were observed in January at station II. In the year 2012, 3 eggs/100 m3 were observed in July at station II and (22 eggs/100 m3) in February 2012 at station I
Eggs of Thryssa hamiltonii were found to be abundant during the post monsoon and summer months. [7,8,15] also observed the eggs of Thryssa hamiltonii.
Family: Pristigastridae
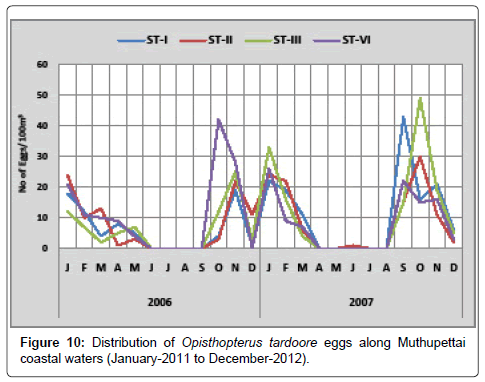
Opisthopterus tardoore: During the first year, the minimum density (1 egg/100 m3) was observed in December at station I and maximum (42 eggs/100 m3) in October at station IV. During the second year, the minimum density (2 eggs/100 m3) was observed in October at station II and maximum (49 eggs/100 m3) in March at station III (Figure 10).
Opisthopterus tardoore eggs were found to be abundant in the Muthupettai waters. These eggs were reported during post monsoon and summer in the Vellar estuary [7,11,15,17] in the Coleroon estuarine.
Family: Chirocentridae
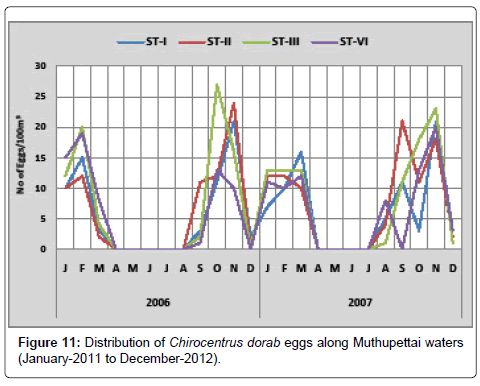
Chirocentrus dorab: The distribution of the eggs of Chirocentrus dorab is depicted in the Figure 11. During the first year of the study (2011), one was observed in September at station IV and 27 eggs/100 m3 in September at station III. In the year 2012, the minimum density of 1 egg/100 m3 was observed in December at station III and a maximum 23 eggs/100 m3 in November at station III.
Chirocentrus dorab eggs were collected during pre monsoon, monsoon and post monsoon seasons from Muthupettai waters. Similar observation made by Manickasundaram [11] in the Coleroon estuarine system as well.
Family: Clupeidae
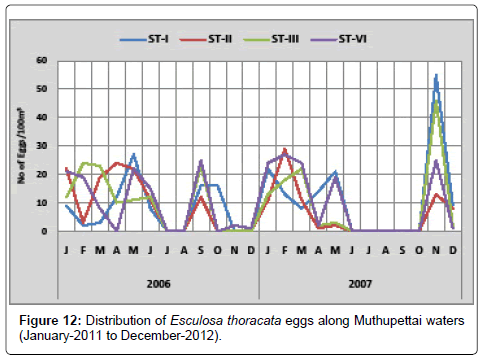
Esculosa thoracata: A single egg observed in December at station IV and maximum (25 eggs/100 m3) in February at station IV. In the second year, the minimum density was in April (1 egg/100 m3) at station II and maximum (55 eggs/100 m3) in October at station II (Figure 12).
Bapat [18] found the abundance of Esculosa thoracata eggs during March, May to August from Mandapam and Kowtal [19] during March to October from Chilka Lake. Venkataramanujam [17] reported their distribution during March to October from Parangipettai. These fishes appear to spawn over an extended period in Muthupettai waters [7] in the Vellar estuary and [11] in the Coleroon estuary.
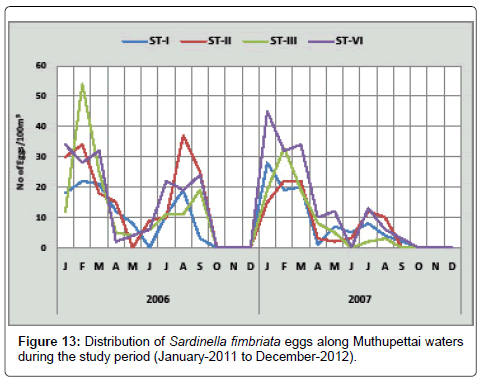
Sardinella fimbriata: During the first year of the study, the minimum density was in September (3 eggs/100 m3) at station I and maximum (54 eggs/100 m3) in February at station III. During the second year of the study, the minimum density (1 egg/100m3) was collected during April at station I and maximum in January (45 eggs/100 m3) at station IV (Figure 13). Sardinella fimbriata eggs were collected during pre monsoon, post monsoon and summer seasons. Similar observations were made by Manickasundaram [11] along Coleroon estuary, [7,8,17] along Parangipettai coastal waters and John [20] along Madras coast.
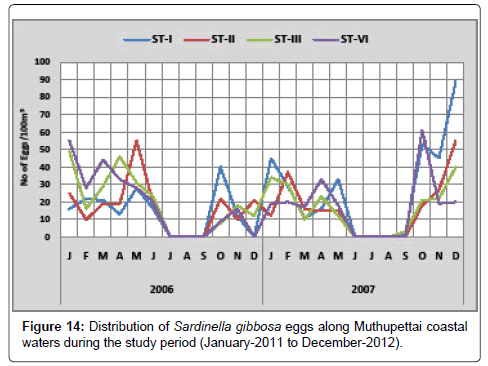
Sardinella gibbosa: Figure 14 shows the monthly variation of Sardinella gibbosa eggs along Muthupettai coastal waters. During the first year of the study period the minimum density (8 eggs/100 m3) were observed in October at station III and maximum (49 eggs/100 m3) in January at station III. But during the second year, the minimum density of these eggs (3 eggs/100 m3) was encountered during September at station I and maximum (45 eggs/100 m3) in January in the same station.
The present findings revealed that the eggs of Sardinella gibbossa were noticed during monsoon, post monsoon and summer seasons with a peak spawning activity in January to May along Muthupettai waters. Similar observations reported by [7,17] from Parangipettai coastal waters and Manickasundaram [11] along Coleroon estuary were agreed with the present observation.
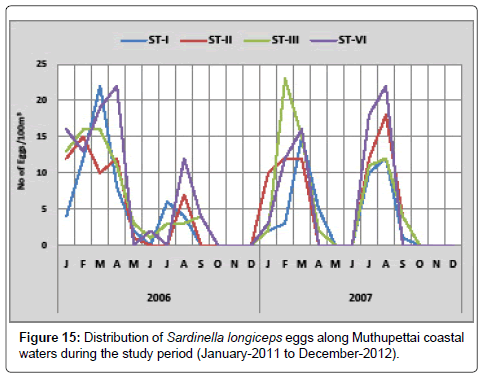
Sardinella longiceps: Figure 15 shows the monthly variation of Sardinella longiceps eggs along Muthupettai coastal waters. During the first year of the study, a single egg observed in May at station II and maximum (22 eggs/100 m3) in March and April at station I. During the second year, single egg observed at station me during September and maximum (23 eggs/100 m3) in station III during February.
Sardinella longiceps eggs were present during post monsoon, summer and early month of pre monsoon seasons along Muthupettai waters. The present result confirms that the discontinuous spawning habit of these fishes. Findings of [20,21] have been noticed these eggs in the plankton sample during post monsoon seasons; Nair [22] collected these eggs during pre monsoon and monsoon seasons in Madras coast. Venkataramanujam [17] observed these eggs during monsoon, post monsoon and summer seasons and Venkataramanujam [7] noticed during post monsoon and summer seasons along Parangipettai coastal waters. Manickasundaram [11] observed during the pre monsoon, post monsoon and summer seasons with peak abundance during February along Coleroon estuary were agreed with the present findings.
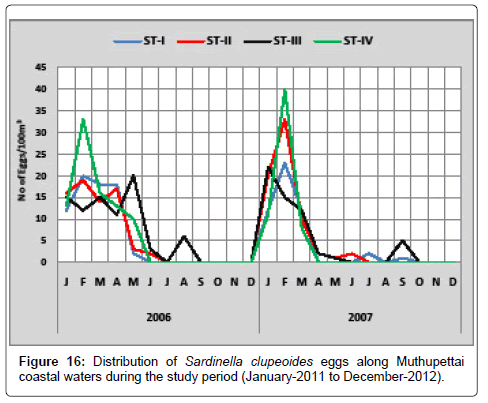
Sardinella clupeoides: Figure 16 shows the monthly variation of Sardinella clupeoides eggs along Muthupettai coastal waters. During the first year of the study, the minimum numbers of eggs (2 eggs/100 m3) were observed in May at station I and maximum (33 eggs/100 m3) in February at station IV. In the second year, single egg was in May at station II and III and maximum (40 eggs/100 m3) in the same month at station VI.
Eggs of Sardinella clupeoides were collected during post monsoon, summer, early month of pre monsoon and monsoon seasons with a peak in February along Muthupettai waters. Similar findings were made by Venkataramanujam [17] who observed these eggs during monsoon, post monsoon and summer seasons, [8,17] made these eggs appeared in post monsoon and summer seasons from Vellar estuary and Manickasundaram [11] observed during monsoon, post monsoon and summer seasons with a peak during February support the present investigation.
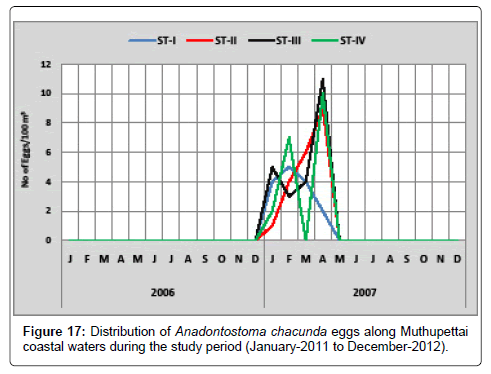
Anadontostoma chacunda: Figure 17 shows the monthly variation of Anadontostoma chacunda eggs along Muthupettai waters. In the first year of the in investigation no egg were observed, but in the second year, single egg was observed in January at station II and maximum (11eggs/100 m3) in April at station III.
Eggs of Anadontostoma chacunda were observed only in the second year of the study period in the post monsoon and the early months of summer. Annual spawning of these fish agrees with the previous works on the east coast by [7,12,17,18,20] have reported similar observation from Parangipettai coastal waters. Manickasundaram [11] observed these eggs during February to May along Coleroon estuary.
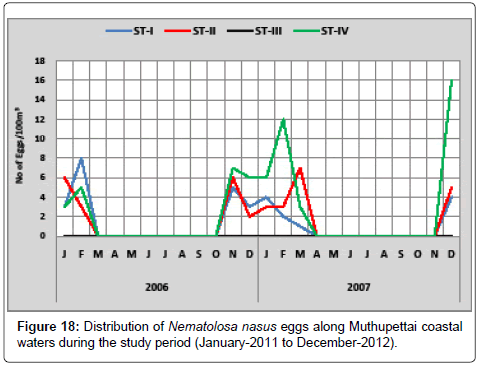
Nematolosa nasus: Figure 18 shows the monthly variation of Nematolosa nasus eggs along Muthupettai waters. During the first year of the study (2 eggs/100 m3) observed in the month of January in the station I and maximum (8 eggs/100 m3) were observed in February at Station I. During the second year of the study a single egg of this species observed during March at station I and Maximum (16 eggs/100 m3) in the station IV in the month of December.
Eggs of Nematolosa nasus were collected during late months of monsoon and post monsoon seasons during the present study. This present observation supported by the previous work of Bensam [8] who noticed these eggs during the post monsoon season from Vellar estuary and [15] observed these eggs in the monsoon and post monsoon season in parangipettai waters.
Family: Synodontidae
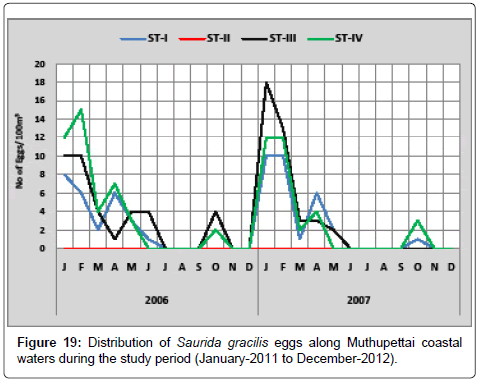
Saurida gracilis: Figure 19 shows the seasonal distribution of Saurida gracilis eggs along Muthupettai coastal waters. During the first year of the study, a single egg observed in the month of June in the station I and maximum (15 eggs/100 m3) in February at station IV. In second year, the minimum number of egg (1 egg/100 m3) was observed in January and February at station I and maximum (18 eggs/100 m3) in January at station IV.
Saurida gracilis eggs were collected during post monsoon, summer, late months of pre monsoon and late month of monsoon seasons gives the picture about the prolonged spawning of these fishes. [7,17,23] noticed during post monsoon and summer along Parangipettai coastal waters, Manickasundaram [11] noticed during post monsoon along Coleroon estuary and Ramaiyan [15] observed these eggs in post monsoon and summer, seasons in Parangipettai waters are in the support of the present investigation
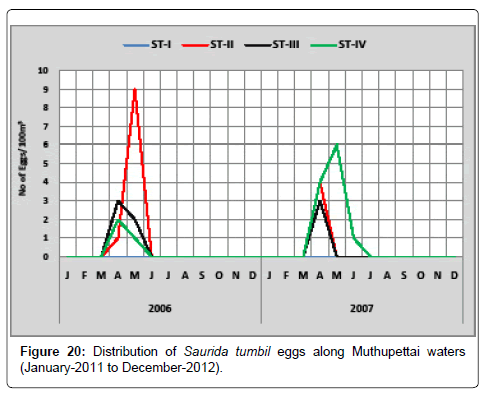
Saurida tumbil: Figure 20 shows the monthly variation of Saurida tumbil eggs along Muthupettai coastal waters. During the first year of the study, the minimum density of these eggs (1 egg/100 m3) was observed in April at station II and maximum (9 eggs/100 m3) in May at station the same station. During the second year of study, a single egg was obtained in June at station IV and the maximum (6 eggs/100 m3) in May at the same station.
Saurida tumbil eggs were collected during post monsoon, summer, pre monsoon and late month of monsoon seasons along Muthupettai coastal waters. However, Venkataramanujam [7] noticed during post monsoon and summer along Parangipettai coastal waters Venkataramanujam [17] observed during post monsoon, summer and pre monsoon seasons, Vijayaraghavan [24] observed during monsoon season along Madras coast.
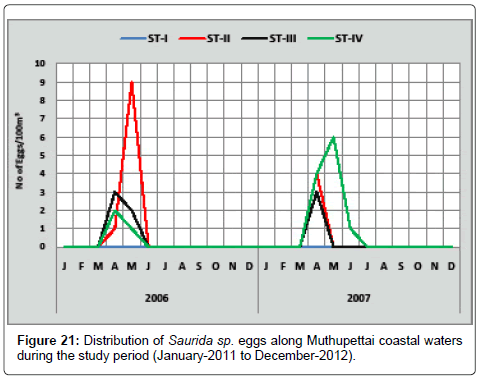
Saurida sp.: Eggs of Saurida sp. (5 eggs/100 m3) observed collected in April at station IV and a single egg was in the same month at station II, in the second year of study period (9 eggs/100 m3) eggs were obtained in the month of April at the station II and (3 eggs/100 m3) collected in the same month at the III and IVth stations along Muthupettai coastal waters (Figure 21). Ramaiyan [15] observed this egg during early months of summer along the Parangipettai coast is the similar to the present findings.
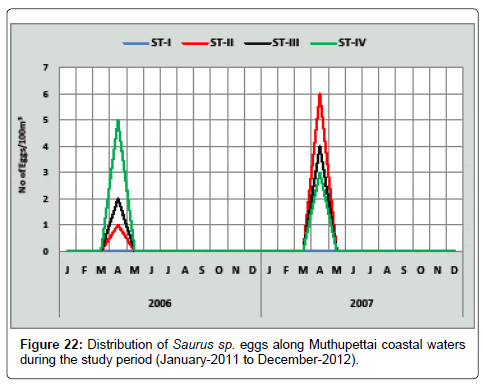
Saurus sp.: A single egg of Saurus sp. was collected during April from station II and 5 eggs /100 m3 eggs were obtained in the same month in the station IV. In the second year of the investigation minimum (3 eggs/100 m3) were observed in the month of April in the station IV and maximum (6 eggs/100m3) were observed in the same month at the station II (Figure 22). Bapat [18] observed during summer season along Mandapam coast and Manickasundaram [11] noticed only during April along Coleroon estuarine are similar to the present findings.
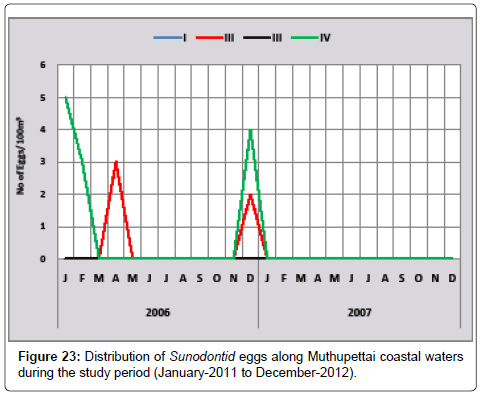
Synodontid eggs: Only few of Synodontid eggs observed in the first year of the investigation. Minimum (3 eggs/100 m3) observed in April at Station II and maximum (5 eggs/100 m3) in the same month of the station IV (Figure 23). Ramaiyan [15] observed the eggs in the month monsoon and pre monsoon in the Parangipettai waters is the support of the present findings.
Family: Mugilidae
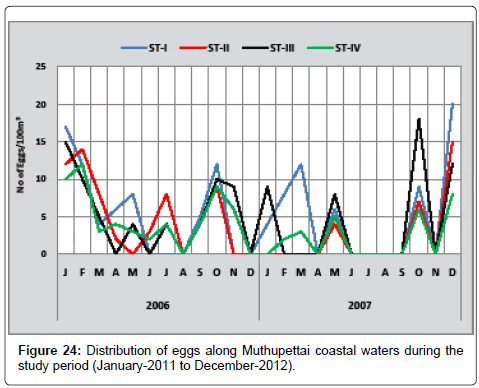
Liza dussumieri: The seasonal distribution of eggs of Liza dussumieri is depicted in Figure 24. The minimum densities (2 eggs/100 m3) were observed in April at station II and maximum (17 eggs/100 m3) in January at station I. During the second year, the minimum number eggs (4 eggs/100 m3) were observed in December and May at station I and II respectively, maximum (20 eggs/100 m3) in December at station I.
However Bensam [8] observed only during pre monsoon season and Ramaiyan [15] observed this egg during post monsoon, summer, and early months of monsoon is the support of the present findings.
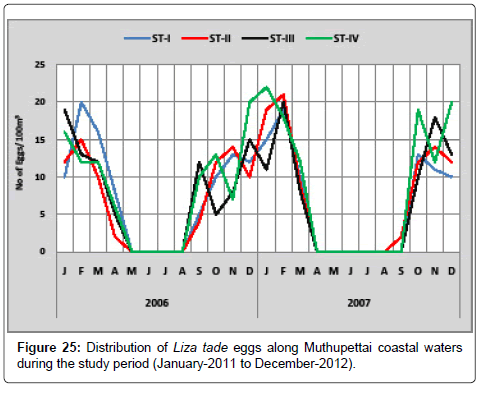
Liza tade: Figure 25 shows the seasonal variation of Liza tade eggs along Muthupettai coastal waters. During the first year, the minimum density was observed in April (2 eggs/100 m3) at station II and maximum (20 eggs/100 m3) in February and December at station I and IV respectively. During the second year, the minimum density (2 eggs/100 m3) was in September at station II and maximum in January (22 eggs/100 m3) at station IV. Eggs of Liza tade were noticed by [8] reported these eggs during pre monsoon season along Parangipettai coastal waters, Ramaiyan [15] observed these eggs in the post monsoon and summer seasons along the Cuddalore coast, Krishnamurthy [25] have reported the occurrence throughout the year in Pitchavaram mangrove area.
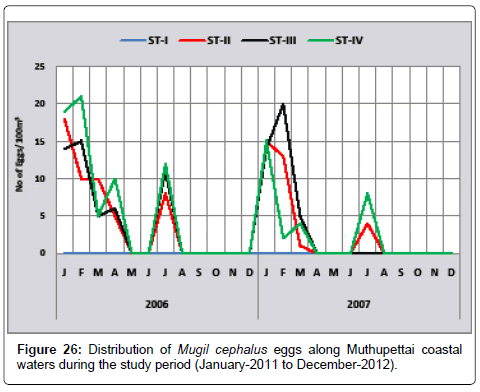
Mugil cephalus: Figure 26 shows the seasonal variation of Mugil cephalus eggs along Muthupettai coastal waters. The minimum density of these eggs were collected during April (5 eggs/100 m3) at station II and maximum density was observed during February (21 eggs/100 m3) at station IV during the study period of first year. In the second year single egg was obtained in March at the station II and maximum (20 eggs/100 m3) in February at station III.
The present observation agrees with the previous findings of Manickasundaram [11] studied during January to April and July to October along Coleroon estuary, Thangaraja [12] recorded during August from Vellar estuary, Venkataramanujam [17] recorded the eggs during pre monsoon and post monsoon seasons, Kowtal [19] noticed that these eggs during July to August from Chilka lake.
Family: Hemiramphidae
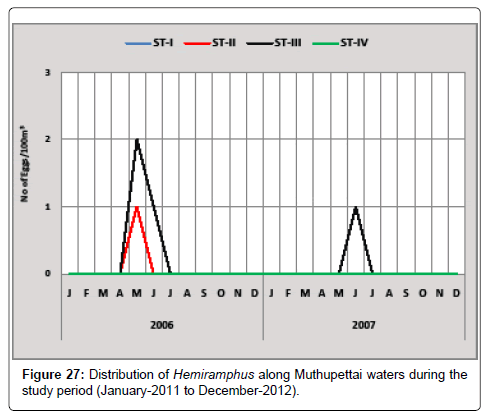
Hemiramphus sp.: A single egg of Hemiramphus sp. was observed in February at station II during the second year of study. Single egg observed in station III during May, and (2 eggs/100 m3) observed in the same month at the station III. During the second year of the investigation single egg was observed in the month of June at the station III (Figure 27).
Manickasundaram [11] reported another type of Hemiramphid eggs during the summer season and Ramaiyan [15] observed this egg during February along Parangipettai coastal waters.
Family: Atherinidae
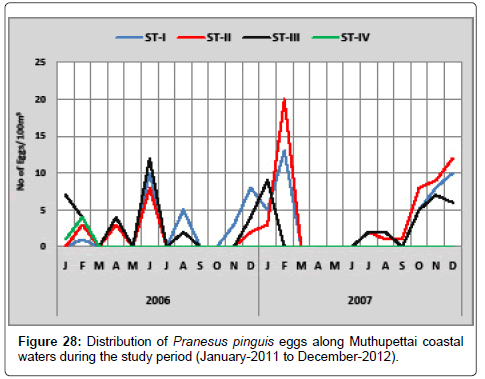
Pranesus pinguis: Figure 28 gives the details of the distribution of Pranesus pinguis eggs along Muthupettai coastal waters. During the first year, single egg was observed in the month of February at station I and maximum (12 eggs/100 m3) in June at station III. However, during the second year, the minimum density of single egg observed in September at station I and maximum (20 eggs/100 m3) in February at station II.
Present observation supported by the previous works made by Thangaraja [12]. But Thangaraja [12] recorded during March, May to September and October from Vellar estuary and Manickasundaram [11] reported during March to June and September to November along Coleroon estuary.
Family: Carangidae
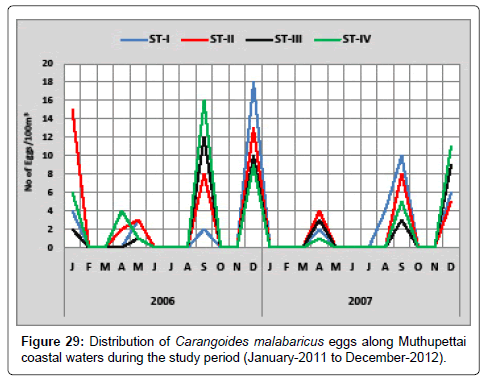
Carangoides malabaricus: The distribution of Carangoides malabaricus eggs is given in Figure 29. During the first year, the minimum density of single egg observed in may at the station III and IV and maximum (18 eggs/100 m3) in December at station I. The minimum density (2 eggs/100 m3) of these eggs was collected during April at station I and maximum (10 eggs/100 m3) during September at the same station I in the second year.
Eggs of Perciformes were numerically abundant and collected almost throughout the year in the present investigation. Same observation was made by Venkataramanujam [17] along the Vellar estuary. During the post monsoon, summer and pre monsoon seasons the carangid eggs were more in number and they were collected in all the stations, as already reported by Manickasundaram [11] noticed during May to June from Coleroon estuary. But Rao [16] reported only during January along Madras coast. Venkataramanujam [17] from Vellar [18] from Rani [26] along Cochin backwaters also. Eggs of Carangoides malabaricus were observed during pre monsoon, monsoon and summer in the present study. Krishnamurthy [27] recorded only in summer from Pichavaram mangrove systems.
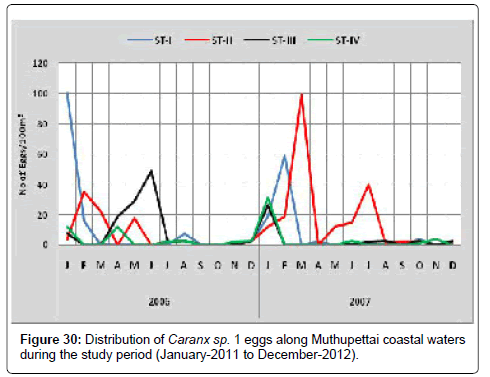
Caranx sp. 1: Figure 30 shows the seasonal distribution of Caranx sp. 1 egg along Muthupettai coastal waters. During the first year, the minimum density (2 eggs/100 m3) was recorded in July at station III and maximum (100 eggs/100 m3) in January at station IV. During second year of the investigation single egg was observed in December at station I and maximum (99 eggs/100 m3) in February at the same station I.
Caranx sp. 1 eggs were available throughout the year in the present study revealed some clear trends. This result obtained in the present study confirms that these fishes are continuous spawners. Similar observation made by Venkataramanujam [7] along Vellar estuary and [11] from Coleroon estuary, [18] from Mandapam region, [19] from Chilka Lake.
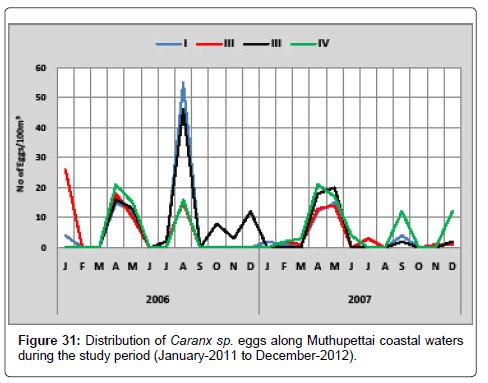
Caranx sp. 2: Figure 31 shows the seasonal distribution of the eggs of Caranx sp. 2 along Muthupettai coastal waters. During the first year of the study period, the minimum density (2 eggs/100 m3) were in July at station II and maximum (55 eggs/100 m3) in August at station I. In the second year of the study a single egg observed in the month of February and March in the station I and maximum (21 eggs/100 m3) in April at station IV.
Manickasundaram [11] observed throughout the year along Coleroon estuary was agreed and Venkataramanujam [17] observed during January to July and September to November along Parangipettai coastal waters with the present investigation.
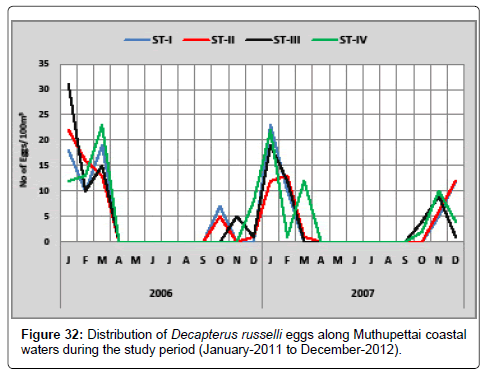
Decapterus russelli: Figure 32 shows the seasonal distribution of the eggs of Decapterus russelli along Muthupettai coastal waters. During the first year, the minimum density (2 eggs/100 m3) was observed in December at station II and maximum (23 eggs/100 m3) in May station IV. During the second year, a single egg was observed in March at station I and maximum density (23 eggs/100 m3) in January at station I.
Decapterus russelli eggs were observed during post monsoon, and monsoon seasons in the present study. However, Manickasundaram [11] noticed during post monsoon season along Coleroon estuary and Ramaiyan [15] observed these eggs in the monsoon and post monsoon seasons was in agreement of the present findings.
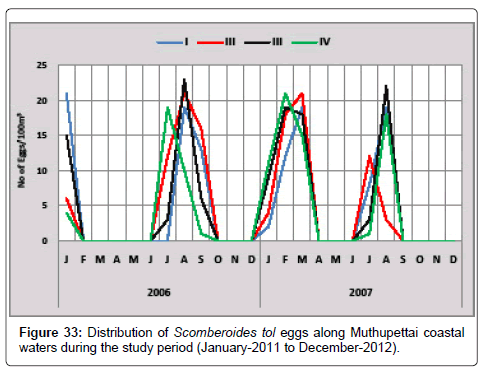
Scomberoides tol: Figure 33 shows the monthly variation of Scomberoides tol eggs along Muthupettai coastal waters. During the first year, a single egg was collected in September at station IV and maximum (23 eggs/100 m3) in April at station III. During the second year, the minimum density (4 eggs/100 m3) was in January at station II and maximum (22 eggs/100 m3) in August at station III.
Scomberoides tol eggs were collected in this investigation during pre monsoon, and post monsoon season. Similar observation made by Manickasundaram [11] along Coleroon estuary and Ramaiyan [15] along Parangipettai waters.
Family: Leiognathidae
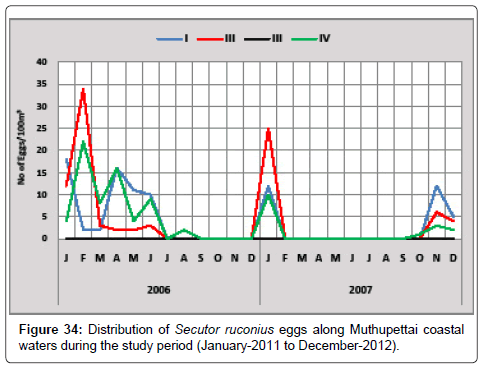
Secutor ruconius: Figure 34 gives the details of the distribution of Secutor ruconius eggs along Muthupettai coastal waters. During the first year, the minimum density (2 eggs/100 m3) was observed in February at station I and maximum (34 eggs/100 m3) in February at station II. The minimum density (4 eggs/100 m3) were in December at station I and maximum (25 eggs/100 m3) in January and February at station I and II respectively in second year of investigation.
Venkataramanujam [7] noticed during post monsoon and summer, Nair [12] observed only during monsoon along Vellar estuary. Krishnamurthy [25] observed in monsoon, post monsoon and summer seasons along Pitchavaram mangrove systems support the present study.
Family: Gerreidae
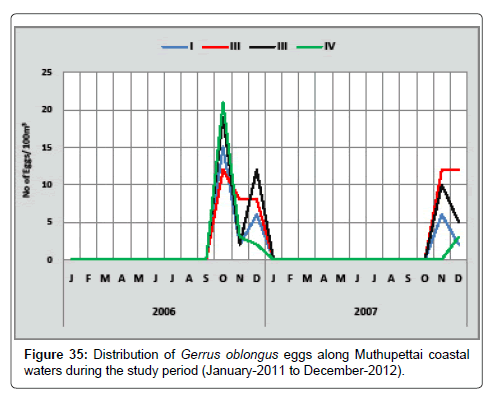
Gerrus oblongus: The distribution of the eggs of Gerrus oblongus is detailed below (Figure 35). During the first year, the minimum number of eggs (2 eggs/100 m3) was collected in December at station IV and maximum (21 eggs/100 m3) in October at station IV. During the second year, the minimum density (2 eggs/100 m3) was in December at station I and maximum (12 eggs/100 m3) in November and December at station II.
Gerrus oblongus eggs were observed monsoon season only in the study period. It is with the agreement of [8] along the Parangipettai waters. But Ramaiyan [15] noticed this egg during monsoon and post monsoon season.
Family: Teraponidae
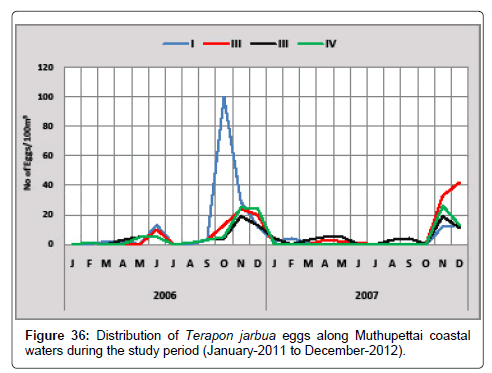
Terapon jarbua: Figure 36 gives the details of the distribution of eggs of Terapon jarbua along Muthupettai coastal waters. The minimum number of eggs (2 eggs/100m3) was observed in March at station I and maximum (100 eggs/100m3) in October at the same station. In the second year minimum density (2 eggs/100 m3) was observed in May at station I and maximum (42 eggs/100 m3) in December at station II.
Venkataramanujam [17] observed during post monsoon and summer season and annual spawning of these fish agrees with the previous works on the east coast by Bensam [8] observed during post monsoon, Manickasundaram [11] noticed almost throughout the year along Coleroon estuary, Thangaraja [12] noticed almost throughout the year along Vellar estuary [18-20] and this present study agreed with the observation was already made by Krishnamurthy [25] along Pitchavaram mangrove systems and Thangaraja [28] observed during April to June, August to October.
Family: Scombridae
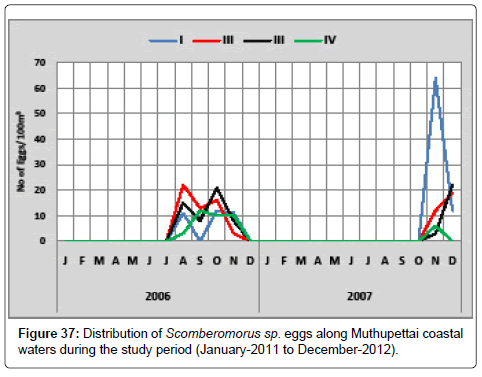
Scomberomorus sp.: The distribution of the eggs of Scomberomorus sp. is given in Figure 37. During the first year, the minimum density (3 eggs/100 m3) was observed during November at station II and maximum (22 eggs/100 m3) were in August at station II. During the second year, the minimum density (3 eggs/100 m3) were observed in November at station III and maximum density (64 eggs/100 m3) in November at station I.
Scomberomorus sp. eggs were collected during pre monsoon, and monsoon months from Muthupettai coastal waters. But Venkataramanujam [17] noticed only during November along Vellar estuary, Ramaiyan [15] noticed these eggs pre monsoon and summer along Parangipettai waters.
Family: Bothidae
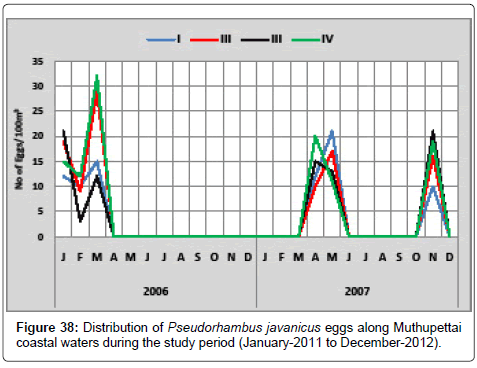
Figure 38 gives the summary of Pseudorhambus javanicus eggs observed during the study period along Muthupettai coastal waters. During the first year, the minimum density (3 eggs/100 m3) was observed during February at station IV and maximum (32 eggs/100 m3) in March at station IV. In the second year of the investigation minimum (10 eggs/100 m3) observed in the month of April at the station II and maximum (21 eggs/100 m3) observed May and November at the station IV and I respectively.
Pseudorhambus javanicus eggs were collected only in the post monsoon and summer seasons from Muthupettai waters. Similar observations were made by Manickasundaram [11] along Coleroon estuary and [7,17] along Vellar estuary.
Family: Pleuronectidae
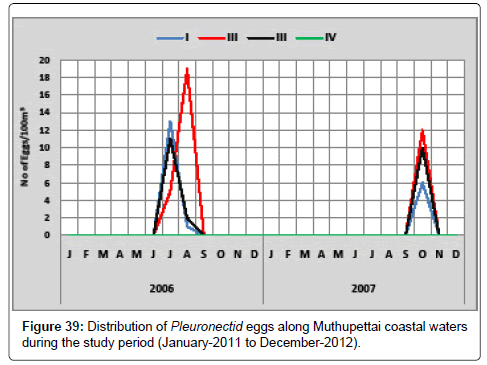
Distribution of Pleuronectid eggs in the study period (January-2011 to December-2012) along Muthupettai coastal waters is given below (Figure 39). Pleuronectid eggs were observed during April, minimum (1 egg/100 m3) at station I and maximum (19 eggs/100 m3) at station II, during the second year minimum. (6 eggs/100 m3) were observed in the month of October at the station I and maximum (12 eggs/100 m3) collected in the month of September at station II.
Ramaiyan [15] observed this egg during early month of summer is the support of the present investigation.
Family: Soleidae
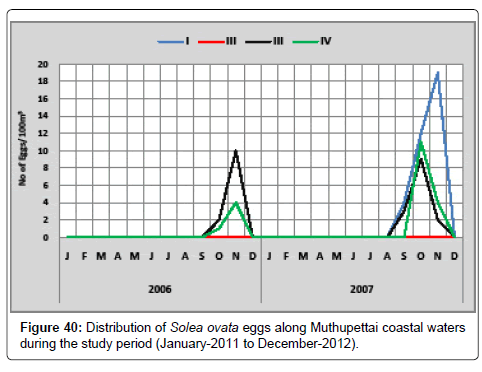
Solea ovate: The details of the distribution of eggs of Solea ovata during the study period of two years along Muthupettai coastal waters are given in Figure 40. During the first year, single egg observed during October at station IV and maximum (10 eggs/100 m3) was in December at station III. During the second year, the minimum density (2 eggs/100 m3) was observed in November at station III and maximum (11 eggs/100 m3) in October station IV.
Few Solea ovata eggs were noticed during the in monsoon and last months of pre monsoon season. Thangaraja [12] observed these eggs in summer season along Parangipettai waters. Ramaiyan [Rengarajan K, David raj I (1984) On ichthyoplankton of the Cochin Backwaters during spring tides. J Mar Biol Ass India 21: 111-118.
Citation: Selvam JD, Varadharajan AB, Balasubramanian T (2013) Distribution and Abundance of Finfish Eggs from Muthupettai, South East Coast of India. J Earth Sci Clim Change 5: 166. DOI: 10.4172/2157-7617.1000166
Copyright: ©2013 Selvam JD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16424
- [From(publication date): 1-2014 - Nov 21, 2025]
- Breakdown by view type
- HTML page views: 11334
- PDF downloads: 5090