Research Article Open Access
Enhancement of Intact- Ion Yield and Surface Sensitivity by Argon-cluster SIMS
Kozo Mochiji *Graduate School of Engineering, University of Hyogo, 2167 Shosha, Himeji, Hyogo 671-2280, Japan
- *Corresponding Author:
- Mochiji Kozo
Graduate School of Engineering
University of Hyogo, Japan
E-mail: mochiji@eng.u-hyogo.ac.jp
Received date: July 04, 2011; Accepted date: August 10, 2011; Published date: Augutst 13, 2011
Citation: Mochiji K (2011) Enhancement of Intact- Ion Yield and Surface Sensitivity by Argon-cluster SIMS. J Anal Bioanal Tech S2:001. doi: 10.4172/2155-9872.S2-001
Copyright: © 2011 Mochiji K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
In secondary ion mass spectrometry (SIMS) of organic samples, damages induced by the primary ions are crucial. The mass of intact ions (secondary ions not dissociated) currently detectable is only as high as the 1,000 Da at best, which prevents the technique from being extended to apply to organic molecules with larger mass. We developed SIMS equipment in which the primary ions were argon cluster ions having a kinetic energy per atom controlled down to 1eV. By applying this equipment to several peptides and proteins, the intensity of fragment ions was decreased by a factor of 102 when the kinetic energy per atom was decreased below 5 eV, and intact ions of cytochrome C (molecular weight: 12,327) and chymotrypsin (molecular weight: 25,000) were detected without using any matrix. Furthermore, we found that argon cluster ions sputter ultra thin layer (~ 2 nm) of organic material and enhance surface sensitivity in SIMS. Based upon the above results, a future prospect of argon-cluster projectile for SIMS is discussed.
Keywords
SIMS; Mass spectrometry; Cluster ion; Time of flight; Protein
Introduction
Various efforts have been made to apply secondary ion mass spectrometry (SIMS) to organic substances [1-3]. Recently, it is reported that use of polyatomic ions and cluster ions as the primary ions in place of atomic ions increases secondary ion intensity and is effective in suppressing damages to sample molecules [4-9]. When an accelerated cluster-ion collides with solid surface, it can induce multiple collisions by numerous constituent atoms, thereby making it possible to improve sputter yield and increase secondary ion intensity. The average level of kinetic energy per atom in the cluster ion is the value of the energy obtained by accelerating the cluster ion divided by the number of constituent atoms of the cluster ion (hereafter called cluster size). For this reason, the higher the number of constituent atoms is, the smaller will be the kinetic energy per atom, thus making it possible to decrease damage imparted to the sample. Until now, cluster ions of Aun + (typically n=1-5), SF5 +, and C60 + etc have been primarily used [4-7,9], but because of the small number of constituent atoms of these cluster ions, using applied acceleration techniques to decrease kinetic energy per atom down to ~10 eV has been difficult.
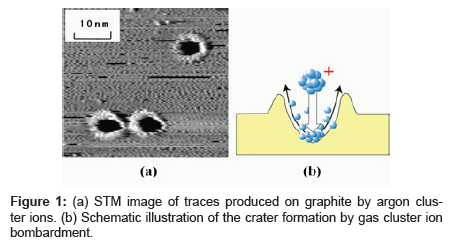
A gas cluster ion such as argon (Ar) cluster ion consists of gaseous atoms aggregated by Van der Waals forces and can be made from approximately 10,000 constituent atoms [10-12]. Contamination possibility is extremely low, as the Ar cluster ion quickly evaporates after bombarding the target, and the Ar atom is chemically inert. We analyzed the traces caused by argon (Ar) cluster ions bombarded on graphite as shown in Figure 1 [13,14]. The kinetic energy per atom of the cluster ion used for bombardment will almost completely determine the rate at which traces are produced as shown in Figure 2. A certain level of kinetic energy per atom or greater is required for causing traces, and this threshold energy is determined by the cohesive energy of the carbon atoms that constitute graphite. This result suggests that adjusting the kinetic energy per atom can suppress damage on the sample. For example, in using polymer as a target, decreasing kinetic energy per atom to the level below the dissociation energy of the chemical bond (3~5 eV) of the polymer suppresses polymer fragmentation.
We have newly developed SIMS equipment in which the cluster size of Ar cluster ions was selected to adjust the kinetic energy per atom of the cluster ion [15]. Because the cluster size of a gas cluster ion varies substantially in the formation stage, bombarding without selecting the size of the clusters may allow the variety of different cluster sizes to affect the sample. As a result of applying the new equipment to several peptides and proteins, the intensity of fragment ions of the sample molecules was decreased by a factor of 102 when the kinetic energy per atom of the incident Ar cluster ions was decreased below 5 eV, and intact ions of cytochrome C (molecular weight: 12,327) and chymotripsin (molecular weight: ~25,000) were detected without using any matrix [16].
Gas-cluster SIMS equipment
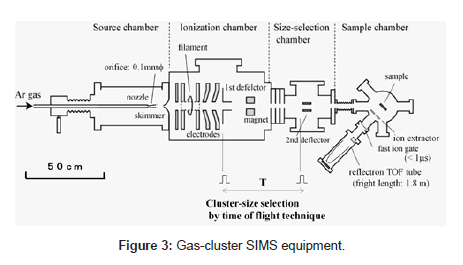
Details of the developed SIMS equipment, consisting of four chambers as shown in Figure 3, have been reported in other papers [15]. We summarize them here. The Neutral Ar cluster was generated by supersonic expansion from a nozzle with a 0.1-mm diameter aperture and a 60-mm expansion and cooling zone. The Ar gas was introduced into the nozzle at the stagnation pressure of 0.5~1.2 MPa. The cluster beam was collimated by a conical skimmer with an opening of 0.5 mm in diameter and then flowed into the ionizer. The neutral clusters were ionized by electron impact from a tungsten filament, and the cluster ions were then extracted at an accelerated voltage of 5~10 kV. Mono atomic ions and small ion components were removed from the original cluster ion beam with the magnet. Cluster-size selection by the TOF (time-of-flight) technique was performed using the two pairs of ion deflectors. The ion beam was chopped in a width of 10 ms by the two ion deflectors. The cluster size was determined from the flight time between the first ion deflector and the second ion deflector. The cluster size before size selection was widely distributed, from 500 to 7000 atoms/cluster. The cluster size distribution after size selection was significantly narrowed, and peak cluster-size became larger when the flight time was longer. A cluster size resolution (M/ΔM) over 10 was achieved by adjusting the pulse widths on the ion deflectors. As a result, we could control the kinetic energy per atom of incident cluster ions in the range of 1 eV and 25 eV.
The size-selected cluster ion beam entered the sample chamber through a 5-mm diameter aperture and focusing ion lens. Primary ion beam application to the samples and measurement of secondary ion were carried out under a pressure of 3 x 10-6 Pa and at room temperature. The secondary ions are measured by a reflectron with a flight tube length of 1.8 m (KNTOF-1800-SL203, Toyama, Kanagawa, Japan). For the precise measurement of ion flight time, an interleaved comb-type ion gate was installed in front of the reflectron. The secondary ions passing thorough the ion gate were detected by an MCP and the ion signals were accumulated by the multi-channel scalar, MCS (P7888-1, FAST ComTec, Oberhaching, Germany). A logic-pulse signal was applied to produce high-voltage pulses to the ion gate, and this signal was used as a start signal for the TOF measurement. In these conditions, the mass resolution (m/Δm) of the reflectron was approximately 200 at m/z of 700 when the pulse width of the ion gate was 100ns.
Detection of intact ions with high mass
We applied the gas-cluster SIMS equipment to some kind of organic samples including peptides and proteins. All samples were thin films formed on a silicon substrate and no matrix agent was used. A water solution of each chemical (1~2 mg/l) was dropped on a silicon substrate and dried at room temperature in air, or freeze-dried in a vacuum. The film thickness of each sample was found to be in the range of 50~500 nm by measuring with a step profiler.
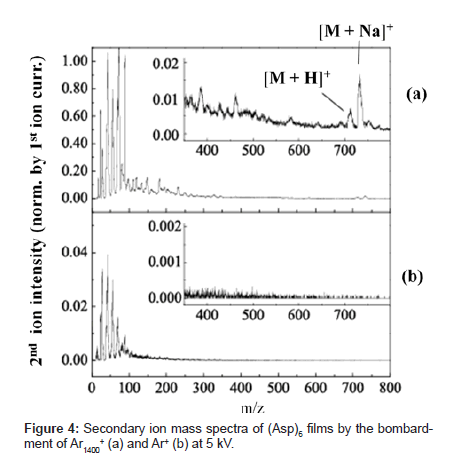
Figure 4 shows secondary ion mass spectra produced when the same acceleration voltage of 5kV is applied to Ar cluster ions in a cluster size of 1,400 (Ar1400+) and Ar mono-atomic ion (Ar+) and these ions bombard a 6-mer peptide of aspartic acid (Asp)6 (molecular weight: 709). The primary ion current applied to each sample normalizes the secondary ion intensity referred in this report. With cluster ion bombardment, secondary ion intensity increased as compared with mono-atomic ion bombardment. The rate of increase was approximately 10 times for the fragment near m/z 100 and below, but the rate was much higher with a greater fragment. Several ion peaks appeared near m/z 700 in the spectrum created by cluster ion bombardment. Of these peaks, the peaks at m/z 710 and 732 indicate intact ions ([M+H]+ and [M+Na]+) with H+ or Na+ attached to (Asp)6 molecules (Figure 4 insert). With mono-atomic ion bombardment, the signal of these intact ions was not distinguishable from the background level.
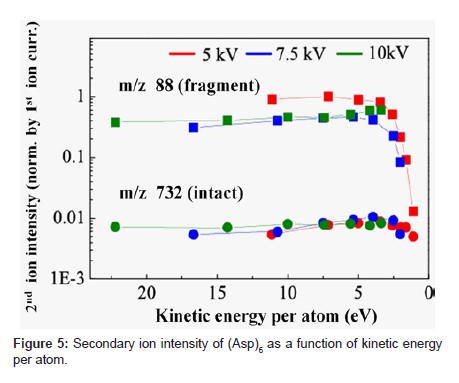
Figure 5 shows a plot of the intensity of fragment ion (m/z: 88) and intact ion (m/z: 732) when bombarded with three different acceleration voltages (5, 7.5, and 10 kV) in relation to kinetic energy per atom. We interpreted the result as follows. The secondary ion yield (Ym +) of species m, when excluding analyzer-related factors, can be expressed as a product of sputtering yield (Ym), ionization probability to positive ions (α+) and fractional concentration of the species m in the surface layer (θm) or (Ym + = Ym • α+ • θm). Ionization of biomolecules such as peptides often occurs by chemical interactions as represented by protonation or attachment with metal ions like Na+, K+ [17,18]. Probability of these interactions hardly depends on the kinetic energy of primary ions. Therefore, the value of α+ may not be changed much within a small range of kinetic energy per atom. The rate of contribution to Ym per atom decreases with the decrease in kinetic energy per atom. The decrease in kinetic energy per atom, however, means the bombardment with a larger cluster size when the acceleration voltage is constant. This means that more constituent atoms can interact with the sample surface and work together for the emission of the sample molecule from the surface. This multiple atom effect works toward increasing Ym. Because molecule dissociation is less likely to occur with decreased kinetic energy per atom, θm of small fragments becomes lower. We think that the 102 factor decrease in intensity of fragment ion of m/z 88 at 5 eV or below of kinetic energy per atom as shown in Figure 5 resulted from substantial suppression of dissociation of the peptide. We think that the decrease in intact ion intensity below 3 eV was due to the overriding influence of the decrease in Ym.
Figure 6 shows the secondary ion mass spectra measured with thin films of cytochrome C (molecular weight: 12,327), lysozyme (molecular weight: 14,307) and chymotripsin (molecular weight: ~25,000). Cluster size of Ar cluster ion was 1,500 atoms and the acceleration voltage 5 kV, making the kinetic energy per atom 3.3 eV. The relative intensity of fragment ions was low in the mass spectra of all samples and clearly showed the peaks considered to be molecular ions (M+) , doubly charged molecular ions (M2+), and dimer ions (2M+).
Ultra thin layer analysis
Cluster projectiles cannot penetrate into the bulk as easily as atoms. Hence, the damage caused by cluster projectiles is confined to a shallower volume. Furthermore, the sputtering yields can be enhanced when an atomic projectile is replaced by a cluster ion with the same incident energy, because the cluster would be shattered upon impact and many individual atoms bombard the sample simultaneously, each creating its own cascade of moving atoms. The simultaneous collision of many low-energy atoms caused by a cluster projectile enables effective sputter removal of surface contaminants, suggesting an improved surface sensitivity of SIMS analysis
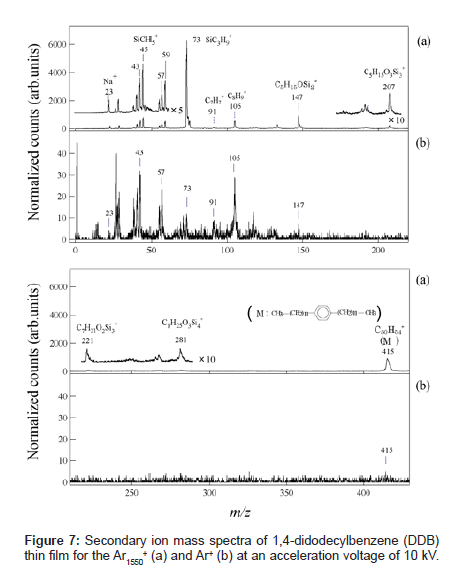
Figures 7(a) and (b) show typical SIMS spectra for 1,4-didodecylbenzene (DDB) (C30H54, molecular weight: 415) thin film bombarded by Ar1550 + and Ar+ ion beams, respectively. The secondary ion intensities were normalized by the primary ion current. The inset depicts the molecular structure of the DDB. Although the characteristic fragment ions of hydrocarbons such as C2H5 + (m/z=29), C3H7 + (m/ z=43), C4H9 + (m/z=57), C7H7 + (m/z=91), and C8H9 + ( m/z=105), were detected in the both spectra, the fragmentation patterns obtained by Ar gas cluster and atomic Ar+ projectiles show remarkable differences. Intact DDB ion was detected much more for the Ar-gas cluster SIMS than for the Ar+ SIMS, which was attributed to the low-energy irradiation effect of the GCIB, as reported in the previous section. The most significant difference in these spectra was that the secondary ions at m/z=45, 59, 73, 147, 207, 221 and 281 which were difficult to assign as hydrocarbon fragments from DDB sample, were detected with a very high intensity in the spectrum obtained by the Ar gas cluster projectile.
These fragment peaks are reasonably assigned to poly-dimethylsiloxane (PDMS) contaminant. These contaminant species are presumably from the air because they increased after exposure to the ambient laboratory air [19]. Table 1 compares the DDB SIMS spectra for Ar-gas cluster and Ar+. The peak intensity of the hydrocarbon fragment species from DDB in the gas cluster SIMS was intensified 10 to 20 times relative to those in the atomic SIMS. On the other hand, the peak intensities of the fragment species of PDMS were enhanced more than 100-500 times in gas cluster SIMS. This result indicates that the PDMS contaminants should be confined near the surface and their fragment ion intensities are more enhanced by the sputtering with gas cluster ions [14]. Thickness of the contaminant layer was roughly estimated to be ~2 nm by analyzing detailed angle dependent XPS data. Thus a large gas cluster projectile can sputter a much shallower volume of organic material than small projectiles, resulting in an extremely surface-sensitive analysis of organic thin films.
| m/z | assignment | Secondary ion Yield (Normalized counts) |
Ratio 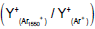 |
|
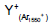 |
 |
|||
| 1 23 29 43 45 57 59 73 91 105 147 207 415 |
H+ Na+ C2H5+ C3H7+, CH3Si+ CH5Si+ C4H9+ C2H7Si+ C3H9Si+ C7H7+ C8H9+ C5H15OSi2+ C6H19Si3O2+ C30H54+ |
â?? ã?? 215 ã?? 229ã?? 574ã?? 800 ã?? 300 ã?? 513 ã?? 6268 ã?? 121 ã?? 530 ã?? 942ã?? 228ã?? 910ã?? |
40 2 22 32 3 23 2 12 9 30 7 â?? 5 |
â?? 108 10 18 267 13 257 522 13 18 134 â?? 182 |
Table 1: Intensity of major fragment peaks of DDB for Ar1550 + and Ar+ bombardment
Summary
We controlled the kinetic energy per atom of the Ar cluster ions used as primary ions for SIMS and successfully detected intact ions of proteins with a molecular weight of 20,000 or greater. Currently, SIMS can detect the molecular weight of intact ions up to approximately the 1,000 level. Certainly other studies have shown that larger molecular ions are detectable by making samples in matrix, etc [3,20]. But the most current pressing need is for measuring solid or thin film samples as they are, particularly with the development of bio-molecular nano-devices. Furthermore, we demonstrated that the extremely effective sputtering of shallow volume by Ar cluster irradiation enhances the surface contaminants species in the mass spectra, which should significantly improve the surface sensitivity of SIMS.
Acknowledgements
I acknowledge Dr. K. Moritani for his cooperation on this work. This work was partially supported by Development of System and Technology for Advanced Measurement and Analysis, Japan Science and Technology Agency.
References
- Vickerman CJ, Briggs D (2001) ToF-SIMS; Surface analysis by mass spectrometry: IM Publications, UK.
- Benninghoven A, Sichtermann KW (1978) Detection, identification and structural investigation of biologically important compounds by secondary ion mass spectrometry. Annal Chem 50: 1180-1184.
- Barber M, Bordoil SR, Sedgwick DR, Tyler NA (1981) Fast atom bombardment of solids as an ion source in mass spectrometry. Nature 293: 270-275.
- Gillen G, Fahey A (2003) Secondary ion mass spectrometry using cluster primary ion beams. Appl Surf Sci 203/204: 209-213.
- Wong CCS, Hill R, Blenkinsopp P, Lockyer NP, Weibel DE et al. (2003) Development of a C60 + ion gun for static SIMS and chemical imaging. Appl Surf Sci 203/204: 219-222.
- Davies N, Davies N, Weibel DE, Blenkinsopp P, Lockyer N, et al. (2003) Development and experimental application of a gold liquid metal ion source. Appl Surf Sci 203/204: 223-227.
- Brunelle A, Della-Negra S, Depauw J, Jacquet D, Le Beyec Y, et al. (2001) Enhanced secondary-ion emission under gold-cluster bombardment with energies from keV to MeV per atom. Phys Rev A 63: 022902.
- Toyoda N, Jiro Matsuo, Takaaki Aoki, Isao Yamada, David B Fenner, et al . (2003) Secondary ion mass spectrometry with gas cluster ion beams. Appl Surf Sci 203/204: 214-218.
- Wucher A (2006) Molecular ion formation under cluster bombardment: A fundamental review. Appl Surf Sci 252: 6482-6489.
- Yamada I, Jiro Matsuo, Zinetulla Insepov, Takaaki Aoki, Toshio Seki, et al. (2000) Nano-processing with gas cluster ion beams. Nucl Instr and Phys Res B 164/165: 944-959.
- Toyoda N, Matsuo J, Yamada I (2004) Surface modification with gas cluster ion beams from fundamental characteristics to applications. Nucl Instr and Phys Res B 216: 379-389.
- Toyoda N, Houzumi S, Yamada I (2006) Development of size-selected cluster ion irradiation system. Nucl. Instr. and Phys. Res. B 242: 466-468.
- Moritani K, Shingo Houzumi, Keigo Takeshima, Noriaki Toyoda, Kozo Mochiji (2008) Preferntial sputtering of DNA molecules on a graphite surface by Ar cluster ion beam. J Phys Chem C 112: 11357-11362.
- Houzumi S, Mochiji K, Toyoda N, Yamada I (2005) Scanning tunneling microscopy observation of graphite surfaces irradiated with size-selected Ar cluster ion beams. Jap J Appl Phys 44: 6252-6254.
- Moritani K, Michihiro H, Jun N, Takahiro K, Noriaki T, et al. (2008) Extremely low energy projectiles for SIMS using size- selected gas cluster ions. Appl Surf Sci 255: 948-950.
- Mochiji K, Hahinokuchi M, Moritani K, Toyoda N (2009) Matrix-free detection of intact ions from proteins in argon-cluster secondary ion mass spectrometry. Rapid Commun Mass Spectrom 23: 648-652.
- Lange W, Jirikowsky M, Benninghoven A (1984) Secondary ion emission from UHV-deposited amino acid over layers on metals. Surf Sci 136: 419-436
- Delcorte A, Bertrand P (1998) Sputtering of parent-like ions from large organic adsorbates on metals under keV ion bombardment. Surf Sci 412/413: 97-124.
- Tanaka M, Kousuke M, Tomokazu H, Noriaki T, Isao Y (2010) Enhanced surface sensitivity in secondary ion mass spectrometric analysis of organic thin films using size-selected Ar gas-cluster ion projectiles. Rapid Commun. Mass Spectrom 24: 1405-1410.
- Seki S, Kambara H, Naoki H (1985) Sequence analysis for an unknown peptide by molecular secondary ion mass spectrometry. Organic Mass Spectrom 20: 18-24.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15249
- [From(publication date):
specialissue-2014 - Dec 23, 2025] - Breakdown by view type
- HTML page views : 10443
- PDF downloads : 4806