Research Article Open Access
Effect of Dye Structure on the Decolorisation Efficiency of Candida Tropicalis and Bacillus Firmus
| Sucharita Arora1*, Harvinder Singh Saini2 and Kamaljit Singh1 | |
| 1Department of Applied Chemical Sciences and Technology, Guru Nanak Dev University, India | |
| 2Department of Microbiology, Guru Nanak Dev University, Amritsar – 143 005, India | |
| Corresponding Author : | Dr. Sucharita Arora Department of Applied Chemical Sciences and Technology Guru Nanak Dev University Amritsar-143 005, India. Tel: +91-183-2258853 Fax: +91183-2258819-20 E-mail: sucharitaarora@yahoo. co.in |
| Received: October 13, 2011; Accepted: November 18, 2011; Published: November 20, 2011 | |
| Citation: Arora S, Saini HS, Singh K (2011) Effect of Dye Structure on the Decolorisation Efficiency of Candida Tropicalis and Bacillus Firmus. J Bioremed Biodegrad 2:131. doi:10.4172/2155-6199.1000131 | |
| Copyright: © 2011 Arora S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Heterocyclic monoazo disperse dyes constitute an important class of textile colorants and are used in the coloration of hydrophobic substrates, with a large share of all the disperse dyes being used for coloration of polyester and its blends. In view of the dearth of effective methods, the need to develop protocols for decolorisation/ degradation of these dyes is fuelled. Decolorisation/degradation of heterocyclic monoazo disperse dyes were tested using Candida tropicalis and Bacillus firmus respectively isolated from dye contaminated soil samples and sludge of domestic seweage drain and the correlation of decolorisation with dye molecular structure has been drawn with the microbial decolorisation efficiency. The results reveal that there is a significant difference between the decolorizing ability of Candida tropicalis and Bacillus firmus respectively, under their respective conditions. Degradation of dyes by both these cultures was driven by a qualitatively visualized hydrophilic-hydrophobic balance of the chromophores as well as the extent to which a dye was adsorbed on cell mass.
| Keywords |
| Heterocyclic monoazo disperse dyes; Structure correlation; Microbial decolorisation; Biodegradation |
| Introduction |
| Dyes are complex aromatic molecular structures which are intended to be stable and consequently are difficult to degrade. It is estimated that approximately 2% of the dyes produced are discharged directly in aqueous effluent, and 10% is subsequently lost during the coloration process. Owing to the intense colors many dyes are visible in water at concentrations as low as 1 ppm [1]. The chemical classes of dyes employed more frequently on industrial scale are azo, anthraquinone, sulphur, indigoid, triphenylmethyl, and phthalocyanine derivatives. Among these, heterocyclic monoazo disperse dyes constitute an important chemical class of textile colorants and are used in the coloration of hydrophobic substrates, with a large share of all the disperse dyes being used for the coloration of polyester and its blends. In comparison with their benzenoid and/or anthraquinonoid counterparts, heterocyclic dyes are technically superior in terms of tinctorial strength, brightness, and ease in dischargeability and wider color gamut. However, a fairly large amount of disperse dyes (10-15%) is released in the effluent during wet processing, and the dyes being hydrophobic resist degradation in aqueous medium. The stringent requirements of environmental agencies for adopting green processes has initiated developments in effective treatment technologies ranging from purely physical to chemical; which however, are limited by the disadvantages of higher implementation cost, generation of greater volumes of solid waste etc. Interest is therefore focused on the microbial biodegradation of dyes. Over the past decade, biological decolorisation has been investigated as an economic method to transform, degrade or mineralize the dyes. A variety of microorganisms (bacteria, fungus, algae, yeast, etc.) have been implicated in decolorizing a range of dyes [2]. As a result of the high stability to microbial attack, many of these dyes/by-products remain colored for a long period of time in wastewaters. Significant amounts of partially degraded disperse dyes are released in the effluent even after biological treatment. Thus, attention to the development of effective means of their disposal and decolorisation is urgently warranted. |
| In general, dye decolorisation/degradation using microorganisms depends strongly on the dye structure and the nature and position of substituents on the chromophore. Dyes with simple structure and low molecular weight exhibits higher rates of color removal in comparison with highly substituted, high molecular weight dyes [3]. It is further supported by the study conducted by Hsueh et al. [4] on understanding the effects of chemical structure on azo dye decolorisation characteristic by Aeromonas hydrophila which revealed that both the positions of substituents on the aromatic ring and the electronic characteristics of substituents in azo dyes significantly affect the performance of biodecolorisation of A. hydrophila [4] Although, there are many structure based methods to reveal biodegradation as well as biodegradability, there is always a need for significant database from experiments to obtain quantitative correlations. The specificity of Orange II reductase isolated from Pseudomonas strain KF46 towards various orange azo dyes was investigated by Zimmermann et al. and it has been reported that specificity of Orange II reductase is strongly dependent on the properties of functional groups in the proximity of azo linkage and thus determined whether the dye is susceptible to biodecolorisation [5]. A number of researchers have correlated the level of color removal with the dye class rather than with molecular features [6,7]. The qualitative/ quantitative correlation between the dye molecular structure and biodegradability is thus required to understand the mechanism of dye molecule bio-reduction and also to predict the biodegradability of correlative dyes in the biological system [8]. |
| In comparison with their water-soluble counterparts, reports on the degradation of disperse dyes are extremely limited and to date, biological decolorisation of a heterocyclic monoazo disperse dyes and the correlation of biodecolorisation with dye molecular structure of disperse dyes has received only scanty attention. In view of this, the present investigation on decolorisation/degradation of heterocyclic monoazo disperse dyes has been achieved using microbial cultures and the correlation of decolorisation with dye molecular structure has been drawn with the microbial decolorisation efficiency. The choice of the dyes was guided by the fact that these model heterocyclic monoazo disperse dyes possess structural features of the commercial dyes and were purified, and characterized unambiguously, for facilitating identification of the degradation products. |
| Materials and Methods |
| Dyes and chemicals |
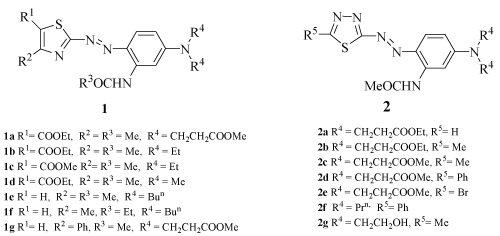
| Fourteen pure hetrerocyclic monoazo disperse dyes (1a-1g and 2a-2g) selected for present investigation were synthesized and characterized in our lab. The synthesis and characterization of dyes 1/2 has already been reported [9-11]. All other chemicals used in this investigation were of LR/AR grade and used as procured from Merck Ltd., Hi-media Labs, Bombay (India), CDH & Qualigens etc. Heterocyclic monoazo disperse dye 1a (Figure 1) was used as model dye for isolation and storing the cultures on agar slants. |
| The stock solution (3000 ppm) of dyes were made in ethanol and were sterilized by passing through (0.2μm pore size, 5mm diameter) Acrodisc sterile syringe filters (Pall Corporation, USA). For drawing structure decolorisation correlations, some dye intermediates were required, the same have also been synthesized using reported methods [10]. |
| Medium composition |
| The mineral salt medium (MSM) was used for decolorisation studies. The composition of MSM in g/L is as follows: Na2HPO4 (3.6), (NH4)2SO4 (1.0), KH2PO4 (1.0), MgSO4 (1.0), Fe(NH4) citrate (0.01), CaCl2 (0.10), containing 10 ml/L of the trace element solution consisting (mg/L) of ZnSO4.7H2O (10.0), MnCl2.4H2O (3.0), CoCl2.6H2O (1.0), NiCl2.6H2O (2.0), Na2MoO42H2O (3.0), H3BO3 (30.0), CuCl2.2H2O (1.0). The stock solutions of glucose (50% w/v) and yeast extract (10% w/v) were sterilized separately and added to MSM to maintain final concentration of 0.5% (w/v) and 0.01% (w/v), respectively. The final pH of the MSM solution was adjusted to 7.0 using 1N NaOH or 1N HCl solution. The sterilization of the medium was carried out in autoclave at 15 psi pressure at 120Ã?Â?C for 15-20 min to ensure the complete killing of all the microorganisms in the medium. |
| Microorganisms |
| The microbial cultures, Culture A (identified as Candida tropicalis, MTCC number 4690) and Culture B (identified as Bacillus firmus, MTCC number 7634), used in the study were isolated from the homogenized dye contaminated soil samples of a local wet processing house and from sludge of a domestic sewage drain by routine microbiological techniques. Dye 1a was chosen as model dye for enrichment and isolation of potential microbial isolates. The pure cultures were stored on MSM dye 1a agar, glucose yeast extract agar and nutrient agar dye 1a slants at 4Ã?Â?C and were regularly sub-cultured on fresh MSM at an interval of fifteen days. |
| Cultivating conditions |
| Culture A and Culture B were grown in 50ml MSM for 16h (log phase) and 12h (log phase), respectively at 30Ã?Â?C on orbital shaker at 100rpm. For the sake of uniformity, the initial optical density (OD600) of the cultures was maintained at 3.96 for Culture A and 1.2 for Culture B throughout further experimentation. |
| Decolorisation |
| For static and anoxic decolorisation experiments, the freshly grown colonies of Culture A from glucose yeast extract agar plate were inoculated into sterilized MSM. The initial pH of the MSM was set at 7.0. After 16 h of incubation at 30Ã?Â?C, under shaking conditions, the activated culture was transferred into the flasks and supplemented with sterilized dye (50 mg/L). The flasks sealed with parafilm were incubated at 35Ã?Â?C, under static condition for 12 days. |
| For aerobic decolorisation, the sterilized MSM supplemented with sucrose (0.125% w/v), ammonium sulphate (0.05% w/v) and yeast extract (0.0025% w/v) was inoculated with overnight grown Culture B and the sterilized dye (50 mg/L) was introduced. The initial pH was set at 6.0. The flasks were shaken on orbital shaker (100 rpm) at temperature of 35Ã?Â?C for 96 hours. The abiotic controls containing dye and MSM without culture were also studied in both the cases. |
| At the end of the decolorisation experimentation, the flask contents were centrifuged at 8000 rpm for 10min and analyzed spectrophotometrically. The decolorized product was analyzed as described below. |
| Spectrophotometric and chromatographic analysis |
| The cell-free supernatant and pellet were separated and the pellet was extracted with n-butanol. The absorbance was recorded at 530 nm, against MSM as reference for cell-free supernatant and n-butanol as reference for butanol extract from pellet. |
| The biotic decolorisation (%) is deduced by subtracting the sum of the residual dye left in the cell-free supernatant (Cs) and adsorbed on the pellet (Cp) from the initial dye concentration (Ci) using equation 1. |
 |
| where Cs = Concentration of dye in the cell-free supernatant (mg/L) |
| Cp = Concentration of dye adsorbed on the pellet (mg/L). |
| The progress of the biotransformation during the decolorisation experiments was monitored by thin layer chromatography (TLC). Also comparison of the reaction products with authentic samples was made using TLC. The cell free extract was extracted with ethyl acetate to extract the biotransformed intermediates of dyes. Precoated aluminium sheets from Merck (60F254, 0.2mm) were used. TLC was run in hexane, ethyl acetate, chloroform, methanol and their mixtures and the chromatograms developed were visualized under UV light. |
| Results and Discussions |
| Isolation and identification |
| The effectiveness of the isolated cultures to decolorize the dyes was assessed by plating the enriched culture on dye agar plates which showed clear zones around the colonies indicating the decolorisation of the dye. Further characterization and identification of these cultures was done at Microbial Type Culture Collection and Gene Bank (MTCC), Institute of Microbial Technology (IMTECH), Chandigarh, India. Culture A was identified to be Candida tropicalis (MTCC number 4690) and Culture B was identified as Bacillus firmus (MTCC number 7634). |
| Decolorisation of disperse dyes with Culture A under static and anoxic conditions |
| Fourteen heterocyclic monoazo disperse dyes (1/2) were selected. The potential of Culture A to decolorize these dyes under static and anoxic conditions as well as Culture B under aerobic conditions are presented in table 1. With Culture A, Dye 1a showed complete decolorisation after 12 days of incubation. The cell-free supernatant as well as n-butanol extract of pellet did not show any residual dye. The detailed characterization of decolorised product formed has already been reported [12]. Dyes 1b and 1c showed maximum biotic decolorisation (92.4-98%) after 12 days of incubation. Dye 1f showed 67.5% of biotic decolorisation. However, dyes 1d, 1e and 1g were found adsorbed on the pellet maximum to the extent of 41.6%, 56.6% and 50% respectively. Dyes 2a-c, 2e and 2g showed more than 90% biotic decolorisation while dye 2d showed 75.1% dye adsorbed on the pellet. |
| Likewise with Culture B under aerobic conditions, dyes 1a, 1c showed maximum biotic decolorisation (above 80%) after 96 hours of incubation while dyes 2a-c, 2e showed more than 90% biotic decolorisation. Dyes 1b, 1d, 1g and 2g were found adsorbed to the extent of more than 84%. |
| Correlation of decolorisation of disperse dyes with molecular structures |
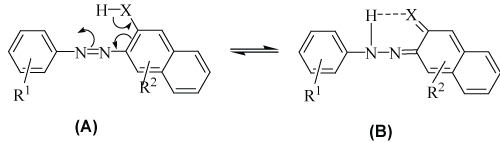
| There is a wide variability in the chemical structures of azo dyes. Both the diazo as well as coupling components may be aromatic or heterocyclic or a combination of the both. A number of hybrid molecules encompassing a heterocyclic diazo component and a benzenoid coupler have been successfully implemented and have enjoyed relatively greater success owing to their technical superiority over the former two categories. Such structural variations may alter the color output by inducing ‘push-pull’ of the electron density over the chromophore and also determine the technical performance when applied on textiles. Such features are also reflected in their tendency to undergo a variety of chemical transformations such as reductive cleavage of the azo linkage, especially when the ‘push-pull’ is strong. The substituents on both the diazo and coupler components e.g. o-hydrogen bonding substituents have been found to stabilize the quinone hydrazone tautomer over the corresponding azo tautomer (Figure 2) [13]. |
| In order to draw an insight into the effect of structure on the decolorisation efficiency of the cultures A and B, the dyes 1a-g and 2a-g (Figure 1) were selected which have been previously prepared and characterized [9,10]. |
| In comparison to 1a which, using culture A, under static and anoxic conditions, depicted complete decolorisation, the decolorisation of 1b appended with electron donating ethyl groups at the coupler side chain was in the range 92.4 to 98%. The residual dye both in the aqueous extract as well as adsorbed on pellet was in the range 0.5-1.7% and 1.2 to 5.7%, respectively. Dye 4c with similar substitution pattern as 1b, except for the fact that it was a methyl ester (R1 = COOMe) observed a similar order of decolorisation as 1b (R1 = COOEt). However, the residual dye in the cell pellet was more (4.9%) in comparison to the aqueous extract (1.5%). Since 1b and 1c, in comparison to 1a lack ester functionality on the coupler side chain would not be extracted into aqueous extract as its hydrolyzed carboxylate. Dye 1d also showed a similar extractive behaviour, although it depicted poor decolorisation (57.8%). |
| Dyes 1e-g are more hydrophobic owing to the presence of either of a n-butyl chain, on the coupler (1e and 1f) or a phenyl group at C4 of the thiazole ring in case of 1g were expected to degrade to a smaller extent owing to the hydrophobicity. Indeed, the decolorisation was found to be 43.2%, 67.5% and 48.1%, respectively. |
| Further, in these dyes, degradation seems to be affected by the adsorption on the pellet. When the dye adsorbed on the pellet is more (1d, 1e and 1g), the overall degradation decreases in comparison to other counterparts where dye adsorption on the pellet is less (1a-c, 1f). A similar type of correlation is observed in the 2a-g. Dyes 2a-c, 2e and 2g are less hydrophobic as compared to 2d and 2f, and consequently are degraded to much higher extent as compared to 2d and 2f and are thus expected to degrade more in accordance with the observations of 1a-g. |
| A similar trend is observed in case of decolorisation with culture B, the degradation corresponded to the hydrophobic- hydrophilic balance as well as the extent to which the dye was adsorbed on the cell pellet. A slight deviation in case of 1b and 1d in the decolorisation with culture B, in comparison to the decolorisation with culture A, may be argued to be arising due to toxicity of these dyes to the culture B. |
| Conclusion |
| Thus, from the above investigation it has been concluded that there is a significant difference between the decolorizing ability of both the cultures, under their respective conditions. |
| Another aspect of this investigation was to correlate decolorisation of some heterocyclic monoazo disperse dyes. The results reveal that degradation of dyes by cultures A and B was driven by a qualitatively visualized hydrophilic-hydrophobic balance of the chromophores as well as the extent to which a dye was adsorbed on cell mass. For instance, more than 50% of the dyes 1d, 1e and 1g (table 1) were adsorbed on the biotic mass of culture A and thus depicted lower extent of biotic decolorisation. Similar trends have been revealed by culture B also. |
| Acknowledgements |
| KS thanks Ministry of Environment and Forests for the research grant (No. 19/24/2008-RE). |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 13967
- [From(publication date):
November-2011 - Nov 15, 2025] - Breakdown by view type
- HTML page views : 9253
- PDF downloads : 4714
