Electrode Placement and Electroejaculation of Blue Swimmer Crab, Portunus pelagicus (linnaeus)
Received: 10-Apr-2013 / Accepted Date: 28-Jun-2013 / Published Date: 05-Jul-2013 DOI: 10.4172/2157-7617.1000142
Abstract
Many workers emphasized the usefulness of electroejaculation technique in artificial insemination studies for hybridization and selective breeding programmes in commercially important decapods. Such techniques are also used to improve the seed production. Similar technique is very much lacking in crabs in general and P. pelagicus in particular. So that electrical stimulation on spermatophore expulsion is studied in P. pelagicus. Among six positions the bilateral ejaculation (extruding spermatophores in both sides) was occurred only in positions I & VI. In all three trials the animals were bilaterally ejaculated 100% in position VI. However in position I the bilateral ejaculation was 66.66%. In other four positions (II, III, IV and V) the ejaculation was happened unilaterally (extruding spermatophores in one side) especially in probe attachment side in all three trials. In positions II & IV all the animals were extruded unilaterally in all the three trials whereas in positions III & V the unilateral ejaculation was 66.66% & 83.33% in trial I, 83.33% & 66.66% in trials II & III respectively. The average volts and seconds required for extrusion of spermatophores was ranged from 5.75 to 7.75 volts and 3.83 to 7.70 seconds respectively. Spermatophore weight was ranged from 33.0 to 105.61 mg.
Keywords: Male crabs; P. pelagicus; Electroejaculation; Technique; Spermatophore
9701Introduction
Crab culture gained its importance from the beginning of last decades due to great demand of live crabs and crab products in the export market [1]. The crab culture in India is presently dependent on wild caught seeds that are not sufficient [2]. The natural seed availability is declining due to indiscriminate collection of juveniles for farming. The collected seeds are not uniform in size and availability throughout the year is a big question mark [1]. To solve this problem year round seed production is very much essential. The mating is most essential key factor for the availability of berried females throughout the year for year round seed production. But it is not possible from natural mating with an unresponsive male (or) female. So that electroejaculation and artificial insemination is only solution for practical application of mass seed production. Some of the works are already carried out in electroejaculation of decapod crustaceans [3] in M. rosenbergii [4,5] in H. americanus [6] in P. setiferus and P. vannamei [7] in M. idella; [8,9] in M. malcomsonii. This type of work is lacking in crabs in general and blue swimming crabs in particular. Electroejaculation of spermatophores is the first step towards accomplishing the goal of developing artificial insemination technique. Hence, in the present study an attempt has been made to examine the suitable position for electroejaculation to get spermatophore in P. pelagicus which leads to year round seed production.
Materials and Methods
The adult male crabs of P. pelagicus were collected from Parangipettai landing centre (Lat. 11° 29'N and Long. 79° 46'E) to determine the suitable position for electrode placement for electroejaculation of spermatophores. The crabs were acclimatized for 48hr in sterilized and filtered seawater (salinity 30-32 ppt; temperature 26-31°C; pH 7.6-8.3 and dissolved oxygen close to saturation 5-6 mg L-1, photophase 12/12 h L/D). During this period the crabs were fed with a mixture of shrimp and clam meat. Fifty percent of seawater was exchanged daily. Uneaten and faceas were siphon out every morning while water exchange. Once the acclimation period was over the crabs were electrically stimulated. The apparatus and methodology used for extrusion of spermatophore was as per (Figure 1) [9]. The following positions were tested for electrode placement by applying an electric shock of 3.0V DC. When no expulsion was achieved, the shock was repeated with a progressively increasing intensity of 4.5, 6.0 and 7.5 DC until the expulsion of spermatophores. For each position six crabs and totally thirty six crabs were tested.
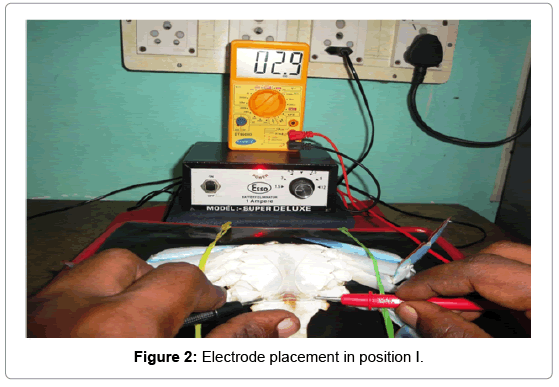
Position I: A single cathode probe was attached to the right side inner coxa of the swimming peddales and an anode was attached to the left side inner coxa of the swimming peddales (Figure 2).
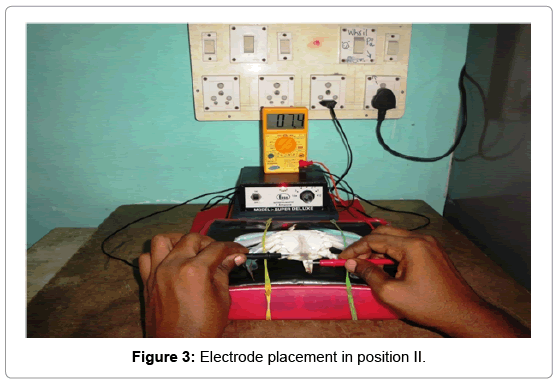
Position II: A single cathode probe was attached to the right side inner coxa of the swimming peddles and anode to the inner abdominal flab upon the hind gut (Figure 3).
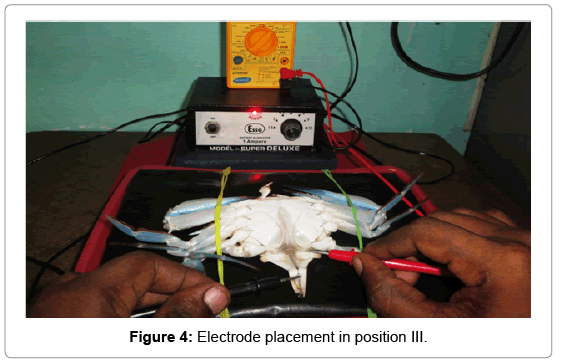
Position III: A single anode probe was attached to the left side inner coxa of the swimming peddles and a cathode to the inner abdominal flab upon the hind gut (Figure 4).
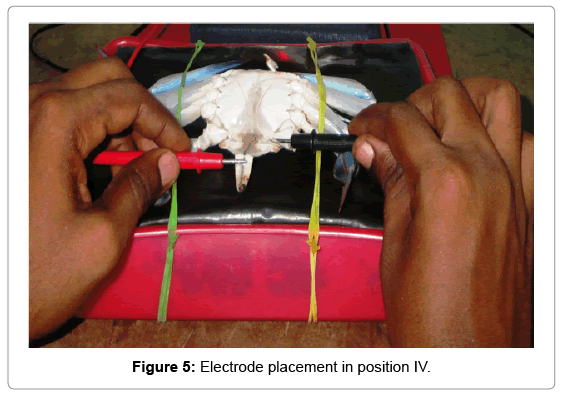
Position IV: A single cathode was attached left side inner coxa of the swimming peddles and an anode to the inner abdominal flab upon the hind gut (Figure 5).
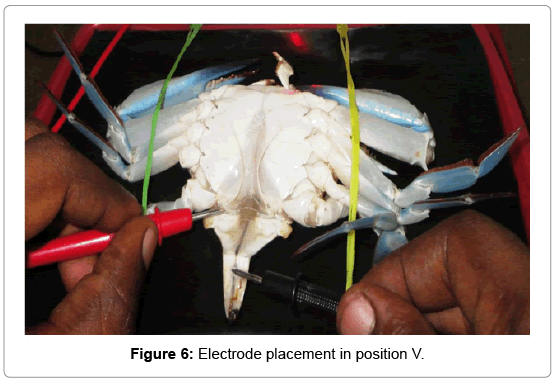
Position V: An anode probe was attached to the right side inner coxa of the swimming peddales and a cathode to the inner abdominal flab upon the hind gut (Figure 6).
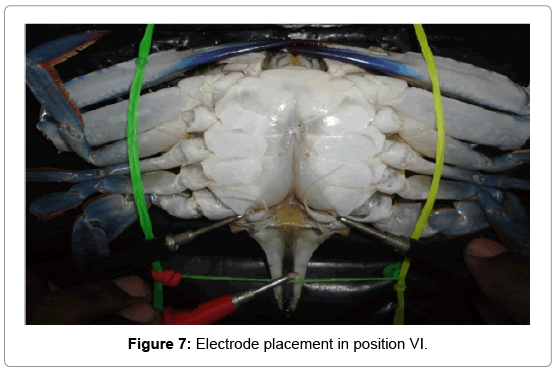
Position VI: A pair of digital cathode probes was attached to the inner areas of coxa of both swimming peddales and anode to the inner abdominal flab upon the hind gut (Figure 7).
The same positions were repeated for another two times with 5 days interval. The spermatophores ejaculated from the stimulated crabs were weighed with digital electronic balance.
Results
Among six positions the bilateral ejaculation (extruding spermatophores in both sides) was occurred only in positions I and VI. In all three trials the animals were bilaterally ejaculated 100% in position VI. However in position I the percentage of bilateral ejaculation was 66.66 in all three trials. In other four positions (II, III, IV and V) the ejaculation was happened unilaterally (extruding spermatophores in one side) especially in probe attachment side in all three trials. In positions II & IV all the animals were extruded unilaterally in all the three trials whereas in positions III & V the percentage of unilateral ejaculation was 66.66 & 83.33 in Trial I, 83.33% & 66.66% in Trials II & III respectively (Tables 1-3).
| Position | Carapace width (mm) | Animal weight (g) | Volts | Seconds | Extrution % | Spermatophore weight (mg) | Total weight (mg) | ||
|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | ||||||
| I | 125.33 ± 4.34 | 122.83 ± 11.88 | 6.00 ± 0.54 | 3.83 ± 0.63 | 83.33 | 66.66 | 43.83 ± 6.21 | 24.16 ± 9.34 | 68.00 ± 13.84 |
| II | 126.83 ± 6.31 | 128.58 ± 23.75 | 5.75 ± 0.60 | 4.16 ± 0.37 | 100 | 0 | 49.50 ± 8.22 | - | 49.50 ± 8.22 |
| III | 128.83 ± 4.12 | 132.33 ± 18.69 | 6.75 ± 0.84 | 6.71 ± 0.87 | 0 | 66.66 | - | 33.00 ± 11.15 | 33.00 ± 11.15 |
| IV | 133.16 ± 4.57 | 148.00 ± 15.85 | 7.25 ± 0.60 | 6.18 ± 0.57 | 0 | 100 | - | 56.50 ± 7.80 | 56.50 ± 7.80 |
| V | 134.16 ± 4.35 | 145.50 ± 16.66 | 7.25 ± 0.90 | 6.59 ± 0.79 | 83.33 | 0 | 44.50 ± 10.92 | - | 44.50 ± 10.92 |
| VI | 128.66 ± 6.41 | 143.00 ± 21.79 | 6.25 ± 0.71 | 5.78 ± 1.09 | 100 | 100 | 53.11 ± 7.75 | 52.50 ± 8.32 | 105.61 ± 15.96 |
Table 1: Electroejaculation volts, seconds, spermatophore extrution and weight in trial I at various positions (The values are in mean ± SE).
| Position | Carapace width (mm) | Animal weight (g) | Volts | Seconds | Extrution % | Spermatophore weight (mg) | Total weight (mg) | ||
|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | ||||||
| I | 125.33 ± 4.34 | 126.16 ± 11.85 | 6.25 ± 0.46 | 4..41 ± 0.71 | 83.33 | 66.66 | 41.00 ± 4.97 | 20.16 ± 8.08 | 61.16 ± 12.17 |
| II | 126.83 ± 6.31 | 131.50 ± 24.48 | 5.75 ± 0.60 | 4.20 ± 0.27 | 100 | 0 | 45.16 ± 7.40 | - | 45.16 ± 7.40 |
| III | 128.83 ± 4.12 | 136.50 ± 18.94 | 7.25 ± 0.71 | 6.97 ± 0.89 | 0 | 83.33 | - | 38.33 ± 8.71 | 38.33 ± 8.71 |
| IV | 133.16 ± 4.57 | 151.33 ± 15.68 | 7.25 ± 0.60 | 6.36 ± 0.54 | 0 | 100 | - | 51.33 ± 7.09 | 51.33 ± 7.09 |
| V | 134.16 ± 4.35 | 149.33 ± 16.91 | 7.75 ± 1.18 | 7.34 ± 1.14 | 66.66 | 0 | 34.83 ± 12.17 | - | 34.83 ± 12.17 |
| VI | 128.66 ± 6.41 | 147.00 ± 21.97 | 6.50 ± 0.63 | 6.03 ± 1.11 | 100 | 100 | 48.00 ± 7.13 | 47.66 ± 7.66 | 95.66 ± 14.62 |
Table 2: Electroejaculation volts, seconds, spermatophore extrution and weight in trial II at various positions (The values are in mean ± SE).
| Position | Carapace Width (mm) | Animal Weight (g) | Volts | Seconds | Extrution % | Spermatophore weight (mg) | Total Weight (mg) | ||
|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | ||||||
| I | 125.33 ± 4.34 | 129.00 ± 11.81 | 6.75 ± 0.51 | 5.20 ± 0.65 | 83.33 | 66.66 | 37.83 ± 4.43 | 18.83 ± 7.76 | 56.66 ± 11.56 |
| II | 126.83 ± 6.31 | 135.00 ± 24.67 | 6.00 ± 0.54 | 4.48 ± 0.47 | 100 | 0 | 40.50 ± 7.22 | - | 40.50 ± 7.22 |
| III | 128.83 ± 4.12 | 141.00 ± 19.05 | 7.75 ± 0.90 | 7.17 ± 0.96 | 0 | 83.33 | - | 36.33 ± 8.43 | 36.33 ± 8.43 |
| IV | 133.16 ± 4.57 | 155.66 ± 15.82 | 7.50 ± 0.67 | 6.70 ± 0.49 | 0 | 100 | - | 46.66 ± 5.93 | 46.66 ± 5.93 |
| V | 134.16 ± 4.35 | 153.50 ± 16.82 | 7.75 ± 1.18 | 7.70 ± 0.97 | 66.66 | 0 | 33.00 ± 11.61 | - | 33.00 ± 11.61 |
| VI | 128.66 ± 6.41 | 149.50 ± 21.80 | 6.50 ± 0.83 | 6.21 ± 1.09 | 100 | 100 | 46.33 ± 7.15 | 45.66 ± 7.89 | 92.00 ± 14.86 |
Table 3: Electroejaculation volts, seconds, spermatophore extrution and weight in trial III at various positions (The values are in mean ± SE).
From the results the average volts and seconds required for extrusion of spermatophores was ranged from 5.75 to 7.75 volts and 3.83 to 7.70 seconds respectively. Spermatophore weight was ranged from 33.0 to 105.61 mg.
Discussion
In general very limited studies are available on electroejaculation in decapods crustaceans (i.e. M. rosenbergi [3]; Homarus americanus [4,5]; Penaeus setiferus P. vennamei [6]; M. malcomsonii [9]). There is no published work on elctroejculation in crabs in general and commercially important portunid crabs in particular. Electrical stimulation proved to be an effective and simple method to noninvasively obtain spermatophores from male blue swimming crab P. pelagicus. From the observations of [3-10] the position of electrode placement seems to be an important factor for spermatophore expulsion.
In the present study, bilateral ejaculation was occurred in all the animals in position VI which attached a pair of digital probes as cathode to the inner area of coxa of the both swimming peddles and an anode to the inner abdominal flab upon the hind gut. So it is recognized as an ideal position for electrode placement. Similar type of electrode placement was reported to be suitable for electroejaculation by [10,11]. However some of the scientists reported that a cathode attached to the left side and anode to the right side of the coxa of the fifth pair of pereiopods is the suitable position for the expulsion of spermatophores [3-10].
By using the electroejaculation apparatus, it is possible to obtain spermatophores from majority of males in less than a few minutes. Because the technique used is to have no adverse side effects - at least in short term. It is quite possible to collect spermatophores from a single male on numerous occasions. Although electro-ejaculation procedure permitted the repeated spermatophore expulsion, the frequent application of electrical stimuli induced melanization of the reproductive tissue. There are two types of reproductive tissue melanizations. One is black melanization which is restricted to gonophores of ejaculam and the other one is red type of melanization which seemed to affect gonophores, ejaculatory duct and vas deferens. No infectious organisms or mortalities were associated with the melanized condition; the disorder is probably not fatal. However, melanized individuals were often impotent and frequently produced simple spermatophores. [7] observed tissues lysis in terminal ampoule of M. idella. [12] reproted only red type melanization which affected gonophores and ejaculum in M. malcomsonii. In the present study also melanization was observed in vas deferens, ejaculatory duct and gonophores after the final trial.
The relatively low success rate in complete expulsion of spematophore with electrical stimulation in positions II, III IV and V may be due to reproductive anatomy of the crab. Interestingly, [6,9] also attributed the same for the low success of complete expulsion of spermatophore with electrical stimulus in penaeid sp. and M. malcolmsonii respectively.
According to [4] when an electrical stimulus was applied to the musculature of vas deferens, it stimulated its contraction and induced spermatophore expulsion. In the present study positions II and V have only right side response, whereas in positions III and IV have only left side response. It may be attributed to the contraction of vas deferens right side in positions II and V and left side in positions III and IV. Whereas in all trials the animals were unilaterally ejaculated in positions II and IV, however in positions III and V has the percentage of unilateral ejaculation was 66.66% and 83.33% in Trial I, 83.33% and 66.66% in both Trials II & III. It’s clearly shows that the cathode position has more stimulation than the anode. So that, it is possible to suggest that cathode position supports more ejaculation than the anode side in positions III & V.
Each species of decapods crustaceans required a particular voltage to induce ejaculation, M. rosenbergii - 5 to 6 V [3], P. penicilatus 1.6 - 3.2 V (Lin, 1986), M. idella - 4.5 V [7] and M. malcolmsonii - 3.2 to 11.1 V [9]. The average volts required for extrusion of spermatophores in the present study was ranged from 5.75 to 7.75. A subthreshold current is also problematic, if insufficient stimulation is used to expel spermatophores. The spermatophores will be partially extruded and a portion will remain and harden in the distal portion of vas deferens, unless this plug is removed, further ejaculation will not be possible. Hardened plugs from partial ejaculations have never been observed in males allowed to mate normally [13]. Obtaining spermatophores from male crabs by electrical stimulation is the first step towards accomplishing the goal of developing artificial insemination and artificial fertilization techniques. However, to achieve this goal, further work is required to develop techniques for cryopreservation of electroejaculated spematophores.
References
- Soundarapandian P, Tamizhazhagan T (2009) Embryonic development of commercially important swimming crab Portunus pelagicus (Linnaeus). Cur Res J Biol Sci 1: 106-108.
- Keenan CP (1999) Aquaculture of the mud crab, genus Scylla - past, present and future. In: Mud crab aquaculture and biology. Proc Intl Sci Forum Darwin, Australia.
- Sandifer PA, Lynn JW (1980) Artificial insemination of caridean shrimp. Advances in Invertebrate Reproduction. Elsevier, North-Holland, Amsterdam, The Netherlands.
- Kooda-Cisco MJ, Talbot P (1983) A technique for electrically stimulating extrusion of spermatophores from the lobster, Homarus americanus. Aquacult 30: 221-227.
- Aiken DE, Waddy SL, Moreland K, Polar SM (1984) Electrically induced ejaculation and artificial insemination of the American lobster Homarus americanus. J Crust Biol 4: 519-527.
- Sandifer PA, Lawrence AL, Harris SG, Chamberlain GW, Stokes AD, et al. (1984) Electrical stimulation of spermatophore expulsion in marine shrimp Penaeus spp. Aquacult 41: 181-187.
- Joshi VP, Diwan AD (1992) Artificial insemination studies in Macrobrachium idella (Hilgendorf, 1898). Freshwater prawns: Proceedings of the National symposium on freshwater prawns (Macrobrachium spp.). Kerala Agricultural University, Trissur, India.
- Kannupandi T (1995) Development of technology for hatchery production in Macrobrachium malcolmsonii and M. rosenbergii. TNSCS&T Project Technical Report 51.
- Samuel MJ, Kannupandi T, Soundarapandian P (1998) Assessing the suitable position for electrode placement and electroejaculation on different size males of cultivable prawn Macrobrachium malcolmsonii (H. Milne Edwards). Indian J Exp Biol 36: 115-117.
- Lin MN (1986) Electrically induced ejaculation and spermatophore transplantation in the red tailed shrimp Penaeus penicillatus Alock. Proceedings of the symposium on Marine Biological Science, Biology Research Center, National Science Council Monograph series 14: 209-221.
- Lin MN (1985) Electrically induced ejaculation in the grass shrimp, Penaeus monodon Fabricius. (Unpublished).
- Samuel MJ (1996) Male reproductive biology, electroejaculation, invitro embryo culture and fatty acid profile during embryogenesis in freshwater cultivable prawn Macrobrachium malcolmsonii (H. Milne Edwards). Ph.D. Thesis, Annamalai University, Tamil Nadu, India.
- Talbot P (1984) Problems and progress in controlling reproductive biology in the American lobster (Homarus). Elsevier Advan Invert Rep 3: 473-480.
Citation: Soundarapandian P, Varadharajan D, Anand T (2013) Electrode Placement and Electroejaculation of Blue Swimmer Crab, Portunus pelagicus (linnaeus). J Earth Sci Clim Change 4: 142. DOI: 10.4172/2157-7617.1000142
Copyright: ©2013 Soundarapandian P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 18811
- [From(publication date): 7-2013 - Dec 23, 2025]
- Breakdown by view type
- HTML page views: 13730
- PDF downloads: 5081